Mechanism of Enzyme Activity Regulation and Strain-Specific Response of Lentinula edodes Cultivation Adaptability Under Peach Wood Substrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Strains and Test Materials
2.2. Medium Formulation
2.3. Cultivation Management
2.4. Yield and Agronomic Traits Determination
2.5. Dynamic Changes in Extracellular Enzyme Activity
2.6. Nutritional Composition of Fruiting Bodies
2.7. Pesticide Residue and Heavy Metal of Fruiting Bodies
2.8. Statistical Analysis
3. Results
3.1. Appropriate Proportion of Peach Wood Promotes Mycelial Growth
3.2. Enzyme Activity Reveals the Adaptation Mechanism of Peach Wood
3.3. Strain-Specific Yield Enhancement Effect
3.4. Three-Dimensional Effects of Peach Wood on Quality Enhancement
3.5. Security Verification
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manzi, P.; Gambelli, L.; Marconi, S.; Vivanti, V.; Pizzoferrato, L. Nutrients in edible mushrooms: An inter-species comparative study. Food Chem. 1999, 65, 477–482. [Google Scholar] [CrossRef]
- Ma, N.L.; Khoo, S.C.; Peng, W.; Ng, C.M.; Teh, C.H.; Park, Y.K.; Lam, S.S. Green application and toxic risk of used diaper and food waste as growth substitute for sustainable cultivation of oyster mushroom (Pleurotus ostreatus). J. Clean. Prod. 2020, 268, 122272. [Google Scholar] [CrossRef]
- Okuda, Y. Sustainability perspectives for future continuity of mushroom production: The bright and dark sides. Front. Sustain. Food Syst. 2022, 6, 1026508. [Google Scholar] [CrossRef]
- Royse, D.J.; Baars, J.; Tan, Q. Current overview of mushroom production in the world. Edible Med. Mushrooms Technol. Appl. 2017, 5, 5–13. [Google Scholar]
- Megersa, S.; Tolessa, A. Enhancing yields of Pleurotus ostreatus and Lentinula edodes mushrooms using water hyacinth (Eichhornia crassipes [Mart.] Solms) supplemented with locally available feedstock as substrate. Heliyon 2024, 10, e39113. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Zheng, Y.; Stelling, J.M.; Loisel, J.; Huang, Y.; Yu, Z. Environmental controls on the carbon and water (H and O) isotopes in peatland Sphagnum mosses. Geochim. Cosmochim. Acta 2020, 277, 265–284. [Google Scholar] [CrossRef]
- Fenibo, E.O.; Ijoma, G.N.; Matambo, T. Biopesticides in sustainable agriculture: A critical sustainable development driver governed by green chemistry principles. Front. Sustain. Food Syst. 2021, 5, 619058. [Google Scholar] [CrossRef]
- Raghavarao, K.S.; Ranganathan, T.V.; Karanth, N.G. Some engineering aspects of solid-state fermentation. Biochem. Eng. J. 2003, 13, 127–135. [Google Scholar] [CrossRef]
- Graminha, E.B.; Gonçalves, A.Z.; Pirota, R.D.; Balsalobre, M.A.; Da Silva, R.; Gomes, E. Enzyme production by solid-state fermentation: Application to animal nutrition. Anim. Feed. Sci. Technol. 2008, 144, 1–22. [Google Scholar] [CrossRef]
- Li, W.; Chen, W.; Yang, Y.; Zhang, J.; Feng, J.; Yu, H.; Zhou, S.; Li, X.; Liu, Y. Effects of culture substrates on taste component content and taste quality of Lentinula edodes. Int. J. Food Sci. Technol. 2017, 52, 981–991. [Google Scholar] [CrossRef]
- Nitta, T.; Kamei, I.; Sugamoto, K.; Meguro, S. Shiitake (Lentinula edodes) cultivation in sawdust media consisting of kunugi (Quercus acutissima) mixed with sugi (Cryptomeria japonica): Optimization of gaseous phase rate in media by three-phase-structure analysis. J. Wood Sci. 2016, 62, 452–459. [Google Scholar]
- Kilpatrick, M.; Murray, D.J.; Ward, F. on Lentinula edodes production. Sci. Cultiv. Edible Fungi 2000 2000, 1, 803. [Google Scholar]
- Leifa, F.; Pandey, A.; Soccol, C.R. Solid state cultivation—An efficient method to use toxic agro-industrial residues. J. Basic. Microb. 2000, 40, 187–197. [Google Scholar] [CrossRef]
- Azman, N.F.; Yamauchi, M.; Yamada, M.; Ikeda, S.; Yamaguchi, T.; Yagi, F.; Hara, H. Utilization of distillation waste of sweet potato Shochu lees for Lentinula edodes cultivation. J. Mater. Cycles Waste 2019, 21, 336–344. [Google Scholar]
- Salmones, D.; Mata, G.; Ramos, L.M.; Waliszewski, K.N. Cultivation of shiitake mushroom, Lentinula edodes, in several lignocellulosic materials originating from the subtropics. Agronomie 1999, 19, 13–19. [Google Scholar]
- Elisashvili, V.I.; Kachlishvili, E.; Asatiani, M.D. Shiitake medicinal mushroom, Lentinus edodes (higher basidiomycetes) productivity and lignocellulolytic enzyme profiles during wheat straw and tree leaf bioconversion. Int. J. Med. Mushrooms 2015, 17, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Q. Study on the optimal grouping of sawdust-saving cultivation substrates for Lentinula edodes. Acta Edulis Fungi 2002, 9, 35–40. [Google Scholar]
- Cabrera, R.; López-Peña, D.; Asaff, A.; Esqueda, M.; Valenzuela-Soto, E.M. Bioavailability of compounds susceptible to enzymatic oxidation enhances growth of shiitake medicinal mushroom (Lentinus edodes) in solid-state fermentation with vineyard prunings. Int. J. Med. Mushrooms 2018, 20, 291–303. [Google Scholar] [CrossRef]
- Ding, C.; Wang, X.; Li, M. Evaluation of six white-rot fungal pretreatments on corn stover for the production of cellulolytic and ligninolytic enzymes, reducing sugars, and ethanol. Appl. Microbiol. Biot. 2019, 103, 5641–5652. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Yu, C.X.; Dong, H.R.; Zhou, F.; Li, Z.P.; Li, Y. Effects of illumination of different lights on agronomic traits and texture quality of fruiting bodies of Lentinula edodes. Mycosystema 2021, 40, 3169–3181. [Google Scholar]
- Pei, J.Q.; Meng, R.Z.; Chen, Y.H.; Wu, X.L. Discovery and Validation of a Functional Gene Affecting the Growth and Development of Pleurotus eryngii. Biotechnol. Bull. 2025, 41, 327–334. [Google Scholar]
- GH/T 1013:2015; Lentinula edodes (Berk.) Pegler. China-Industry Standards-Supply and Marketing Cooperatives: Beijing, China, 2015.
- Vetchinkina, E.P.; Gorshkov, V.Y.; Ageeva, M.V.; Gogolev, Y.V.; Nikitina, V.E. Activity and expression of laccase, tyrosinase, glucanase, and chitinase genes during morphogenesis of Lentinus edodes. Microbiology 2015, 84, 49–58. [Google Scholar] [CrossRef]
- NY/T 912-2004; Determination of Cellulase Activity in Feed Additives—Speetrophotometry. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2004.
- GB/T 23874-2009; Determination of Xylanase Activity in Feed Additives—Spectrophotometric Method. China International Standardization Administration: Beijing, China, 2009.
- Mattila, P.; Salo-Väänänen, P.; Könkö, K.; Aro, H.; Jalava, T. Basic composition and amino acid contents of mushrooms cultivated in Finland. J. Agric. Food Chem. 2002, 50, 6419–6422. [Google Scholar] [CrossRef]
- GB 5009.4-2016; National Food Safety Standard—Determination of Ash Content in Food. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.6-2016; National Food Safety Standards—Determination of Fat in Food. China Food and Drug Administration: Beijing, China, 2016.
- GB/T 5009.10-2003; Determination of Crude Fiber in Vegetable Foods. China Food and Drug Administration: Beijing, China, 2003.
- Mæhre, H.K.; Dalheim, L.; Edvinsen, G.K.; Elvevoll, E.O.; Jensen, I.J. Protein determination—Method matters. Foods 2018, 7, 5. [Google Scholar] [CrossRef]
- GB 23200.12-2016; National Food Safety Standards—Determination of 440 Pesticides and Related Chemicals Residues in Mushrooms Liquid Chromatography-Mass Spectrometry. China International Standardization Administration: Beijing, China, 2016.
- GB 5009.268-2016; National Food Safety Standard—Determination of Multiple Elements in Food. China Food and Drug Administration: Beijing, China, 2016.
- Sheng, C.; Wang, Y.; Pan, C.; Shi, L.; Wang, Y.; Ma, Y.; Wang, J.; Zhao, J.; Zhang, P.; Liu, Z.; et al. Evaluation of rice straw, corncob, and soybean straw as substrates for the cultivation of Lepista sordida. Life 2024, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, Y.; Nakade, K.; Yoshida, K.; Natsume, S.; Miyazaki, K.; Sato, S.; van Peer, A.F.; Konno, N. Grouping of multicopper oxidases in Lentinula edodes by sequence similarities and expression patterns. AMB Express 2015, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, F.; Fu, Y.; Li, D.; Sun, X.; Li, C.; Song, B.; Li, Y. Effects of mixed agro-residues (corn crop waste) on lignin-degrading enzyme activities, growth, and quality of Lentinula edodes. RSC Adv. 2020, 10, 9798–9807. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, Y.; Li, F.; Liu, P. Active polysaccharides from Lentinula edodes and Pleurotus ostreatus by addition of corn straw and xylosma sawdust through solid-state fermentation. Int. J. Biol. Macromol. 2023, 228, 647–658. [Google Scholar] [CrossRef]
- Lee, J.; Gwak, K.; Kim, S.; Kim, M.; Choi, D.; Choi, I. Characterization of xylanase from Lentinus edodes M290 cultured on waste mushroom logs. J. Microbiol. Biotechnol. 2007, 17, 1811. [Google Scholar]
- Pedri, Z.C.; Lozano, L.M.; Hermann, K.L.; Helm, C.V.; Peralta, R.M.; Tavares, L.B. Influence of nitrogen sources on the enzymatic activity and grown by Lentinula edodes in biomass Eucalyptus benthamii. Braz. J. Biol. 2015, 75, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Shang, X.; Zhang, M.; Yu, H.; Zhang, D.; Tan, Q.; Song, C. Cultivation methods and biology of Lentinula edodes. Appl. Microbiol. Biotechnol. 2025, 109, 63. [Google Scholar] [CrossRef] [PubMed]
- Grassino, A.N.; Brnčić, M.; Vikić-Topić, D.; Roca, S.; Dent, M.; Brnčić, S.R. Ultrasound assisted extraction and characterization of pectin from tomato waste. Food Chem. 2016, 198, 93–100. [Google Scholar] [CrossRef]
- Wu, F.; Jia, X.; Yin, L.; Cheng, Y.; Miao, Y.; Zhang, X. The effect of hemicellulose and lignin on properties of polysaccharides in Lentinus edodes and their antioxidant evaluation. Molecules 2019, 24, 1834. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.P.; Serrano, C.; Pires, T.; Mestrinho, E.; Dias, L.; Teixeira, D.M.; Caldeira, A.T. Degradation of terbuthylazine, difenoconazole and pendimethalin pesticides by selected fungi cultures. Sci. Total Environ. 2012, 435, 402–410. [Google Scholar] [CrossRef]
- Pinto, A.P.; Rodrigues, S.C.; Caldeira, A.T.; Teixeira, D.M. Exploring the potential of novel biomixtures and Lentinula edodes fungus for the degradation of selected pesticides. Evaluation for use in biobed systems. Sci. Total Environ. 2016, 541, 1372–1381. [Google Scholar] [CrossRef]
- Huang, Q.; Jia, Y.; Wan, Y.; Li, H.; Jiang, R. Market survey and risk assessment for trace metals in edible fungi and the substrate role in accumulation of heavy metals. J. Food Sci. 2015, 80, H1612–H1618. [Google Scholar] [CrossRef] [PubMed]
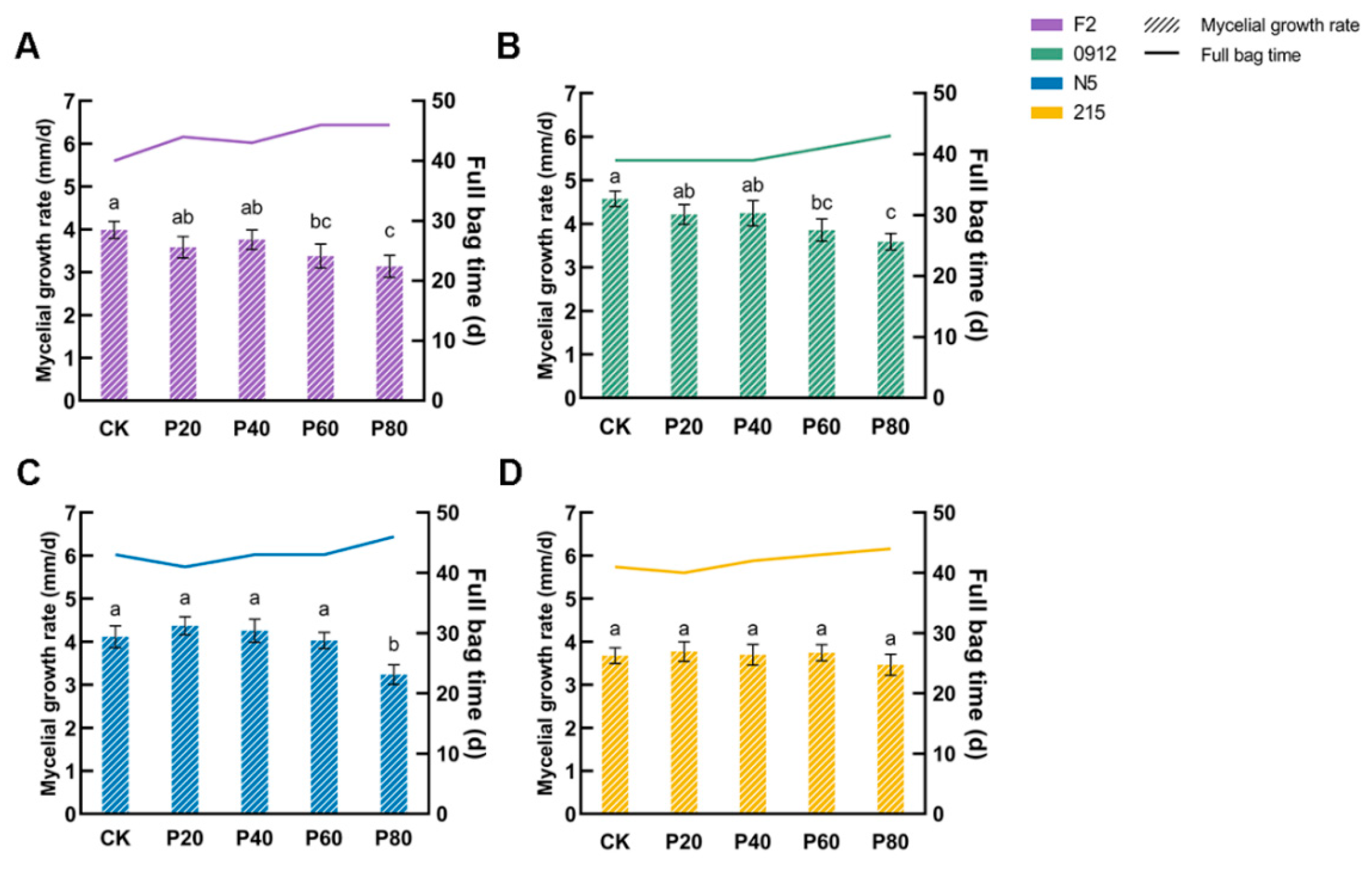

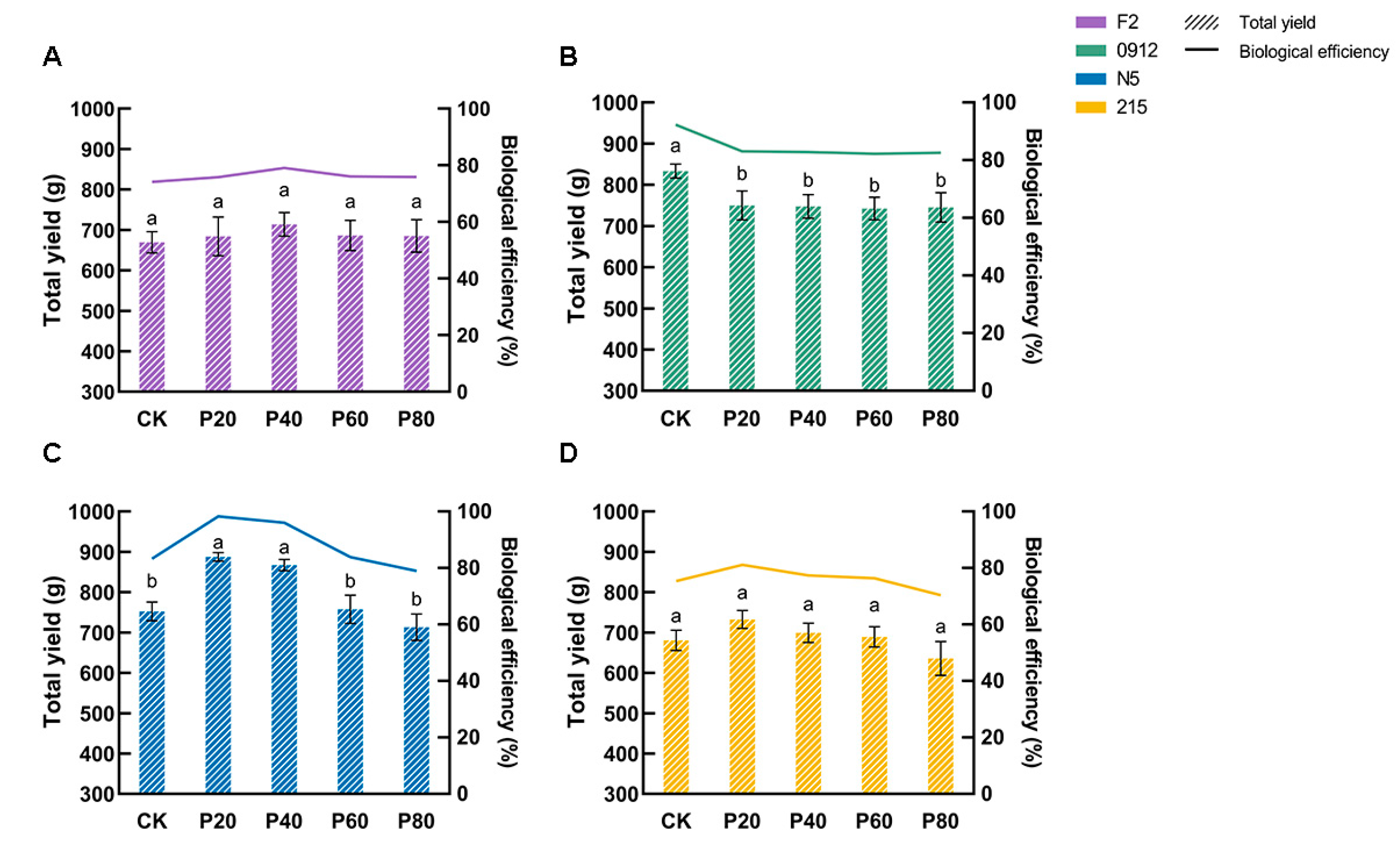
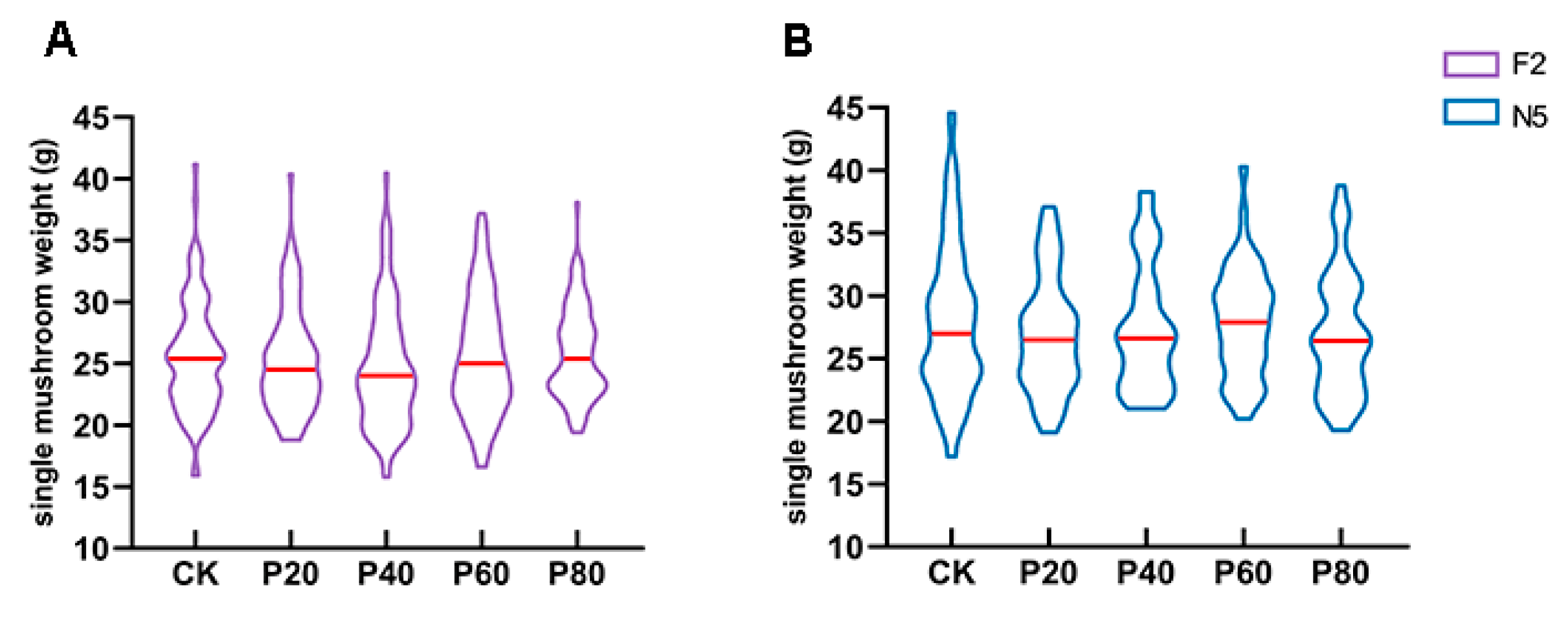
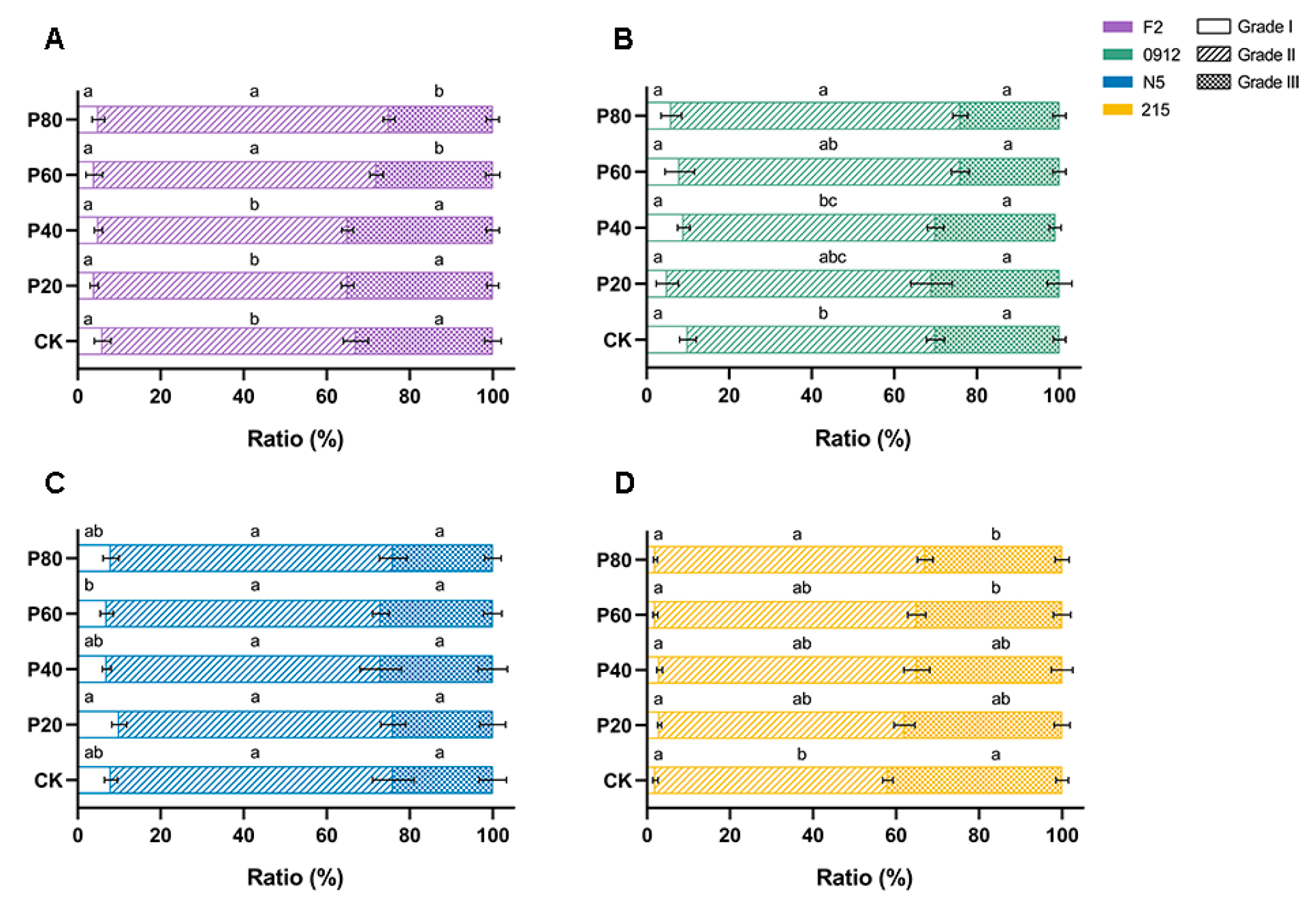
| Formulation | Oak Wood Chips | Peach Wood Chips | Wheat Bran | Gypsum |
|---|---|---|---|---|
| CK | 80% | 0 | 18% | 2% |
| P20 | 60% | 20% | 18% | 2% |
| P40 | 40% | 40% | 18% | 2% |
| P60 | 20% | 60% | 18% | 2% |
| P80 | 0 | 80% | 18% | 2% |
| Strain | Formulation | Pileus Diameter (cm) | Pileus Thickness (cm) | Stipe Length (cm) | Stipe Diameter (cm) | Cap Mass Proportion (cm) |
|---|---|---|---|---|---|---|
| F2 | CK | 5.78 ± 0.02 a | 1.31 ± 0.01 a | 3.38 ± 0.05 b | 1.51 ± 0.06 a | 0.7889 a |
| P20 | 5.77 ± 0.04 a | 1.29 ± 0.01 a | 3.39 ± 0.06 b | 1.51 ± 0.03 a | 0.7845 a | |
| P40 | 5.69 ± 0.04 a | 1.27 ± 0.02 a | 3.39 ± 0.04 b | 1.42 ± 0.03 b | 0.7934 a | |
| P60 | 5.72 ± 0.09 a | 1.29 ± 0.02 a | 3.56 ± 0.04 a | 1.45 ± 0.06 ab | 0.7949 a | |
| P80 | 5.76 ± 0.05 a | 1.30 ± 0.02 a | 3.51 ± 0.03 a | 1.44 ± 0.05 ab | 0.7935 a | |
| 0912 | CK | 5.91 ± 0.09 a | 1.33 ± 0.02 a | 3.55 ± 0.04 b | 1.55 ± 0.09 a | 0.7884 a |
| P20 | 5.94 ± 0.02 a | 1.34 ± 0.02 a | 3.67 ± 0.05 a | 1.61 ± 0.07 a | 0.7703 b | |
| P40 | 5.88 ± 0.09 ab | 1.35 ± 0.04 a | 3.39 ± 0.06 cd | 1.56 ± 0.12 a | 0.7896 a | |
| P60 | 5.75 ± 0.09 b | 1.31 ± 0.02 ab | 3.46 ± 0.02 c | 1.47 ± 0.02 a | 0.7949 a | |
| P80 | 5.76 ± 0.08 b | 1.28 ± 0.02 b | 3.33 ± 0.05 d | 1.52 ± 0.02 a | 0.7884 a | |
| N5 | CK | 5.87 ± 0.09 a | 1.39 ± 0.03 b | 3.48 ± 0.11 a | 1.49 ± 0.04 a | 0.8023 b |
| P20 | 5.82 ± 0.14 a | 1.42 ± 0.04 ab | 3.46 ± 0.02 a | 1.42 ± 0.07 a | 0.8089 b | |
| P40 | 5.85 ± 0.08 a | 1.44 ± 0.01 a | 3.37 ± 0.05 a | 1.43 ± 0.03 a | 0.8155 a | |
| P60 | 5.91 ± 0.08 a | 1.39 ± 0.02 ab | 3.37 ± 0.04 a | 1.43 ± 0.02 a | 0.8138 a | |
| P80 | 5.83 ± 0.08 a | 1.32 ± 0.02 c | 3.23 ± 0.04 b | 1.49 ± 0.08 a | 0.8125 a | |
| 215 | CK | 5.54 ± 0.09 a | 1.34 ± 0.03 a | 4.04 ± 0.03 b | 1.44 ± 0.02 bc | 0.7153 c |
| P20 | 5.57 ± 0.05 a | 1.33 ± 0.02 a | 4.34 ± 0.09 a | 1.43 ± 0.04 c | 0.7305 b | |
| P40 | 5.55 ± 0.12 a | 1.30 ± 0.02 ab | 4.11 ± 0.06 b | 1.41 ± 0.03 c | 0.7452 ab | |
| P60 | 5.60 ± 0.12 a | 1.27 ± 0.03 bc | 4.44 ± 0.15 a | 1.50 ± 0.03 a | 0.7497 a | |
| P80 | 5.44 ± 0.10 a | 1.25 ± 0.03 c | 3.83 ± 0.03 c | 1.49 ± 0.03 ab | 0.7420 ab |
| Formulation | Total Polysaccharides (mg/g) | Crude Protein (g/kg) | Ash Content (g/kg) | Crude Lipid (g/kg) | Crude Fiber (g/kg) |
|---|---|---|---|---|---|
| CK | 25.27 ± 1.70 c | 250.44 ± 2.86 c | 66.53 ± 0.87 bc | 64.20 ± 2.45 d | 12.03 ± 0.82 c |
| P20 | 23.65 ± 1.73 c | 261.99 ± 1.48 b | 67.23 ± 0.17 b | 71.84 ± 0.51 c | 19.58 ± 0.61 b |
| P40 | 24.01 ± 0.45 c | 264.86 ± 0.79 b | 71.93 ± 0.49 a | 85.03 ± 0.93 a | 30.68 ± 4.47 a |
| P60 | 29.42 ± 2.50 b | 275.81 ± 2.35 a | 72.88 ± 0.58 a | 78.90 ± 0.50 b | 19.29 ± 0.47 b |
| P80 | 33.20 ± 1.23 a | 241.38 ± 3.89 d | 65.76 ± 1.01 c | 63.90 ± 1.26 d | 14.39 ± 0.96 c |
| Analyte | Peach Wood Chips (mg/kg) | Fruiting Bodies (mg/kg) |
|---|---|---|
| Difenoconazole | 0.089 | ND (<0.01) |
| Abamectin | ND (<0.05) | ND (<0.05) |
| Acetamiprid | ND (<0.01) | ND (<0.01) |
| Pyraclostrobin | ND (<0.01) | ND (<0.01) |
| Methyl parathion | ND (<0.01) | ND (<0.01) |
| Pyrimethanil | ND (<0.01) | ND (<0.01) |
| Imidacloprid | 0.038 | ND (<0.01) |
| Malathion | - | ND (<0.01) |
| Dimethoate | - | ND (<0.01) |
| Cyhalothrin (including λ-cyhalothrin) | - | ND (<0.01) |
| Cyfluthrin (including β-cyfluthrin) | - | ND (<0.01) |
| Deltamethrin | - | ND (<0.01) |
| Cypermethrin (including θ-cypermethrin) | - | ND (<0.01) |
| Flucythrinate | - | ND (<0.01) |
| Prochloraz (including prochloraz-manganese) | - | ND (<0.01) |
| Carbendazim | - | ND (<0.01) |
| Chlorothalonil | - | ND (<0.01) |
| Formulation | Arsenic (μg/kg) | Cadmium (μg/kg) | Lead (μg/kg) | Mercury (μg/kg) |
|---|---|---|---|---|
| CK | 186.17 ± 4.53 b | 1295.42 ± 37.45 a | 343.20 ± 9.84 b | 6.27 ± 0.27 b |
| P20 | 125.76 ± 3.53 d | 973.12 ± 49.14 d | 388.19 ± 16.53 a | 5.55 ± 0.19 c |
| P40 | 143.58 ± 4.77 c | 1067.41 ± 43.12 bc | 301.11 ± 8.89 c | 5.47 ± 0.02 c |
| P60 | 141.29 ± 4.09 c | 1090.15 ± 24.69 b | 261.19 ± 8.67 d | 6.26 ± 0.28 b |
| P80 | 311.13 ± 12.39 a | 1021.82 ± 8.35 cd | 221.12 ± 5.82 e | 7.00 ± 0.34 a |
| Limited standard | ≤1000 | ≤2000 | ≤2000 | ≤200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, N.; Dong, H.-R.; Tian, L.; Xin, T.-Z.; Wang, S.-X.; Li, Y.; He, M.-N.; Yu, H.-L. Mechanism of Enzyme Activity Regulation and Strain-Specific Response of Lentinula edodes Cultivation Adaptability Under Peach Wood Substrate. J. Fungi 2025, 11, 684. https://doi.org/10.3390/jof11090684
Jiang N, Dong H-R, Tian L, Xin T-Z, Wang S-X, Li Y, He M-N, Yu H-L. Mechanism of Enzyme Activity Regulation and Strain-Specific Response of Lentinula edodes Cultivation Adaptability Under Peach Wood Substrate. Journal of Fungi. 2025; 11(9):684. https://doi.org/10.3390/jof11090684
Chicago/Turabian StyleJiang, Ning, Hao-Ran Dong, Long Tian, Tai-Zeng Xin, Shou-Xian Wang, Yu Li, Mei-Na He, and Hai-Long Yu. 2025. "Mechanism of Enzyme Activity Regulation and Strain-Specific Response of Lentinula edodes Cultivation Adaptability Under Peach Wood Substrate" Journal of Fungi 11, no. 9: 684. https://doi.org/10.3390/jof11090684
APA StyleJiang, N., Dong, H.-R., Tian, L., Xin, T.-Z., Wang, S.-X., Li, Y., He, M.-N., & Yu, H.-L. (2025). Mechanism of Enzyme Activity Regulation and Strain-Specific Response of Lentinula edodes Cultivation Adaptability Under Peach Wood Substrate. Journal of Fungi, 11(9), 684. https://doi.org/10.3390/jof11090684






