The Transformation and Protein Expression of the Edible Mushroom Stropharia rugosoannulata Protoplasts by Agrobacterium-tumefaciens-Mediated Transformation
Abstract
1. Introduction
2. Results
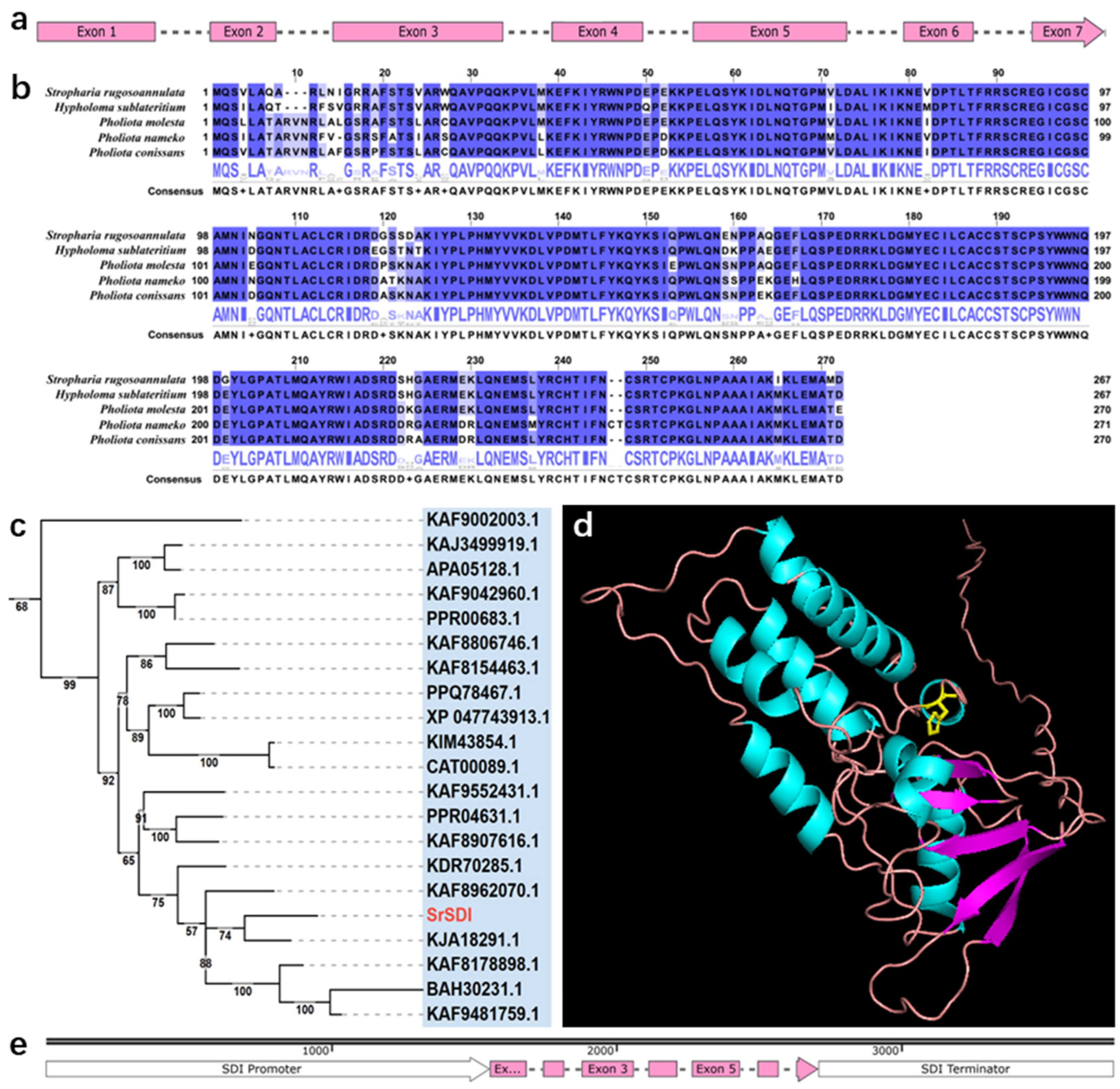
2.1. Characterization of the Wild-Type GPD and SDI Gene from S. rugosoannulata
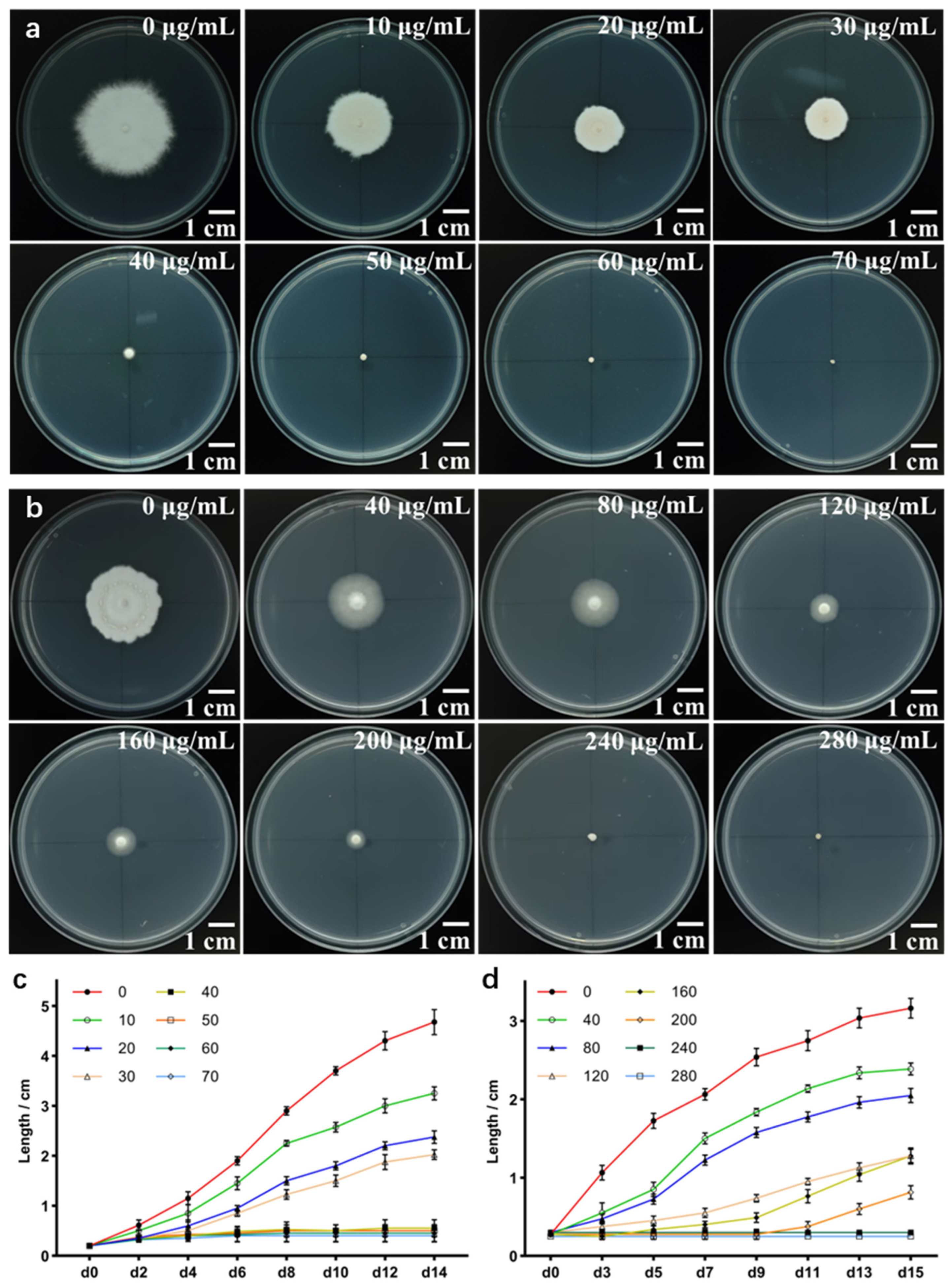
2.2. The Monokaryon Mycelium of S. rugosoannulata Exhibits Sensitivity to Hygromycin B and Carboxin
2.3. Establishment of a Transformation Protocol for HC7
2.4. Identification of the Inserted T-DNA Flanking Sequences in Transformants
2.5. Detection and Visualization of mCherry
2.6. Detection of GUS Gene Expression
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Strains and Culture Conditions
5.2. Identification of Candidate Genes and Their Regulatory Sequences
5.3. Extraction of Genomic DNA and Long-Read RNA-Seq
5.4. Construction of Plasmid
5.5. Test of Fungal Sensitivity Toward Hygromycin B and Carboxin
5.6. Protoplast Preparation
5.7. Agrobacterium-tumefaciens-Mediated Transformation of S. rugosoannulata
5.8. PCR Analysis of Putative Transformants
5.9. Fluorescence Observation and GUS Activity Analysis
5.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Hu, C.-F.; Feng, X.; Cheng, L.; Ibrahim, S.A.; Wang, C.-T.; Huang, W. Isolation, characterization and antioxidant of polysaccharides from Stropharia rugosoannulata. Int. J. Biol. Macromol. 2020, 155, 883–889. [Google Scholar] [CrossRef]
- Huang, L.; He, C.; Si, C.; Shi, H.; Duan, J. Nutritional, bioactive, and flavor components of Giant Stropharia (Stropharia rugoso-annulata): A Review. J. Fungi 2023, 9, 792. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Sun, J.; Luo, Z.-Y.; Rao, S.-Q.; Su, Y.-J.; Xu, R.-R.; Yang, Y.-J. Chemical composition of five wild edible mushrooms collected from Southwest China and their antihyperglycemic and antioxidant activity. Food Chem. Toxicol. 2012, 50, 1238–1244. [Google Scholar] [CrossRef]
- Weng, M. Extraction, Structure and Bioactivity of Polysaccharides from Stropharia rugodo-annulata. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2010. [Google Scholar]
- Sato, S.; Fukuda, Y.; Ogura, Y.; Kwon, E.; Kuwahara, S. Synthesis of the epimeric secosteroids strophasterols A and B. Angew. Chem. 2017, 129, 11051–11054. [Google Scholar] [CrossRef]
- Kodama, N.; Kakuno, T.; Nanba, H. Nanba Stimulation of the natural immune system in normal mice by polysaccharide from maitake mushroom. Mycoscience 2003, 44, 257–261. [Google Scholar] [CrossRef]
- Jiang, L. The Research on Preparation, Structural Identification and Biological Activity of Polysaccharide from Stropharia rugosoannulata (SR-1) and Polysaccharide from Tricholoma lascivum (Fr.) Gillet (TLG-1). Master’s Thesis, China West Normal University, Nanchong, China, 2019. [Google Scholar]
- Zhang, W.; Tian, G.; Geng, X.; Zhao, Y.; Ng, T.B.; Zhao, L.; Wang, H. Isolation and characterization of a novel lectin from the edible mushroom Stropharia rugosoannulata. Molecules 2014, 19, 19880–19891. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y. Nutritional Quality Analyse, Extraction and Application of Flavonoids of Stropharia rugoso-annulata Farlow. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2010. [Google Scholar]
- Meyer, V.; Mueller, D.; Strowig, T.; Stahl, U. Comparison of different transformation methods for Aspergillus giganteus. Curr. Genet. 2003, 43, 371–377. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, Q.; Li, H. Advances in genetic modification technologies. Chin. J. Biotechnol. 2015, 31, 1162–1174. [Google Scholar]
- Chen, B.-X.; Wei, T.; Ye, Z.-W.; Yun, F.; Kang, L.-Z.; Tang, H.-B.; Guo, L.-Q.; Lin, J.-F. Efficient CRISPR-Cas9 gene disruption system in edible-medicinal mushroom Cordyceps militaris. Front. Microbiol. 2018, 9, 1157. [Google Scholar] [CrossRef] [PubMed]
- Barcellos, F.G.; Fungaro, M.H.P.; Furlaneto, M.C.; Lejeune, B.; Pizzirani-Kleiner, A.A.; Lúcio de Azevedo, J. Genetic analysis of Aspergillus nidulans unstable transformants obtained by the biolistic process. Can. J. Microbiol. 1998, 44, 1137–1141. [Google Scholar] [CrossRef]
- Magaña-Ortíz, D.; Coconi-Linares, N.; Ortiz-Vazquez, E.; Fernández, F.; Loske, A.M.; Gómez-Lim, M.A. A novel and highly efficient method for genetic transformation of fungi employing shock waves. Fungal Genet. Biol. 2013, 56, 9–16. [Google Scholar] [CrossRef]
- Herzog, R.; Solovyeva, I.; Bölker, M.; Lugones, L.G.; Hennicke, F. Exploring molecular tools for transformation and gene expression in the cultivated edible mushroom Agrocybe aegerita. Mol. Genet. Genom. 2019, 294, 663–677. [Google Scholar] [CrossRef]
- Liu, G.; Cao, L.; Rao, Z.; Qiu, X.; Han, R. Identification of the genes involved in growth characters of medicinal fungus Ophiocordyceps sinensis based on Agrobacterium tumefaciens–mediated transformation. Appl. Microbiol. Biotechnol. 2020, 104, 2663–2674. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Liu, W.-T.; Stark, H.; Huang, C.-T. Expression of enterovirus 71 virus-like particles in transgenic enoki (Flammulina velutipes). Appl. Microbiol. Biotechnol. 2015, 99, 6765–6774. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liu, D.; Wang, Y.; Ren, D.; Zheng, L.; Chen, L.; Ma, A. Use of the yeast-like cells of Tremella fuciformis as a cell factory to produce a Pleurotus ostreatus hydrophobin. Biotechnol. Lett. 2017, 39, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- John, M.A.; Peberdy, J.F. Transformation of Aspergillus nidulans using the argB gene. Enzym. Microb. Technol. 1984, 6, 386–389. [Google Scholar] [CrossRef]
- Tran, V.-T.; Thai, H.-D.; Vu, T.X.; Vu, H.H.; Nguyen, G.T.; Trinh, M.T.; Tran, H.T.T.; Pham, H.T.T.; Le, N.T.H. An efficient Agrobacterium-mediated system based on the pyrG auxotrophic marker for recombinant expression in the filamentous fungus Penicillium rubens. Biotechnol. Lett. 2023, 45, 689–702. [Google Scholar] [CrossRef]
- Yelton, M.M.; Hamer, J.E.; Timberlake, W.E. Transformation of Aspergillus nidulans by using a trpC plasmid. Proc. Natl. Acad. Sci. USA 1984, 81, 1470–1474. [Google Scholar] [CrossRef] [PubMed]
- Narasipura, S.D.; Ren, P.; Dyavaiah, M.; Auger, I.; Chaturvedi, V.; Chaturvedi, S. An efficient method for homologous gene reconstitution in Cryptococcus gattii using URA5 auxotrophic marker. Mycopathologia 2006, 162, 401–409. [Google Scholar] [CrossRef]
- Balabanova, L.A.; Shkryl, Y.N.; Slepchenko, L.V.; Yugay, Y.A.; Gorpenchenko, T.Y.; Kirichuk, N.N.; Khudyakova, Y.V.; Bakunina, I.Y.; Podvolotskaya, A.B.; Bulgakov, V.P. Development of host strains and vector system for an efficient genetic transformation of filamentous fungi. Plasmid 2019, 101, 1–9. [Google Scholar] [CrossRef]
- Ahuja, M.; Punekar, N.S. Phosphinothricin resistance in Aspergillus niger and its utility as a selectable transformation marker. Fungal Genet. Biol. 2008, 45, 1103–1110. [Google Scholar] [CrossRef]
- Yang, X.; Peng, J.; Pan, J. Nourseothricin N-acetyl transferase (NAT), a new selectable marker for nuclear gene expression in Chlamydomonas. Plant Methods 2019, 15, 140. [Google Scholar] [CrossRef]
- Arentshorst, M.; Niu, J.; Ram, A.F.J. Efficient generation of Aspergillus niger knock out strains by combining NHEJ mutants and a split marker approach. In Genetic Transformation Systems in Fungi; van den Berg, M.A., Maruthachalam, K., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 1, pp. 263–272. [Google Scholar]
- Djulic, A.; Schmid, A.; Lenz, H.; Sharma, P.; Koch, C.; Wirsel, S.G.; Voegele, R.T. Transient transformation of the obligate biotrophic rust fungus Uromyces fabae using biolistics. Fungal Biol. 2011, 115, 633–642. [Google Scholar] [CrossRef]
- Crespo-Sempere, A.; López-Pérez, M.; Martínez-Culebras, P.V.; González-Candelas, L. Development of a green fluorescent tagged strain of Aspergillus carbonarius to monitor fungal colonization in grapes. Int. J. Food Microbiol. 2011, 148, 135–140. [Google Scholar] [CrossRef]
- Wu, L.; Conner, R.L.; Wang, X.; Xu, R.; Li, H. Variation in growth, colonization of maize, and metabolic parameters of GFP-and DsRed-labeled Fusarium verticillioides strains. Phytopathology 2016, 106, 890–899. [Google Scholar] [CrossRef]
- Wang, Q.; Coleman, J.J. CRISPR/Cas9-mediated endogenous gene tagging in Fusarium oxysporum. Fungal Genet. Biol. 2019, 126, 17–24. [Google Scholar] [CrossRef]
- Liu, D.; Garrigues, S.; de Vries, R.P. Heterologous protein production in filamentous fungi. Appl. Microbiol. Biotechnol. 2023, 107, 5019–5033. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Li, H.; Xu, Y.; Zhang, C.; Wu, J.; Lei, Y. Establishment of an efficient Agrobacterium tumefaciens-mediated transformation system for an Armillaria species, a host of the fully mycoheterotrophic plant Gastrodia elata. Folia Microbiol. 2024, 69, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bao, D.; Huang, Z.; Li, Y.; Zhou, C.; Wan, J.; Tang, L.; Mao, W.; Wang, Y.; Gong, M.; Zou, G.; et al. Agrobacterium-mediated transformation of arthroconidia obtained from the edible mushroom Hypsizygus marmoreus. J. Microbiol. Methods 2020, 171, 105878. [Google Scholar] [CrossRef]
- Punt, P.J.; Oliver, R.P.; Dingemanse, M.A.; Pouwels, P.H.; van den Hondel, C.A. Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene 1987, 56, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Van De Rhee, M.D.; Graça, P.M.A.; Huizing, H.J.; Mooibroek, H. Transformation of the cultivated mushroom, Agaricus bisporus, to hygromycin B resistance. Mol. Genet. Genom. 1996, 250, 252–258. [Google Scholar] [CrossRef]
- Zhu, X.; Xiong, L.; Li, H.; Song, X.; Liu, J.; Yang, G. Computational and experimental insight into the molecular mechanism of carboxamide inhibitors of Succinate-ubquinone oxidoreductase. ChemMedChem 2014, 9, 1512–1521. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.T.; Matsuyama, T.; Irie, T.; Watanabe, T.; Kuwahara, M. Carboxin resistance transformation of the homobasidiomycete fungus Pleurotus ostreatus. Curr. Genet. 2000, 37, 209–212. [Google Scholar] [CrossRef]
- Guo, M.; Zhu, X.; Li, H.; Tan, L.; Pan, Y. Development of a novel strategy for fungal transformation based on a mutant locus conferring carboxin-resistance in Magnaporthe oryzae. AMB Express 2016, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Chen, X.; Mou, C.; Dai, S.; Bian, Y.; Kang, H. Agrobacterium-mediated transformation of the ascomycete mushroom Morchella importuna using polyubiquitin and glyceraldehyde-3-phosphate dehydrogenase promoter-based binary vectors. World J. Microbiol. Biotechnol. 2018, 34, 148. [Google Scholar] [CrossRef]
- Lichius, A.; Ruiz, D.M.; Zeilinger, S. Genetic transformation of filamentous fungi: Achievements and challenges. In Grand Challenges in Fungal Biotechnology; Springer: Cham, Switzerland, 2020; pp. 123–164. [Google Scholar]
- Wang, S.; Chen, H.; Wang, Y.; Tang, X.; Zhang, H.; Chen, W.; Chen, Y.Q. Optimization of Agrobacterium tumefaciens-mediated transformation method of oleaginous filamentous fungus Mortierella alpina on co-cultivation materials choice. J. Microbiol. Methods 2018, 152, 179–185. [Google Scholar] [CrossRef]
- Fu, J.; Brockman, N.E.; Wickes, B.L. Optimizing transformation frequency of Cryptococcus neoformans and Cryptococcus gattii using Agrobacterium tumefaciens. J. Fungi 2021, 7, 520. [Google Scholar] [CrossRef] [PubMed]
- D’spain, S.; Andrade, P.I.; Brockman, N.E.; Fu, J.; Wickes, B.L. Agrobacterium tumefaciens-Mediated Transformation of Candida glabrata. J. Fungi 2022, 8, 596. [Google Scholar] [CrossRef]
- Bruhn, J.N.; Abright, N.; Mihail, J.D. Forest farming of wine-cap Stropharia mushrooms. Agrofor. Syst. 2010, 79, 267–275. [Google Scholar] [CrossRef]
- Okamoto, T.; Yamada, M.; Sekiya, S.; Okuhara, T.; Taguchi, G.; Inatomi, S.; Shimosaka, M. Agrobacterium tumefaciens-mediated transformation of the vegetative dikaryotic mycelium of the cultivated mushroom Flammulina velutipes. Biosci. Biotechnol. Biochem. 2010, 74, 2327–2329. [Google Scholar] [CrossRef]
- Lei, M.; Wu, X.; Zhang, J.; Wang, H.; Huang, C. Establishment of an efficient transformation system for Pleurotus ostreatus. World J. Microbiol. Biotechnol. 2017, 33, 214. [Google Scholar] [CrossRef]
- Martínez-Cruz, J.; Romero, D.; de Vicente, A.; Pérez-García, A. Transformation of the cucurbit powdery mildew pathogen Podosphaera xanthii by Agrobacterium tumefaciens. New Phytol. 2017, 213, 1961–1973. [Google Scholar] [CrossRef]
- Mora-Lugo, R.; Zimmermann, J.; Rizk, A.M.; Fernandez-Lahore, M. Development of a transformation system for Aspergillus sojae based on the Agrobacterium tumefaciens-mediated approach. BMC Microbiol. 2014, 14, 247. [Google Scholar] [CrossRef]
- Spanu, P.D.; Abbott, J.C.; Amselem, J.; Burgis, T.A.; Soanes, D.M.; Stüber, K.; van Themaat, E.V.L.; Brown, J.K.M.; Butcher, S.A.; Gurr, S.J.; et al. Genome expansion and gene loss in powdery mildew fungi reveal tradeoffs in extreme parasitism. Science 2010, 330, 1543–1546. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, A.J.; Wei, K.H.-C.; Huang, Y.; Lee, Y.C.G. Causes and consequences of varying transposable element activity: An evolutionary perspective. Annu. Rev. Genom. Hum. Genet. 2024, 25, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.H.; Mokhtar, M.M.; El Allali, A. Transposable elements: Multifunctional players in the plant genome. Front. Plant Sci. 2024, 14, 1330127. [Google Scholar] [CrossRef]
- Tao, Y.; Xie, B.; Yang, Z.; Chen, Z.; Chen, B.; Deng, Y.; Jiang, Y.; van Peer, A.F. Identification and expression analysis of a new glycoside hydrolase family 55 exo-β-1,3-glucanase-encoding gene in Volvariella volvacea suggests a role in fruiting body development. Gene 2013, 527, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Muraguchi, H.; Fujita, T.; Kishibe, Y.; Konno, K.; Ueda, N.; Nakahori, K.; Yanagi, S.O.; Kamada, T. The exp1 gene essential for pileus expansion and autolysis of the inky cap mushroom Coprinopsis cinerea (Coprinus cinereus) encodes an HMG protein. Fungal Genet. Biol. 2008, 45, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Zeng, Y.; Li, X.; Zhao, Z.; Shen, N.; Zhou, Y.; Bian, Y.; Xiao, Y. Molecular profiling of rice straw degradability discrepancy in Stropharia rugosoannulata core germplasm. J. Agric. Food Chem. 2024, 72, 25379–25390. [Google Scholar] [CrossRef]
- Gu, M.; Chen, Q.; Zhang, Y.; Zhao, Y.; Wang, L.; Wu, X.; Zhao, M.; Gao, W. Evaluation of Genetic Diversity and Agronomic Traits of Germplasm Resources of Stropharia rugosoannulata. Horticulturae 2024, 10, 213. [Google Scholar] [CrossRef]
- Hu, X.; Hoffmann, D.S.; Wang, M.; Schuhmacher, L.; Stroe, M.C.; Schreckenberger, B.; Elstner, M.; Fischer, R. GprC of the nematode-trapping fungus Arthrobotrys flagrans activates mitochondria and reprograms fungal cells for nematode hunting. Nat. Microbiol. 2024, 9, 1752–1763. [Google Scholar] [CrossRef]
- Tsai, M.-C.; Barati, M.T.; Kuppireddy, V.S.; Beckerson, W.C.; Long, G.; Perlin, M.H. Characterization of Microbotryum lychnidis-dioicae secreted effector proteins, their potential host targets, and localization in a heterologous host plant. J. Fungi 2024, 10, 262. [Google Scholar] [CrossRef]
- Sato, M.; Kurahashi, A.; Nishibori, K.; Fujimori, F. Development of a transformation system for the edible mushroom Grifola frondosa: Demonstrating heterologous gene expression and RNAi-mediated gene silencing. Mycoscience 2015, 56, 364–372. [Google Scholar] [CrossRef]
- Liu, Q.; Meng, G.; Wang, M.; Li, X.; Liu, M.; Wang, F.; Yang, Y.; Dong, C. Safe-harbor-targeted CRISPR/Cas9 system and Cmhyd1 overexpression enhances disease resistance in Cordyceps militaris. J. Agric. Food Chem. 2023, 71, 15249–15260. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; An, J.Y.; Choi, Y.-J.; Oh, Y.-L.; Ro, H.-S.; Ryu, H. Construction of a CRISPR/Cas9-Mediated Genome Editing System in Lentinula edodes. Mycobiology 2021, 49, 599–603. [Google Scholar] [CrossRef]
- Kamiya, A.; Ueshima, H.; Nishida, S.; Honda, Y.; Kamitsuji, H.; Sato, T.; Miyamoto, H.; Sumita, T.; Izumitsu, K.; Irie, T. Development of a gene-targeting system using CRISPR/Cas9 and utilization of pyrG as a novel selectable marker in Lentinula edodes. FEMS Microbiol. Lett. 2023, 370, fnad042. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Yang, H.; Tang, T.; Ma, C.; Kang, C. An effective CRISPR-Cas9 technology for efficiently isolating transgene-free mutants in Arabidopsis, Brassica napus, Strawberry, and Soybean. In CRISPR-Cas Methods; Islam, M.T., Bhowmik, P.K., Molla, K.A., Eds.; Springer: New York, NY, USA, 2020; pp. 99–115. [Google Scholar]
- Yan, P.-S.; Jiang, J.-H.; Cui, W.-S. Characterization of protoplasts prepared from the edible fungus, Stropharia rugoso-annulata. World J. Microbiol. Biotechnol. 2004, 20, 173–177. [Google Scholar] [CrossRef]
- Zhou, F.; Yang, H.-L.; Zhao, Y.; Jiang, S.-X.; Li, Z.-P.; Zha, L.; Li, Y. Characteristics of mononuclear properties and the protoplast monokaryogenesis of different varieties of Stropharia rugosoannulata. Acta Agric. Shanghai 2023, 39, 33–37. [Google Scholar]
- Tan, J.; Gong, Q.; Yu, S.; Hou, Y.; Zeng, D.; Zhu, Q.; Liu, Y.-G. A modified high-efficiency thermal asymmetric interlaced PCR method for amplifying long unknown flanking sequences. J. Genet. Genom. 2019, 46, 363–366. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, D.; Xiong, H. The Transformation and Protein Expression of the Edible Mushroom Stropharia rugosoannulata Protoplasts by Agrobacterium-tumefaciens-Mediated Transformation. J. Fungi 2025, 11, 674. https://doi.org/10.3390/jof11090674
Yin D, Xiong H. The Transformation and Protein Expression of the Edible Mushroom Stropharia rugosoannulata Protoplasts by Agrobacterium-tumefaciens-Mediated Transformation. Journal of Fungi. 2025; 11(9):674. https://doi.org/10.3390/jof11090674
Chicago/Turabian StyleYin, Dongjie, and Hairong Xiong. 2025. "The Transformation and Protein Expression of the Edible Mushroom Stropharia rugosoannulata Protoplasts by Agrobacterium-tumefaciens-Mediated Transformation" Journal of Fungi 11, no. 9: 674. https://doi.org/10.3390/jof11090674
APA StyleYin, D., & Xiong, H. (2025). The Transformation and Protein Expression of the Edible Mushroom Stropharia rugosoannulata Protoplasts by Agrobacterium-tumefaciens-Mediated Transformation. Journal of Fungi, 11(9), 674. https://doi.org/10.3390/jof11090674





