Abstract
In the main growing areas in Serbia, plants with symptoms of stem blight were sampled in nine orchards with American highbush blueberry (Vaccinium corymbosum), cultivar ‘Duke’, with high disease incidence, and 153 samples were taken. A total of 128 Botryosphaeriaceae isolates were characterized on the basis of morphology, sequence analysis, multilocus phylogeny based on ITS, TEF1-α and TUB2 sequences and pathogenicity, and belonged to one of the four species Neofusicoccum parvum, Botryosphaeria dothidea, Diplodia seriata and Lasiodiplodia iraniensis. Both D. seriata and L. iraniensis were detected for the first time on blueberries in Serbia, and L. iraniensis was detected for the first time on blueberries worldwide. Comparative morphological and TEF1-α sequence analyses allowed a clear separation of L. iraniensis from the phylogenetically closely related L. fujianensis, L. thailandica and L. endophytica. Of the nine blueberry cultivars ‘Aurora’, ‘Barbara Ann’, ‘Bluecrop’, ‘Bluejay’, ‘Draper’, ‘Duke’, ‘Huron’, ‘Patriot’ and ‘Spartan’ inoculated with L. iraniensis (isolate 421-19), the cultivar ‘Duke’ was the most susceptible. In our study, the majority of orchards were in their second or third year of production, implying that the planting material is likely to be the source of infection, emphasizing the importance of pathogen-free planting material.
1. Introduction
The American highbush blueberry (Vaccinium corymbosum L., Fam. Ericaceae) is commercially cultivated worldwide under various climatic conditions [1] as its fruits have exceptional nutritional properties and positive effects on health [2]. As it is a very profitable crop, the global production of blueberries is constantly increasing, with more than 1.2 million tonnes being produced in 2022, and America being the largest producer (979,668 tonnes), followed by Europe (207,915 tonnes) (https://www.fao.org/faostat, accessed on 15 May 2025). In Serbia, the production of highbush blueberries is also increasing very rapidly, much faster than the production of any other fruit species [3] and is currently grown on over 2500 ha [4].
Blueberry production worldwide can be affected by a number of biotic and abiotic factors, and among these, fungi from the Botryosphaeriaceae family are considered one of the most important and devastating factors limiting blueberry production [5,6,7,8,9]. In New Zealand, diseases caused by Botryosphaeriaceae are considered particularly devastating in newly planted orchards, where nearly 20% of plants are infected and significant annual costs are incurred due to yield loss and replanting costs [6]. The most important factor contributing to the rapid spread of Botryosphaeriaceae disease is probably related to the health status of the planting material [10]. Due to the broad host range, latent infections, the ability to infect plants via wounds and the limited possibilities of efficient disease control [11], Botryosphaeriaceae pose a major challenge to the production of numerous host plants, including blueberries. The fungal family Botryosphaeriaceae currently comprises 24 defined genera with diverse lifestyles, saprobes, endophytes and pathogens associated with a wide range of host plants. Among the Botryosphaeriaceae, the genera Botryosphaeria, Diplodia, Lasiodiplodia, Neofusicoccum, Dothiorella and Neoscytalidium are the most important and best-studied plant pathogens [12].
Although previously considered a possible synonym of Diplodia [13], the fungal genus Lasiodiplodia Ellis & Everh has long been recognized and is well defined based on the morphology of the pycnidia, the longitudinal striation of the mature conidia and phylogenetic studies [14,15]. Lasiodiplodia is a very dynamic genus with over 47 established species to date, with new species being described relatively frequently [16,17]. Some Lasiodiplodia species are even considered to be of quarantine importance, such as L. pseudotheobromae [18] and more recently, L. iraniensis [19]. Lasiodiplodia iraniensis is a relatively newly described species that occurs as a pathogen of Salvadora persica, Juglans spp., mango, Eucalyptus spp., Citrus spp. and tropical almonds in Iran [20]. Several studies have shown that the status of isolates identified as L. jatrophicola as a closely related but distinct species from L. iraniensis is not justified, and it is currently synonymized with L. iraniensis [19,21,22,23]. After the initial description, L. iraniensis was found on mango in Western Australia [24], the United Arab Emirates [25], Brazil [26] and Peru [22], on mandarins in the United Arab Emirates [25], Bougainvillea spectabilis in southern China [27], Anacardium occidentale in Brazil [28], Persian lime in Mexico [23] and, more recently, on Adansonia digitata in Mozambique [29], Eucaliptus in India [30], bananas in Brazil [31] and yam and sweet oranges [19,32] in the USA. In all these regions and on all host plants, L. iraniensis has been described as an aggressive and economically important pathogen.
The intensive increase in blueberry production in Serbia has been accompanied by the appearance of various symptoms of stem blight of a largely unknown origin, which has triggered research that has recently confirmed the presence of blueberry strain pathogens including Macrophomina phaseolina [33], Fusarium sporotrichioides [34], Neopestalotiopsis clavispora [35] and N. vaccinii, N. rosae, Diaporthe eres, D. foeniculina and Neofusicoccum parvum [36]. During the study of blueberry stem diseases, we obtained a considerable number of Botryosphaeriaceae isolates from symptomatic plants, and our main objectives were as follows: (i) to identify the causal pathogens; (ii) to investigate morphological characteristics and the ability to grow at extreme temperatures; (iii) to determine the taxonomic position of the obtained isolates based on the sequences of ITS rDNA, translation elongation factor 1α (TEF1-α) and β-tubulin (TUB2); (iv) to determine the phylogenetic relationship between the Botryosphaeriaceae and, in particular, between the Lasiodiplodia spp. isolates and the relationship with newly detected species in Serbia; and (v) to evaluate the susceptibility of nine blueberry cultivars grown in Serbia and worldwide to selected discovered Lasiodiplodia sp.
2. Materials and Methods
2.1. Sampling and Isolations
The blueberry stem disease survey was conducted from 2011 to 2022, and the field inspections covered nine production fields/locations in four administrative districts in Serbia. The blueberry cultivar ‘Duke’ was grown in all orchards and a total of 153 samples were collected (Table 1). Disease incidence was calculated in each orchard (by zigzag inspections and random assessment of 100 plants in three replicates), and 5–25 samples were collected, depending on size of the orchard and symptoms. Small woody fragments of necrotic tissue were taken from each sample, surface sterilized with 2% sodium hypochlorite, placed on potato dextrose agar (PDA; 200 g potato, 20 g dextrose, 17 g agar and 1 litre distilled H2O) [37] and incubated at 24 °C for 5 days. One or more representative colonies with the same morphology were selected from each of the nine growing fields, from which monosporial isolates were obtained for further characterization. The isolates were stored on sealed PDA slants at 4 °C in the fungal collection of the Department of Phytopathology, Institute of Phytomedicine, University of Belgrade—Faculty of Agriculture.

Table 1.
Geographic distribution of isolates collected in Serbia.
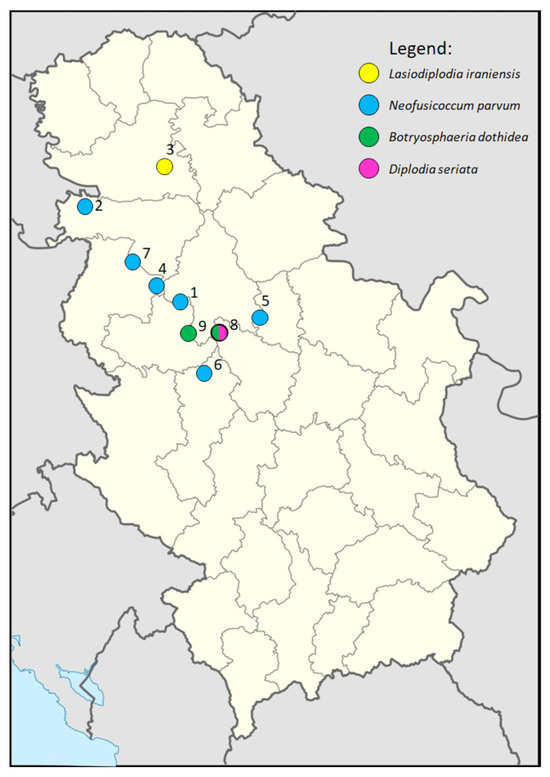
Figure 1.
Geographic distribution of localities in Serbia included in the survey and detected isolates.
2.2. Morphological and Ecological Characterization
Colony appearance, including colour and shape, was assessed 14 dpi (days post inoculation) on PDA at 24 °C in the dark. Growth rate was determined by measuring two perpendicular colony diameters in five replicates per isolate and calculating an average value for each isolate. To induce sporulation, isolates were cultured on pine needle agar (PNA: 17 g agar, 1 litre distilled H2O and sterilized pine needles placed onto the medium) [38]. The presence and appearance of pycnidia and conidia were observed at 14, 21, 28 and 35 dpi using a compound microscope (Olympus CX41, Olympus Europa SE & Co. KG), and the dimensions of pycnidia and immature and mature conidia were measured (50 and 100 randomly selected, respectively). The selected Lasiodiplodia spp. isolates were physiologically characterized based on colony appearance and ability to grow on PDA at temperatures of 5, 10, 15, 25, 35, 37.5 and 40 °C, as determined by measuring two perpendicular colony diameters in five replicates per isolate and calculating an average value for each temperature. Data were analyzed with SPSS (version 29, IBM, NY, USA) using one-way ANOVA followed by Duncan’s multiple range test at p < 0.05.
2.3. DNA Amplification and Sequencing
Total genomic DNA was extracted using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany) from 100 mg of dry mycelium from 7-day-old cultures of 38 selected isolates grown in potato dextrose broth (PDB; 200 g potato, 20 g dextrose and 1 L distilled H2O), following the manufacturer’s instructions. PCR amplification of three genomic regions, including ITS rDNA (38 isolates), TEF1-α (24 isolates) and TUB2 (24 isolates), was performed using the primers ITS1F/ITS4 [39,40], Bt2A/Bt2B [41] and EF1-728/EF1-986 [42], on annealing temperatures 52 °C, 55 °C and 58 °C, respectively. All reactions were performed in a total volume of 25 μL consisting of 12.5 μL of 2× PCR Master Mix (Fermentas, Lithuania), 6.5 μL of RNase-free water, 2.5 μL of both forward and reverse primers (working solution with a final concentration of 100 pmol/μL, Metabion International, Germany) and 1 μL of template DNA. The amplification conditions were as follows: initial denaturation at 94 °C for 5 min, followed by 40 cycles of denaturation at 94 °C for 30 s, variable recommended annealing conditions, elongation at 72 °C for 1 min and final elongation for 10 min at 72 °C. The amplicons obtained were stained with ethidium bromide, analyzed by 1% agarose gel electrophoresis and visualized with a UV transilluminator. The PCR products of all genomic regions were sequenced directly in both directions with an automatic sequencer (Automatic Sequencer Macrogen Inc., The Netherlands) using the same primers as for amplification. The consensus sequences were calculated with ClustalW [43], integrated into the software MEGA X [44], and deposited in GenBank (http://www.ncbi.nlm.nih.gov, accessed on 1 April 2025).
2.4. Sequence and Phylogenetic Analyses
Sequences generated from the selected 38 isolates were compared with each other by calculating nucleotide (nt) similarities, as well as with previously deposited isolates available in the GenBank, using the similarity search tool BLAST (version 2.13.0, NCBI) for identification at the genus level.
Multilocus phylogenetic sequence analyses (ITS rDNA, TEF1-α and TUB2) were performed on two data sets, one to clarify the position of 24 Serbian isolates within the family Botryosphaeriaceae and the other to clarify the position of five Lasiodiplodia isolates within the genus Lasiodiplodia. The targeted analyses of Botryosphaeriaceae included 10 previously listed type-derived species (38 reference isolates) and Melanops tulasnei [45,46,47], while other targeted analyses included 34 previously listed type-derived species (49 reference isolates) and Dothiorella viticola [48] as an outgroup (Table 2) with gaps and missing data treated as missing characters. The phylogenetic trees were inferred using the Maximum Likelihood method implemented in MEGA X software [44]. Gamma distributed Tamura-Nei model (G+I) determined by a Model test implemented in MEGA X was used as the best-fitting model of nucleotide substitution. All sites with gaps were omitted. The reliability of the obtained trees was evaluated with 1000 bootstrap replicates.

Table 2.
Isolates of the Botryosphaeriaceae species used in this study.
The position of the Serbian Lasiodiplodia isolates was further evaluated based on the nucleotide polymorphism within the TEF1-α gene. A total of 19 sequences of the closely related L. iraniensis, L. fujianensis, L. thailandica and L. endophytica were aligned and analyzed using the sequence of L. endophytica as a representative [49].
2.5. Pathogenicity Testing
The pathogenicity of 70 Botryosphaeriaceae isolates (40 N. parvum, 20 B. dothidea, 5 D. seriata and 5 L. iraniensis) was tested by the artificial wound inoculation of branches of a 6-year-old healthy blueberry ‘Duke’ from a collection orchard of the Fruit Research Institute Čačak, Serbia, using mycelial plugs, as previously described [16,30,50]. Well-developed, symptomless blueberry branches were superficially sterilized and a clear cut approximately 0.5 cm long incision was made with a sterile scalpel blade without damaging the underlying cambial tissue. Mycelial plugs (5 mm diameter) from the edge of a 4-day-old PDA culture grown at 24 °C were placed under the bark (mycelial surface facing downwards) and the wound was sealed with sterilized moist cotton wool and Parafilm. As a negative control, branches were inoculated with sterile PDA plugs. Three branches were inoculated with each isolate, and the experiment was repeated twice. The pathogenicity of the isolates was assessed 14 dpi. Re-isolations were made from all symptomatic cuttings using the same methods as for isolation.
2.6. Cultivar Susceptibility Testing
In order to assess the susceptibility of blueberry cultivars to infection, a selected L. iraniensis isolate (421-19) was used for the inoculations of branches of six-year-old healthy plants of nine different blueberry cultivars (‘Aurora’, ‘Barbara Ann’, ‘Bluecrop’, ‘Bluejay’, ‘Draper’, ‘Duke’, ‘Huron’, ‘Patriot’ and ‘Spartan’). The experiment was carried out as previously described for the pathogenicity testing. The disease intensity of the nine blueberry cultivars was assessed after 14 dpi. For the purpose of rating, the following 0–4 scale was established in this study based on symptom intensity: 0—no reaction; 1—surface necrosis near the wounded spot; 2—necrosis length from 2 to 20 mm; 3—necrosis length from 21 to 40 mm; and 4—necrosis length greater than 40 mm. The inoculations were performed in 3 replicates and the entire experiment was performed twice. The data were analyzed with the SPSS Software (version 29, IBM, USA) using one-way ANOVA followed by Duncan’s multiple range test at p < 0.05.
3. Results
3.1. Disease Symptoms and Isolates
During the survey, diseased blueberry plants were observed at the nine locations in Serbia (Figure 1), from which 153 samples were collected (Table 1), resulting in 236 isolates, of which representative monosporial isolates were morphologically categorized into 11 morphogroups, of which one or several representative isolates were identified by sequencing the ITS region to the genus level. A total of 128 Botryosphaeriaceae-like isolates were detected in single (three locations) or mixed infection with several non-Botryosphaeriaceae species (Table 1). Symptomatic plants were randomly distributed in groups along the rows or patches of different sizes in the orchards. All sampled orchards were up to six years old and disease incidence was estimated at sampling and ranged between 10 and 30% (mean 20.6%). The plants showed symptoms such as twig dieback, stem blight and wilt, followed by whole plant decay (Figure 2A,G,M and Figure 3A,C). Cross sections of symptomatic branches showed varying degrees of internal tissue necrosis, which correlated with symptom intensity (Figure 2B,H,N and Figure 3B). The spatial distribution of diseased plants along rows or groups of plants in close proximity is probably due to long-distance dispersal by planting material, which is responsible for the introduction of pathogens into the orchards, as well as the spread of the inoculum over short distances within the orchards by the movement of raindrops. Among the Botryosphaeriaceae-like isolates, four species were detected by ITS sequencing. N. parvum was the most prevalent with a detection frequency of 34.75% (82 isolates out of 236) (six out of nine localities). B. dothidea was detected in two localities with a detection frequency of 13.98% (33/236). D. seriata and L. iraniensis were both represented by five isolates from two individual localities with a detection frequency of 6.25 and 3.91, respectively. No species-specific symptomatology was observed. For further detailed characterization, twenty-four isolates were selected, eight isolates of N. parvum, seven of B. dothidea, four of D. seriata and five of L. iraniensis.

Figure 2.
Symptomatology and morphology of Botryosphaeriaceae isolates from blueberry in Serbia: stem blight, wilting and inner tissue necrosis caused by Neofusicoccum parvum (A,B), Botryosphaeria dothidea (G,H) and Diplodia seriata (M,N); surface and reverse side of two weeks old colonies on PDA of N. parvum (C,D), B. dothidea (I,J) and D. seriata (O,P); pycnidium and conidia four weeks post inoculation on PNA of N. parvum (E,F), B. dothidea (K,L) and D. seriata (Q,R).

Figure 3.
Symptomatology and morphology of Lasiodiplodia iraniensis isolates from Serbia: stem blight and wilting of blueberry (A); inner tissue necrosis (B); numerous pycnidia protruding bark on diseased branches (C); one (D) and two week old colonies on PDA (E); unicellular hyaline immature and pigmented mature conidia four weeks post inoculation on PNA (F); pigmented, 1-septate conidia with longitudinal striations (G), necrosis on inoculated branches of nine different blueberry cultivars: ‘Aurora’, ‘Spartan’, ‘Barbara Ann’, ‘Patriot’, ‘Huron’, ‘Draper’, ‘Bluejay’, ‘Bluecrop’ and ‘Duke’ (H–P).
3.2. Fungal Morphology
The observed morphological characteristics of 70 Botryosphaeriaceae isolates from blueberries in Serbia showed stable uniform morphological characteristics within the four Botryosphaeriaceae species detected.
All 40 N. parvum isolates had a uniform appearance and initially formed white colonies with a grey centre after three days of incubation. With ageing, the colour of the colonies changed in all isolates, so that after seven days they were olive-grey on the surface and greenish grey on the reverse side and finally after two weeks of incubation they were dark grey on the surface and almost black on the reverse side. The aerial mycelium of all isolates was woolly and dense and often grouped in tufts that reached the lid of the Petri dish (Figure 2C,D). All isolates grew fast with average daily growth rates of 14.83 ± 1.09 mm, with no statistical differences between isolates. On PNA, all isolates formed globose blackish pycnidia after two weeks with average dimensions of (250-) 619.80 (-1250) × (200-) 547.75 (-1000) µm (Figure 2E), in which hyaline, fusiform to ellipsoidal, aseptate conidia (16.82–19.06 × 6.54–10.54 µm, average 17.94 ± 1.12 µm × 8.59 ± 2.05 µm) were visible after four weeks of incubation (Figure 2F).
All 20 isolates of B. dothidea also showed a uniform morphology and initially formed white, almost transparent colonies, which became darker in the centre after three days and dark olive-grey after seven days. With ageing after two weeks of incubation, the colonies became dark grey on the surface and dark brown, almost black, on the reverse, all with dense aerial mycelium, and often grouped in tufts that reached the lid of the Petri dish (Figure 2I,J). The average growth rate for all isolates was 13.5 ± 0.92 mm, with no statistical differences. After two weeks of incubation on PNA, all isolates developed blackish pycnidia with an average size of (230-) 365 (-500) × (200-) 287.5 (-375) µm (Figure 2K), with hyaline, fusiform, mostly aseptate conidia (24.91–29.09 × 6.88–9.12 µm, average 27 ± 2.09 × 8 ± 1.12 µm) developed well after four weeks of incubation (Figure 2L).
All five isolates of D. seriata also showed no differences in the appearance of the colonies and initially formed whitish colonies with a visible light olive-brown centre after three days. With further incubation, the colonies became dark olive-grey on the surface and dark grey, almost black, on the reverse after two weeks (Figure 2O,P). The aerial mycelium was dense and fluffy. The colonies of all isolates were fast growing with average growth of 26.2 ± 1.05 mm, overgrowing the entire surface of the Petri dish within two days, with no statistical differences. After two weeks of incubation on PNA, blackish pycnidia (Figure 2Q) could be observed, but they were immersed in the needles, woolly and densely covered with mycelium, making it difficult to determine the exact dimensions. One week after pycnidia formation (three weeks after inoculation on PNA), ovoid to oblong, elliptical, aseptate conidia could be observed (Figure 2R), which were initially hyaline and turned brown with age and were mainly uniseptate (22.95–26.75 × 9.50–10.75 µm, average 25 × 10 ± 1.15 μm), with no statistical differences between the five characterized isolates.
All five isolates of L. iraniensis exhibited uniform morphological characteristics on PDA and formed fast-growing, abundant aerial colonies with an average daily growth rate of 23.6 ± 1.12 mm and overgrew the surface of a 90 mm Petri dish in two days. Initially, the colonies of all isolates were whitish to smoky grey and became grey to olivaceous at the surface and dark, almost black, on the reverse side after two weeks (Figure 3D,E). Sporulation was induced on PNA and blueberry branches, where all isolates formed globose, black pycnidia covered with a dense mycelium (with average dimensions of (520-) 612.5 (-950) × (300-) 400 (-450) µm) after 14 dpi (Figure 3C). The presence of unicellular, hyaline, grey, immature conidia was recorded three weeks after inoculation (19.60–24.40 × 15.0 µm, average 22.00 ± 2.4 × 15.00 ± 0 µm). Approximately 60% of the conidia were pigmented, ellipsoid to ovoid, 1-septate with longitudinal striations (average (20.15–23.35 × 9.75–12.75 µm, 21.75 ± 1.6 × 11.25 ± 1.5 µm) four weeks after inoculation (Figure 3F), and after five weeks, all conidia were mature and pigmented (Figure 3G). There were no statistical differences between the five isolates in terms of growth rate and length of immature versus mature conidia (), except that the immature conidia were wider compared to the mature conidia (Fisher LSD method and 95% confidence).
Ecological characterization of L. iraniensis showed that none of the isolates were able to grow at 5 °C and 40 °C, while growth was recorded at cardinal temperatures of 10 and 37.5 °C (average daily growth for all isolates 6.9 and 2.65 mm, respectively). All isolates grew fastest at 25 and 35 °C (average for all isolates 23.6 and 22.35 mm, respectively). None of the isolates produced pink pigment on PDA at 35 °C in darkness.
3.3. Molecular Identification and Phylogenetic Analyses
BLASTn analyses for each of the ITS, TEF1-α and TUB2 sequences of the morphologically characterized isolates confirmed the identification and proved that eight N. parvum isolates generated in this study share 98.5–100% nucleotide (nt) similarity with N. parvum ex-type isolate CBS 112931, seven isolates of B. dothidea 97.4–100% nt similarity with B. dothidea ex-type isolate CBS 115476 and four isolates of D. seriata 96.1–100% similarity with D. seriata ex-type (CBS 112555). The sequences of five L. iraniensis isolates had 100% nt similarity and 99.6–100% nt sequence similarity to sequences of L. iraniensis (including the ex-type isolate CBS 124710), L. pseudotheobromae, L. theobromae and L. gonubiensis. Similarly, TEF1-α and TUB2 Lasiodiplodia sequence analyses showed that the Serbian isolates have 99.3–99.7% and 97.2–100% nt sequence similarity with sequences of multiple Lasiodiplodia species, respectively.
A multi-locus phylogenetic analysis based on the combined ITS, TEF1-α and TUB2 gene regions using the Maximum likelihood method which included 24 Serbian sequences from four species (N. parvum, B. dothidea, D. seriata, L. iraniensis) and 40 selected isolates from the Botryosphaeriaceae family belonging to 10 species yielded a phylogenetic tree that clearly resolved the topology of several well-supported clades corresponding to N. parvum, B. dothidea, D. seriata, L. iraniensis and other related species. The Serbian isolates clustered within their respective species clades and formed well-supported subclades together with the corresponding ex-type or reference strains (Figure 4). Within the N. parvum clade four subclades are indicated, all comprising 1–4 isolates from Serbia, demonstrating the diversity of blueberry isolates in Serbia.
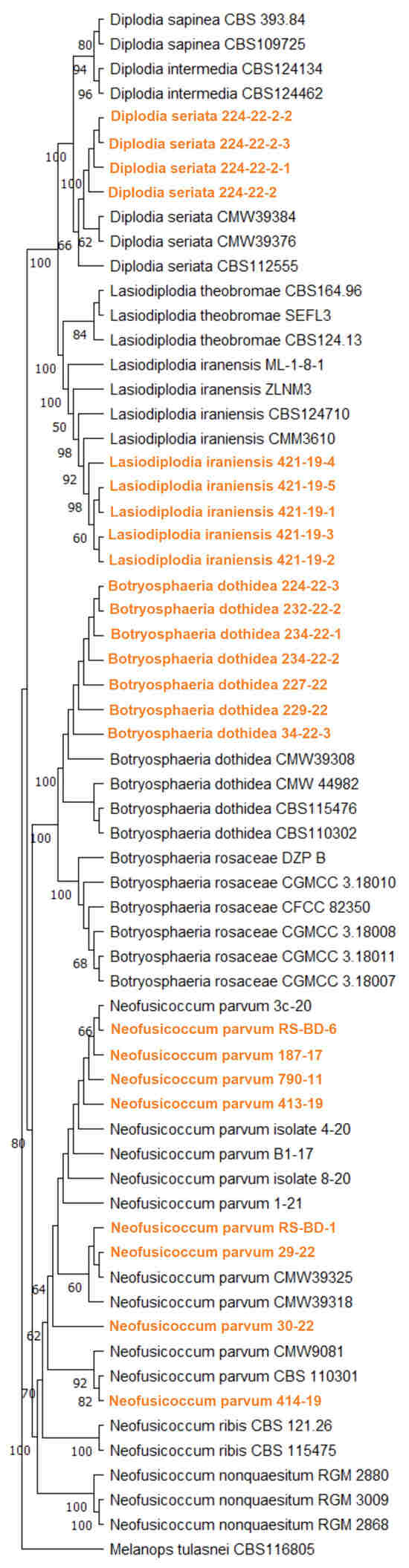
Figure 4.
Maximum likelihood phylogenetic tree inferred from concatenated ITS rDNA, TEF1-α and TUB2 genes of 24 Serbian and 10 previously listed type-derived Botryosphaeriaceae species (38 reference isolates) and Melanops tulasnei as an outgroup. Phylogram was generated with MEGA X using Tamura-Nei model Gamma distributed (G+I) [44]. Bootstrap analysis was performed with 1000 replicates and bootstrap values (>50%) are shown next to relevant branches. The Serbian Botryosphaeriaceae isolates are orange coloured.
Phylogenetic analyses of the ITS, TEF1-α and TUB2 sequences using the Maximum likelihood method, which included five Serbian and fifty selected Lasiodiplodia isolates belonging to 35 species, yielded a phylogenetic tree whose topology and resolution are consistent with previous identification of publicly available isolates (Figure 5). The well-supported branch, which includes the closely related L. iraniensis, L. fujianensis, L. thailandica and L. endophytica, also included all Serbian isolates more closely related to L. iraniensis and L. fujianensis.
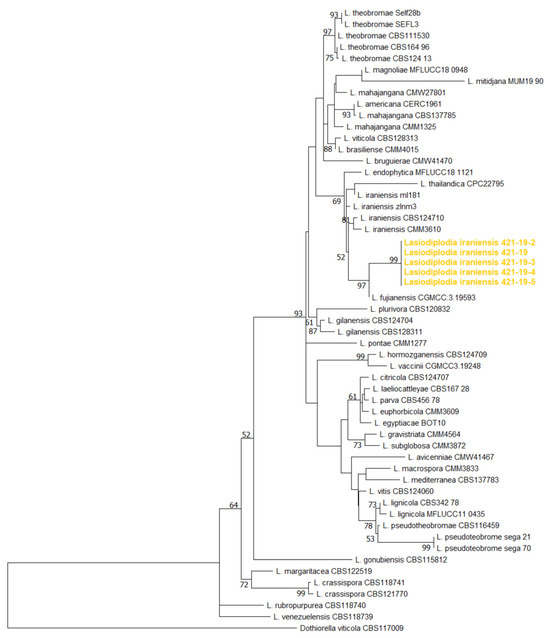
Figure 5.
Maximum likelihood phylogenetic tree inferred from concatenated ITS rDNA, TEF1-α and TUB2 genes of 5 Serbian and 34 previously listed type-derived species (54 reference isolates) Lasiodiplodia spp. and Dothiorella viticola as an outgroup. Phylogram was generated with MEGA X using Tamura-Nei model Gamma distributed (G+I) [44]. Bootstrap analysis was performed with 1000 replicates and bootstrap values (>70%) are shown next to relevant branches. The Serbian Lasiodiplodia iraniensis isolates are orange coloured.
Subsequent TEF1-α sequence analyses of L. iraniensis, L. fujianensis, L. thailandica and L. endophytica revealed polymorphism at several positions (Table 3). In the analyzed set, L. thailandica isolates were unique as they had adenine at positions 14 and 55 and an insertion at positions 60–67, while all isolates of L. iraniensis, including Serbian isolates, were unique as they had adenine at positions 16 and 68. L. iraniensis could be easily distinguished from the closely related L. fujianensis, which is also a pathogen of blueberries [17] and has cytosine and thymine at positions 16 and 68, respectively. In addition, all 15 L. iraniensis isolates available to date form two separate haplotypes based on single nucleotide polymorphism, as they have either cytosine (9 isolates) or thymine (5 isolates) at position 137.

Table 3.
Translation elongation factor 1α gene nucleotide polymorphism of all available isolates of Lasiodiplodia iraniensis, L. fujianensis, L. thailandica and L. endophytica.
3.4. Pathogenicity
All 70 detected isolates of Botryosphaeriaceae caused visible symptoms 14 dpi on all inoculated branches, which completely resembled the symptoms of a natural infection. All isolates showed uniform pathogenicity and caused similar reactions in terms of the appearance and intensity of symptoms on the inoculated branches. A large number of pycnidia were observed in the necrotic area of all inoculated branches. No symptoms were observed on the control plants. All isolates were easily reisolated from all inoculated and symptomatic branches, so that Koch’s postulates were fulfilled.
3.5. Cultivar Susceptibility
When evaluating the response of cultivars to inoculation with the selected L iraniensis isolate 421-19, visible symptoms of necrosis were well developed 14 dpi on all inoculated branches of all nine blueberry cultivars tested, namely ‘Aurora’, ‘Barbara Ann’, ‘Bluecrop’, ‘Bluejay’, ‘Draper’, ‘Duke’, ‘Huron’, ‘Patriot’ and ‘Spartan’. The appearance of symptoms was similar in all cultivars and resembled a natural infection, while the control plants of all inoculated cultivars showed no symptoms. The intensity of necrosis varied from cultivar to cultivar (Figure 2H–P), and statistical analysis revealed that symptom development was significantly dependent on cultivar (p < 0.001). The cultivar ‘Duke’ proved to be the most susceptible cultivar with an average score of 3.17 ± 0.983, while the remaining eight cultivars formed a statistically uniform group with similar disease intensity, with average scores of 1.049 ± 0.105 (‘Aurora’)–2.17 ± 0.983 (‘Bluejay’ and ‘Draper’) (Figure 6).
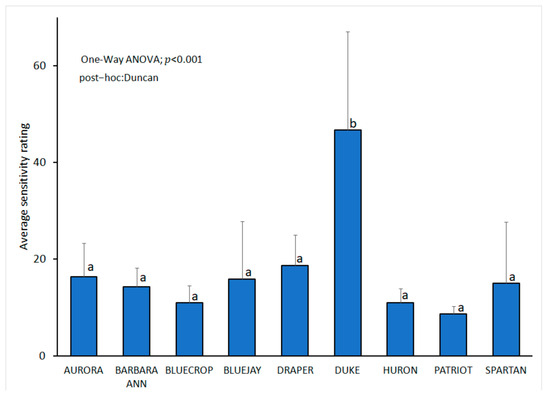
Figure 6.
Lasiodiplodia iraniensis: susceptibility of nine blueberry cultivars analyzed with one-way ANOVA followed by Duncan’s multiple range tests at p < 0.05 using SPSS software (IBM, USA) assessed 14 days post inoculation, following 0–4 scale based on the symptom intensity: 0—no reaction; 1—surface necrosis near wounded spot; 2—necrosis lengths from 2 to 20 mm; 3—necrosis lengths from 21 to 40 mm, 4—necrosis length longer than 40 mm. The bars represent standard deviation. Values labelled with the same letter do not differ significantly.
4. Discussion
As a result of our symptom-based study of blueberry dieback in the main growing areas, we found N. parvum, B. dothidea, D. seriata and L. iraniensis, mainly in the form of mixed infections, causing stem blight and plant decay with an average disease incidence of over 20% in all orchards in Serbia. Both D. seriata and L. iraniensis were detected for the first time on blueberries in Serbia, and L. iraniensis was detected for the first time on blueberries worldwide.
N. parvum and B. dothidea are known to have a broad host range [21,52] and were recently detected on blueberries in Serbia [36]. Our study revealed a high prevalence of N. parvum, which is comparable to other blueberry growing areas in the world [8,38,54]. In Serbia, both N. parvum and B. dothidea are known pathogens of trees and shrubs [45,55,56,57], and in addition, B. dothidea is a known post-harvest pathogen of apples and quinces [58,59,60] and a root rot pathogen of sugar beet [47]. Although D. seriata, the third species detected, is known to infect trees and shrubs in Serbia [55] and after harvest on apples and quinces [59,61], it was not known to infect blueberries prior to our study. D. seriata is not very common in blueberries worldwide and has been found in a couple of samples in New Zealand [6,10] and the United States [62], which is similar to the situation in Serbia.
The fourth species detected, L. iraniensis, is a new pathogen for Serbia and was detected in blueberries for the first time worldwide. L. iraniensis has so far been detected mainly in tropical plants and nuts [19,20,22,23,24,25,26,27,28,29,30,31,32]. To date, at least 10 different Lasiodiplodia species have been described as blueberry pathogens. L. chinensis [16], L. clavispora, L. fujianensis, L. henanica, L. nanpingensis [17] and L. pseudotheobromae have been described in China [63], and L. laeliocattleyae in Peru [50]. L. mediterranea has been recorded in the USA [64], Australia [65] and Mexico [66], while L. theobromae occurs in Spain [9,67], China [8], Peru [50], the USA [7] and Australia [65]. L. vaccinii has been recorded in China [16], which shows that this genus could be associated with blueberries in general.
Infected blueberry plants in Serbia showed typical symptoms of Botryosphaeria stem blight [9,11,17,50] and could not be associated with any of the four detected species. Conventional identification of the Serbian isolates based on morphology and growth rate showed that they share characteristics of N. parvum [9,14,68,69,70,71,72], B. dothidea [69,72,73,74,75,76,77], D. seriata [59,61,72,78,79,80,81] and L. iraniensis [19,20,30,32]
Phylogenetic analyses of all detected Botryosphaeriaceae not only confirmed the identity of N. parvum, B. dothidea and D. seriata, but also revealed considerable diversity among isolates of N. parvum in Serbia, which is comparable to the high genetic variation observed in the New Zealand population from grapevines [82] or in Korea on Japanese bay trees [70] as well as in the production of pathogenicity-related toxins in N. parvum populations in France and Portugal [83]. A previous characterization of the population of N. parvum from blueberries in Serbia [36] did not reveal significant diversity among isolates, possibly due to a lower number of sampled orchards where infection could be due to a single introduction. In our study, diversity was shown to be the likely result of multiple introductions. The reverse situation and low diversity within the B. dothidea branch and between isolates from Serbia were similar to the situation of walnut in France [84] and olive in Croatia [72]. The low diversity among isolates of D. seriata detected in our study is to be expected as all isolates originated from a single field, probably as a result of a single introduction, although some variability in the D. serata population has been observed elsewhere [85,86].
The identity of Serbian L. iraniensis within the Botryosphaeriaceae as well as within Lasiodiplodia spp. could not be fully confirmed by phylogenetic analyses based on three loci, as has already been shown for some Lasiodiplodia isolates [52,62]. The Serbian isolates branched with the closely related L. iraniensis, L. fujianensis, L. thailandica and L. endophytica [17,21,49], but also share some of the morphological characteristics such as colony appearance and growth rate [17,19,20,30,32,87]. All four species can be clearly distinguished by the presence and size of the pycnidium as well as the septation and colour of the mature conidia. The Serbian L. iraniensis isolates form pycnidia with an average size of 612.5 µm (up to 850 µm), which is consistent with previously published values for L. iraniensis (up to 980 µm, [20]) and differs markedly from the larger pycnidia of L. fujianensis (up to 1.3 mm, [17]) and the much smaller pycnidia of L. thailandica [87], while L. endophytica does not sporulate in culture [49]. The morphology of the conidia is also a solid tool to distinguish between these four species. Serbian and all previously published isolates of L. iraniensis form pigmented, dark brown, mature conidia that are 1-septate [19,20,31,32,88,89]. The closely related L. fujianensis can be easily distinguished as it forms pigmented but aseptate mature conidia [17], while L. thailandica is characterized by the fact that most mature conidia remain hyaline [49,87,88].
Sequence analysis of the TEF1-α gene provided further confirmation of the clear distinction of L. iraniensis from the closely related L. fujianensis, which was recently detected as a blueberry pathogen in China [17], and from the phylogenetically closely related L. thailandica and L. endophytica, which were not recorded as blueberry pathogens. All available L. iraniensis, including the five Serbian isolates, shared adenine at positions 16 and 68 of the analyzed fragment of the TEF1-α gene, which is a unique sequence feature. In our study, we also found that the previously characterized population of L. iraniensis consists of two haplotypes based on the presence of cytosine or thymine at position 137 of the TEF1-α gene, which represents the first worldwide population analysis of this pathogen. The Serbian isolates belong to the rarer cytosine–haplotype and are identical to the L. iraniensis isolates from Jatropha curcas in Brazil (described as L. jatrophicola, [17,53]) and sweet orange in the USA [32]. The potential role and importance of this diversity in the L. iraniensis population will likely become clearer as additional data and isolates become available and characterized.
There are no studies on the susceptibility of different blueberry accessions or cultivars to Lasiodiplodia spp. Even the data on cultivars naturally infected with Lasiodiplodia spp. are limited. In China, L. theobromae was isolated from the cultivar ‘Misty’ and L. pseudotheobromae from M6 [8]. Our studies on the susceptibility of nine blueberry cultivars are valuable and provide the first data on the presence of different levels of susceptibility in nine tested cultivars. Blueberry ‘Duke’ was found to be significantly more susceptible compared to the other cultivars, which should be further confirmed under different conditions and in other blueberry growing regions. The tested cultivars ‘Aurora’, ‘Bluecrop’ and ‘Bluejay’, which are predominant in blueberry cultivation in the USA [90], responded well and developed low disease severity. The observed difference between the blueberry cultivars tested and the fact that L. iraniensis was isolated from ‘Duke’ in this study may indicate a possible link between natural infection and susceptibility of a particular cultivar.
In Serbia, blueberry ‘Duke’ as the most commonly grown cultivar [3,4], characterized by high susceptibility, is seriously threatened by Bortyosphaeriaceae and especially the emergence of D. seriata and L. iraniensis as new blueberry pathogens. Limiting options for the overall management of Botryosphaeriaceae stem blight diseases emphasize the use of disease-free planting material and the avoidance of injuring plants [11]. In our study, the majority of orchards were in their second or third year of production, meaning that planting material is a likely source of infection, as has been shown previously for many Botryosphaeriaceae [10,21,91]. It would be beneficial for Serbian producers if the control of production and, above all, the import of blueberry planting material in Serbia were strengthened and improved. In view of the fact that the quarantine status of L. pseudotheobromae and L. iraniensis has been discussed [18,19], the standard procedure in the international trade of blueberry planting material should be analyzed and reconsidered. Our results offered a solution as we identified less or moderately susceptible blueberry cultivars to be grown in the affected areas and even more emphasized the need to use pathogen-free planting material in all blueberry-growing areas worldwide.
Author Contributions
Conceptualization, A.B., M.M., M.V. and D.J.; methodology, A.B., M.V., M.M., M.G., B.V., D.J. and T.V.; software, M.V. and M.M.; validation, A.B., M.V., M.M., M.G., B.V., D.J. and T.V.; formal analysis, M.V., M.M.; investigation, A.B., M.V., M.M. and M.G.; resources, A.B., M.V., M.M. and D.J.; data curation, M.M. and M.V.; writing—original draft preparation, M.M., M.V., M.G. and A.B.; writing—review and editing, A.B., M.V., M.M., M.G., B.V., D.J. and T.V.; visualization, M.M. and M.V.; supervision, A.B.; project administration, A.B.; funding acquisition, A.B. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants 451-03-137/2025-03/200116, 451-03-136/2025-03/200215 and 451-03-137/2025-03/200383 of the Ministry of Science, Technological Development and Innovation of the Republic of Serbia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data set available on request from the authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PDA | Potato Dextrose Agar |
| PNA | Pine Needle Agar |
| PDB | Potato Dextrose Broth |
| ITS | Internal Transcribed Spacer |
| TEF1-α | Translation Elongation Factor 1α |
| TUB2 | Beta Tubulin |
| DNA | Deoxyribonucleic Acid |
| Nt | Nucleotide |
| Bp | Base Pair |
| Dpi | Days Per Inoculation |
| Cv | Cultivar |
| PCR | Polymerase Chain Reaction |
References
- Retamales, J.B.; Hancock, J.F. Blueberries, 2nd ed.; Bell and Bain Ltd.: Glasgow, UK, 2018; pp. 1–323. [Google Scholar]
- Kalt, W.; Dufour, D. Health functionality of blueberries. HortTechnology 1997, 7, 216–221. [Google Scholar] [CrossRef]
- Leposavić, A.; Jevremović, D. Blueberry-Technology of Growing, Protection and Processing; Scientific Pomological Society of Serbia: Belgrade, Serbia, 2020. [Google Scholar]
- Milivojević, J. Jagodaste Voćke; Univerzitet u Beogradu, Poljoprivredni Fakultet: Beograd-Zemun, Serbia, 2022; pp. 339–429. [Google Scholar]
- Milholland, R.D.; Galletta, G.J. Pathogenic variation among isolates of Botryosphaeria corticis on blueberry. Phytopathology 1969, 59, 1540–1543. [Google Scholar]
- Sammonds, J.; Billones, R.; Rocchetti, M.; Ridgway, H.J.; Walter, M.; Jaspers, M.V. Survey of blueberry farms for Botryosphaeria dieback and crown rot pathogens. N. Z. Plant Prot. 2009, 62, 238–242. [Google Scholar] [CrossRef]
- Wright, A.F.; Harmon, P.F. First report of Lasiodiplodia theobromae associated with stem blight of southern highbush blueberries in Florida. Plant Dis. 2009, 93, 962. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, H.; Zhou, Z.; Hu, T.; Wang, S.; Wang, Y.; Cao, K. Identification and distribution of Botryosphaeriaceae species associated with blueberry stem blight in China. Eur. J. Plant Pathol. 2015, 143, 737–752. [Google Scholar] [CrossRef]
- Aviles, M.; De los Santos, B.; Borrero, C. Increase of canker disease severity in blueberries caused by Neofusicoccum parvum or Lasiodiplodia theobromae due to interaction with Macrophomina phaseolina root infection. Eur. J. Plant Pathol. 2021, 159, 655–663. [Google Scholar] [CrossRef]
- Tennakoon, K.M.S.; Ridgway, H.J.; Jaspers, M.V.; Jones, E.E. Botryosphaeriaceae species associated with blueberry dieback and sources of primary inoculum in propagation nurseries in New Zealand. Eur. J. Plant Pathol. 2017, 150, 363–374. [Google Scholar] [CrossRef]
- Ru, S.; Ding, S.; Oliver, J.E.; Amodu, A. A review of Botryosphaeria stem blight disease of blueberry from the perspective of plant breeding. Agriculture 2022, 13, 100. [Google Scholar] [CrossRef]
- Garcia, J.F.; Lawrence, D.P.; Morales-Cruz, A.; Travadon, R.; Minio, A.; Hernandez-Martinez, R.; Rolshausen, P.E.; Baumgartner, K.; Cantu, D. Phylogenomics of plant-associated Botryosphaeriaceae species. Front. Microbiol. 2021, 12, 652802. [Google Scholar] [CrossRef]
- Denman, S.; Crous, P.W.; Taylor, J.E.; Kang, J.C.; Pascoe, I.; Wingfield, M.J. An overview of the taxonomic history of Botryosphaeria, and a re-evaluation of its anamorphs based on morphology and ITS rDNA phylogeny. Stud. Mycol. 2000, 45, 129–140. [Google Scholar]
- Phillips, A.J.L.; Alves, A.; Abdollahzadeh, J.; Slippers, B.; Wingfield, M.J.; Groenewald, J.Z.; Crous, P.W. The Botryosphaeriaceae: Genera and species known from culture. Stud. Mycol. 2013, 76, 51–67. [Google Scholar] [CrossRef]
- Slippers, B.; Crous, P.W.; Jami, F.; Groenewald, J.Z.; Wingfield, M.J. Diversity in the Botryosphaeriales: Looking back, looking forward. Fungal Biol. 2017, 121, 307–321. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; He, W.; Zhang, Y. Stem blight of blueberry caused by Lasiodiplodia vaccinii sp. nov. in China. Plant Dis. 2019, 103, 2041–2050. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Bhoyroo, V.; Rampadarath, S.; Jeewon, R. Multigene phylogenetics and morphology reveal five novel Lasiodiplodia species associated with blueberries. Life 2021, 11, 657. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Plant health horizon scanning newsletter. EFSA Support. Publ. 2023, 20, 7813E. [Google Scholar] [CrossRef]
- Jibrin, M.O.; Liu, Q.; Huang, Y.; Urbina, H.; Gazis, R.; Zhang, S. Lasiodiplodia iraniensis, a new causal agent of tuber rot on yam (Dioscorea species) imported into the United States and implication for quarantine decisions. Plant Dis. 2022, 106, 3027–3032. [Google Scholar] [CrossRef]
- Abdollahzadeh, J.; Javadi, A.; Mohammadi Goltapeh, E.; Zare, R.; Phillips, A.J.L. Phylogeny and morphology of four new species of Lasiodiplodia from Iran. Persoonia 2010, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cruywagen, E.M.; Slippers, B.; Roux, J.; Wingfield, M.J. Phylogenetic species recognition and hybridization in Lasiodiplodia: A case study on species from baobabs. Fungal Biol. 2017, 121, 420–436. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Galvez, E.; Guerrero, P.; Barradas, C.; Crous, P.W.; Alves, A. Phylogeny and pathogenicity of Lasiodiplodia species associated with dieback of mango in Peru. Fungal Biol. 2017, 121, 452–465. [Google Scholar] [CrossRef]
- Bautista-Cruz, M.A.; Almaguer-Vergas, G.; Leyva-Mir, S.G.; Colinas-Leon, M.T.; Correia, K.C.; Camacho-Tapia, M.; Robles-Yerena, L.; Michereff, S.J.; Tovar-Pedraza, J.M. Phylogeny, distribution, and pathogenicity of Lasiodiplodia species associated with cankers and dieback symptoms of Persian lime in Mexico. Plant Dis. 2019, 103, 1156–1165. [Google Scholar] [CrossRef]
- Sakalidis, M.L.; Ray, J.D.; Lanoiselet, V.; Hardy, G.E.S.J.; Burgess, T.I. Pathogenic Botryosphaeriaceae associated with Mangifera indica in the Kimberley Region of Western Australia. Eur. J. Plant Pathol. 2011, 130, 379–391. [Google Scholar] [CrossRef]
- Al-Sadi, A.M.; Al-Wehaibi, A.N.; Al-Shariqi, R.M.; Al-Hammadi, M.S.; Al-Hosni, I.A.; Al-Mahmooli, I.H.; Al-Ghaithi, A.G. Population genetic analysis reveals diversity in Lasiodiplodia species infecting date palm, Citrus, and mango in Oman and the UAE. Plant Dis. 2013, 97, 1363–1369. [Google Scholar] [CrossRef]
- Marques, M.W.; Lima, N.B.; Morals, M.A., Jr.; Barbosa, M.A.G.; Souza, B.O.; Michereff, S.; Phillips, J.A.L.; Câmara, M.P.S. Species of Lasiodiplodia associated with mango in Brazil. Fungal Divers. 2013, 61, 181–193. [Google Scholar] [CrossRef]
- Li, G.Q.; Arnold, R.J.; Liu, F.F.; Li, J.Q.; Chen, S.F. Identification and pathogenicity of Lasiodiplodia species from Eucalyptus urophylla × grandis, Polyscias balfouriana and Bougainvillea spectabilis in Southern China. J. Phytopathol. 2015, 163, 956–967. [Google Scholar] [CrossRef]
- Netto, M.S.B.; Lima, W.G.; Correia, K.C.; Da Silva, C.F.B.; Thon, M.; Marting, R.B.; Miller, R.N.G.; Michereff, S.J.; Câmara, M.P.S. Analysis of phylogeny, distribution, and pathogenicity of Botryosphaeriaceae species associated with gummosis of Anacardium in Brazil, with a new species of Lasiodiplodia. Fungal Biol. 2017, 121, 437–451. [Google Scholar] [CrossRef]
- El-Ganainy, S.M.; Ismail, A.M.; Iqbal, Z.; Elshewy, E.S.; Alhudaib, K.A.; Almaghasla, M.I.; Magista, D. Diversity among Lasiodiplodia species causing dieback, root rot and leaf spot on fruit trees in Egypt, and a description of Lasiodiplodia newvalleyensis sp. nov. J. Fungi 2022, 8, 1203. [Google Scholar] [CrossRef]
- Negi, N.; Krishna, R.; Meena, R.K.; Pandey, A.; Bhandari, M.S.; Pandey, S. First report of Lasiodiplodia iraniensis causing leaf spot disease of Eucalyptus in India. Physiol. Mol. Plant Pathol. 2023, 127, 102113. [Google Scholar] [CrossRef]
- Silva, D.E.M.; Vieira, R.F.B.S.; Inokuti, E.M.; Almeida, M.M.M.; Cordeiro, M.V.M.; Lima, C.S.; Oster, A.H.; Silva, C.F.B. First report of Lasiodiplodia iraniensis causing crown rot on banana fruits in Brazil. Plant Dis. 2023, 107, 3315. [Google Scholar] [CrossRef]
- Piattino, V.; Aiello, D.; Dardani, G.; Martino, I.; Flores, M.; Aćimović, S.G.; Spadaro, D.; Polizzi, G.; Guarnaccia, V. Lasiodiplodia iraniensis and Diaporthe spp. are associated with twig dieback and fruit stem-end rot of sweet orange, Citrus sinensis, in Florida. Horticulturae 2024, 10, 406. [Google Scholar] [CrossRef]
- Popović, T.; Blagojević, J.; Aleksić, G.; Jelušić, A.; Krnjajić, S.; Milovanović, P. A blight disease on highbush blueberry associated with Macrophomina phaseolina in Serbia. Can. J. Plant Pathol. 2018, 40, 121–127. [Google Scholar] [CrossRef]
- Ristić, D.; Vučurović, I.; Živković, S.; Starović, M.; Delibašić, G.; Tanović, B.; Aleksić, G. Fusarium sporotrichioides—Novi patogen borovnice u Srbiji. In Proceedings of the XVI Simpozijum o Zaštiti Bilja u Bosni i Hercegovini, Sarajevo, Bosnia and Herzegovina, 5–7 November 2019; pp. 42–43. [Google Scholar]
- Jevremović, D.; Vasić, T.; Živković, S.; Vasilijević, B.; Marić, M.; Vojvodić, M.; Bulajić, A. Neopestalotiopsis clavispora: A causal agent of twig dieback on highbush blueberries in Serbia. J. Plant Dis. Prot. 2022, 129, 1277–1283. [Google Scholar] [CrossRef]
- Blagojević, J.; Aleksić, G.; Vučurović, I.; Starović, M.; Ristić, D. Exploring the phylogenetic diversity of Botryosphaeriaceae and Diaporthe species causing dieback and shoot blight of blueberry in Serbia. Phytopathology 2024, 114, 1333–1345. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, O.; Sinclair, J. Basic Plant Pathology Methods, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar] [CrossRef]
- Espinoza, J.G.; Briceño, E.X.; Chávez, E.R.; Úrbez-Torres, J.R.; Latorre, B.A. Neofusicoccum spp. associated with stem canker and dieback of blueberry in Chile. Plant Dis. 2009, 93, 1187–1194. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Zlatković, M.; Keča, N.; Wingfield, M.J.; Jami, F.; Slippers, B. Shot hole disease on Prunus laurocerasus caused by Neofusicoccum parvum. Antonie Leeuwenhoek 2016, 109, 543–564. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.P.; Zhang, M.; Dou, Z.P.; Zhang, Y. Botryosphaeria rosaceae sp. nov. and B. ramosa, new botryosphaeriaceous taxa from China. Mycosphere 2017, 8, 162–171. [Google Scholar] [CrossRef]
- Vučković, N.; Duduk, N.; Rekanović, E.; Duduk, B.; Vico, I. First report of Botryosphaeria dothidea causing root rot of sugar beet in Serbia. Plant Dis. 2024, 108, 3658. [Google Scholar] [CrossRef]
- Bezerra, J.D.P.; Crous, P.W.; Aiello, D.; Gullino, M.L.; Polizzi, G.; Guarnaccia, V. Genetic diversity and pathogenicity of Botryosphaeriaceae species associated with symptomatic citrus plants in Europe. Plants 2021, 10, 492. [Google Scholar] [CrossRef]
- de Silva, N.I.; Phillips, A.J.L.; Liu, J.K.; Lumyong, S.; Hyde, K.D. Phylogeny and morphology of Lasiodiplodia species associated with Magnolia forest plants. Sci. Rep. 2019, 9, 14355. Available online: https://www.nature.com/articles/s41598-019-50804-x (accessed on 23 September 2024). [CrossRef]
- Rodriguez-Galvez, E.; Hilário, S.; Lopes, A.; Alves, A. Diversity and pathogenicity of Lasiodiplodia and Neopestalotiopsis species associated with stem blight and dieback of blueberry plants in Peru. Eur. J. Plant Pathol. 2020, 157, 89–102. [Google Scholar] [CrossRef]
- Tennakoon, D.S.; Kuo, C.H.; Maharachchikumbura, S.S.N.; Thambugala, K.M.; Gentekaki, E.; Phillips, A.J.L.; Bhat, D.J.; Wanasinghe, D.N.; De Silva, N.I.; Promputtha, I.; et al. Taxonomic and phylogenetic contributions to Celtis formosana, Ficus ampelas, F. septica, Macaranga tanarius and Morus australis leaf litter inhabiting microfungi. Fungal Divers. 2021, 108, 1–215. [Google Scholar] [CrossRef]
- Rathnayaka, A.R.; Chethana, K.W.T.; Manawasinghe, I.S.; Wijesinghe, S.N.; De Silva, N.I.; Tennakoon, D.S.; Phillips, A.J.L.; Liu, J.K.; Jones, E.B.G.; Wang, Y.; et al. Lasiodiplodia: Generic revision by providing molecular markers, geographical distribution and haplotype diversity. Mycosphere 2023, 14, 1254–1339. [Google Scholar] [CrossRef]
- Machado, A.; Pinho, D.; Pereira, O. Phylogeny, identification and pathogenicity of the Botryosphaeriaceae associated with collar and root rot of the biofuel plant Jatropha curcas in Brazil, with a description of new species of Lasiodiplodia. Fungal Divers. 2014, 67, 231–247. [Google Scholar] [CrossRef]
- Hilario, S.; Lopes, A.; Santos, L.; Alves, A. Botryosphaeriaceae species associated with blueberry stem blight and dieback in the Centre region of Portugal. Eur. J. Plant Pathol. 2020, 156, 31–44. [Google Scholar] [CrossRef]
- Zlatković, M.; Keča, N.; Wingfield, M.J.; Jami, F.; Slippers, B. Botryosphaeriaceae associated with the die-back of ornamental trees in Serbia. Forest Pathol. 2016, 46, 267–282. [Google Scholar] [CrossRef]
- Zlatković, M.; Wingfield, M.J.; Jami, F.; Slippers, B. Genetic uniformity characterizes the Invasive spread of Neofusicoccum parvum. Plant Pathol. 2019, 68, 1153–1163. [Google Scholar] [CrossRef]
- Karličić, V.; Jovičić-Petrović, J.; Marojević, V.; Zlatković, M.; Orlović, S.; Raičević, V. Potential of Trichoderma spp. and Pinus sylvestris bark extracts as biocontrol agents against fungal pathogens residing in the Botryosphaeriales. Environ. Sci. Proc. 2021, 3, 99. [Google Scholar] [CrossRef]
- Vasić, M.; Duduk, N.; Vico, I.; Ivanović, M.S. First report of Botryosphaeria dothidea causing white rot of apple fruit in Serbia. Plant Dis. 2013, 97, 1659. [Google Scholar] [CrossRef]
- Vučković, N.; Vico, I.; Duduk, B.; Duduk, N. Diversity of Botryosphaeriaceae and Diaporthe species associated with postharvest apple fruit decay in Serbia. Phytopathology 2022, 112, 929–943. [Google Scholar] [CrossRef]
- Vučković, N.; Vico, I.; Duduk, N. First report of Botryosphaeria dothidea causing postharvest rot of quince fruits in Serbia. J. Plant Pathol. 2023, 105, 605. [Google Scholar] [CrossRef]
- Vico, I.; Žebeljan, A.; Vučković, N.; Vasić, M.; Duduk, N. First report of Diplodia seriata causing postharvest rot of quince fruit in Serbia. Plant Dis. 2017, 101, 1823. [Google Scholar] [CrossRef]
- Flor, N.C.; Wright, A.F.; Huguet-Tapia, J.; Harmon, P.F.; Liberti, D. Identification of fungi in the Botryosphaeriaceae family associated with stem blight of Vaccinium spp. in the southeastern United States. Fungal Biol. 2022, 126, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.N.; Zhao, H.H.; Yu, Y.Y.; Li, X.D.; Liang, C.; Li, B.D. The pathogen causing Lasiodiplodia twig blight of blueberry. Mycosystema 2016, 35, 657–665. [Google Scholar] [CrossRef]
- Wiseman, M.S.; Serdani, M.; Putnam, M.L. A new cane dieback disease of northern highbush blueberry in the United States caused by Lasiodiplodia mediterranea. Plant Dis. 2017, 101, 1317. [Google Scholar] [CrossRef]
- Scarlett, K.A.; Shuttleworth, L.A.; Collins, D.; Rothwell, C.T.; Guest, D.I.; Daniel, R. Botryosphaeriales associated with stem blight and dieback of blueberry (Vaccinium spp.) in New South Wales and Western Australia. Australas. Plant Pathol. 2019, 48, 45–57. [Google Scholar] [CrossRef]
- Rebollar-Alviter, A.; Boyzo-Marín, J.; Silva-Rojas, H.V.; Ramírez, G. Fungi and oomycete pathogens causing stem blight and root rots on blueberry in Central Mexico. Phytopathology 2013, 103, 119–120. [Google Scholar]
- Borrero, C.; Perez, S.; Aviles, M. First report of canker disease caused by Lasiodiplodia theobromae on blueberry bushes in Spain. Plant Dis. 2019, 103, 10. [Google Scholar] [CrossRef]
- Latorre, B.A.; Díaz, G.A.; Reed, M.P. Effect of water activity on in vitro mycelial growth of Neofusicoccum spp. infecting blueberry. Cienc. Investig. Agrar. 2012, 9, 221–228. [Google Scholar] [CrossRef]
- Babiker, E.M.; Stringer, S.J.; Sakhanokho, H.F.; Smith, B.J. Characterization and pathogenicity of stem blight complex isolates associated with stem blight disease on Vaccinium species. HortScience 2019, 54, 1199–1203. [Google Scholar] [CrossRef]
- Choi, S.; Paul, N.C.; Lee, K.-H.; Kim, H.-J.; Sang, H.M. Morphology, molecular phylogeny, and pathogenicity of Neofusicoccum parvum, associated with leaf spot disease of a new host, the Japanese bay tree (Machilus thunbergii). Forests 2021, 12, 440. [Google Scholar] [CrossRef]
- Milas, P.; Barra-Bucarei, L.; Castro, J.F.; Carrasco-Fernández, J.; Chilian, J.; Tapia, E.; Santelices, C.; Cisterna-Oyarce, V.; Muñoz, V.; Ortiz-Campos, J.; et al. Identification and distribution of species of Neofusicoccum that cause blueberry stem blight in Chile. Mycologia 2023, 115, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Petrović, E.; Vrandečić, K.; Belušić Vozila, A.; Ćosić, J.; Godena, S. Diversity and pathogenicity of Botryosphaeriaceae species isolated from olives in Istria, Croatia, and evaluation of varietal resistance. Plants 2024, 13, 1813. [Google Scholar] [CrossRef]
- Li, X.; Yan, J.; Kong, F.; Qiao, G.; Zhang, Z.; Wang, Z. Botryosphaeria dothidea causing canker of grapevine newly reported in China. Plant Pathol. 2010, 59, 1170. [Google Scholar] [CrossRef]
- Yu, L.; Rarisara, I.; Xu, S.G.; Wu, X.; Zhao, J.R. First report of stem blight of blueberry caused by Botryosphaeria dothidea in China. Plant Dis. 2012, 96, 1697. [Google Scholar] [CrossRef]
- Yan, J.; Xie, Y.; Yao, S.; Wang, Z.; Li, X. Characterization of Botryosphaeria dothidea, the causal agent of grapevine canker in China. Australas. Plant Pathol. 2012, 41, 351–357. [Google Scholar] [CrossRef]
- Marsberg, A.; Kemler, M.; Jami, F.; Nagel, J.H.; Postma-Smidt, A.; Naidoo, S.; Wingfield, M.J.; Crous, P.W.; Spatafora, J.W.; Hesse, C.N.; et al. Botryosphaeria dothidea: A latent pathogen of global importance to woody plant health. Mol. Plant Pathol. 2017, 18, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Kovač, M.; Diminić, D.; Orlović, S.; Zlatković, M. Botryosphaeria Dothidea and Neofusicoccum Yunnanense Causing Canker and Die-Back of Sequoiadendron Giganteum in Croatia. Forests 2021, 12, 695. [Google Scholar] [CrossRef]
- Phillips, A.J.L.; Crous, P.W.; Alves, A. Diplodia seriata, the anamorph of “Botryosphaeria” obtusa. Fungal Divers. 2007, 25, 141–155. [Google Scholar]
- Gonzalez-Dominguez, E.; Alves, A.; León, M.; Armengol, J. Characterization of Botryosphaeriaceae species associated with diseased loquat (Eriobotrya japonica) in Spain. Plant Pathol. 2017, 66, 77–89. [Google Scholar] [CrossRef]
- Arrigoni, E.; Oliveira Longa, C.M.; Angeli, D.; Soini, M.; Pertot, I.; Perazzolli, M. A fast and reliable method for Diplodia seriata inoculation of trunks and assessment of fungicide efficacy on potted apple plants under greenhouse conditions. Phytopathol. Mediterr. 2019, 58, 163–173. [Google Scholar] [CrossRef]
- Manetti, G.; Brunetti, A.; Sciarroni, L.; Lumia, V.; Bechini, S.; Marangi, P.; Reverberi, M.; Scortichini, M.; Pilotti, M. Diplodia seriata Isolated from Declining Olive Trees in Salento (Apulia, Italy): Pathogenicity Trials Give a Glimpse That It Is More Virulent to Drought-Stressed Olive Trees and in a Warmth-Conditioned Environment. Plants 2024, 13, 2245. [Google Scholar] [CrossRef] [PubMed]
- Baskarathevan, J.; Jaspers, M.V.; Jones, E.E.; Cruickshank, R.H.; Ridgway, H.J. Genetic and pathogenic diversity of Neofusicoccum parvum in New Zealand vineyards. Fungal Biol. 2012, 116, 276–288. [Google Scholar] [CrossRef]
- Trotel-Aziz, P.; Robert-Siegwald, G.; Fernandez, O.; Leal, C.; Villaume, S.; Guise, J.F.; Abou-Mansour, E.; Lebrun, M.H.; Fontaine, F. Diversity of Neofusicoccum parvum for the production of the phytotoxic metabolites (-)-Terremutin and (R)-Mellein. J. Fungi 2022, 8, 319. [Google Scholar] [CrossRef]
- Belair, M.; Picot, A.; Lepais, O.; Masson, C.; Hébrard, M.; Moronvalle, A.; Comont, G.; Gabri Martin, V.M.; Tréguer, S.; Laloum, Y.; et al. Genetic diversity and population structure of Botryosphaeria dothidea and Neofusicoccum parvum on English walnut (Juglans regia L.) in France. Sci. Rep. 2024, 14, 19817. [Google Scholar] [CrossRef] [PubMed]
- Elena, G.; Garcia-Figueres, F.; Reigada, S.; Luque, J. Intraspecific variation in Diplodia seriata isolates occurring on grapevines in Spain. Plant Pathol. 2015, 64, 680–689. [Google Scholar] [CrossRef]
- Bhat, A.H.; Shah, M.D.; Padder, B.A.; Shah, Z.A.; Dar, E.A.; Fayaz, U.; Nain, M.S.; Ali, M.A.; Al-Hemaid, F.M.; Stępień, P.; et al. Morphological, pathogenic and genetic diversity in Diplodia seriata associated with black rot canker of apple in India. Sci. Rep. 2023, 13, 15682. [Google Scholar] [CrossRef]
- Trakunyingcharoen, T.; Lombard, L.; Groenewald, J.Z.; Cheewangkoon, R.; To-anun, C.; Crous, P.W. Caulicolous Botryosphaeriales from Thailand. Persoonia 2015, 34, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Crous, P.W.; Correia, A.; Phillips, A.J.L. Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Divers. 2008, 28, 1–13. [Google Scholar]
- Dou, Z.P.; He, W.; Zhang, Y. Does morphology matter in taxonomy of Lasiodiplodia? An answer from Lasiodiplodia hyalina sp. nov. Mycosphere 2017, 8, 1014–1027. [Google Scholar] [CrossRef]
- Gallardo, R.K.; Zhang, Q.; Dossett, M.; Polashock, J.J.; Rodriguez-Saona, C.; Vorsa, N.; Edger, P.P.; Ashrafi, H.; Babiker, E.; Finn, C.E.; et al. Breeding trait priorities of the blueberry industry in the United States and Canada. HortScience 2018, 53, 1021–1028. [Google Scholar] [CrossRef]
- Sakalidis, M.L.; Hardy, G.E.S.J.; Burgess, T.I. Endophytes as potential pathogens of the baobab species Adansonia gregorii: A focus on the Botryosphaeriaceae. Fungal Ecol. 2011, 4, 1–14. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).