Surface Changes Induced by Brushing Increase Candida albicans Biofilms on 3D-Printed Denture Base Resin
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Surface Roughness Before Simulated Brushing (T0)
2.3. Experimental Groups
2.4. Brushing Cycles
2.5. Surface Roughness After Simulated Brushing (Ra)
2.6. Biofilm Formation
2.6.1. Assessment of Cell Proliferation by Counting Colony Forming Units (CFU/mL)
2.6.2. Assessment of Cellular Metabolic Activity Using the alamarBlue® Assay
2.6.3. Qualitative and Quantitative Analysis of Cell Viability Using CFM
2.7. Statistical Analysis
3. Results
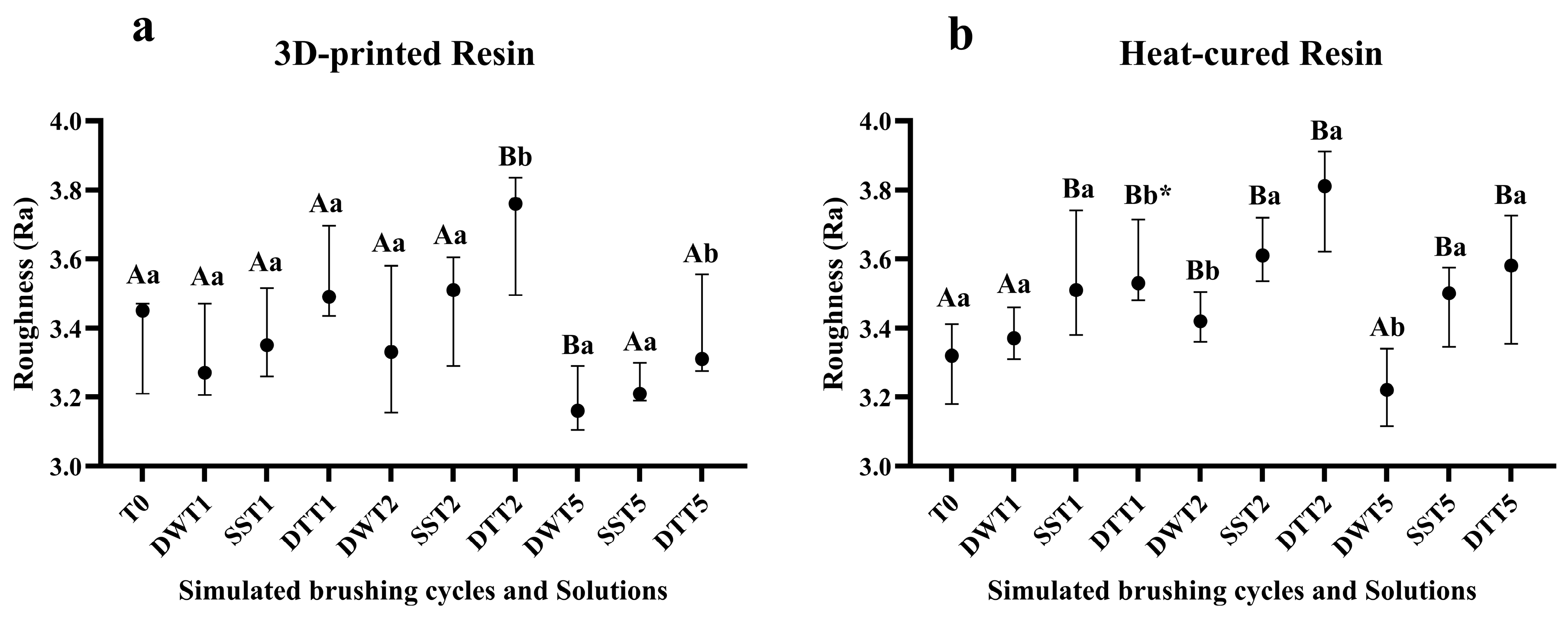
3.1. Surface Roughness
3.2. Assessment of Cell Proliferation by Counting Colony Forming Units (CFU/mL)
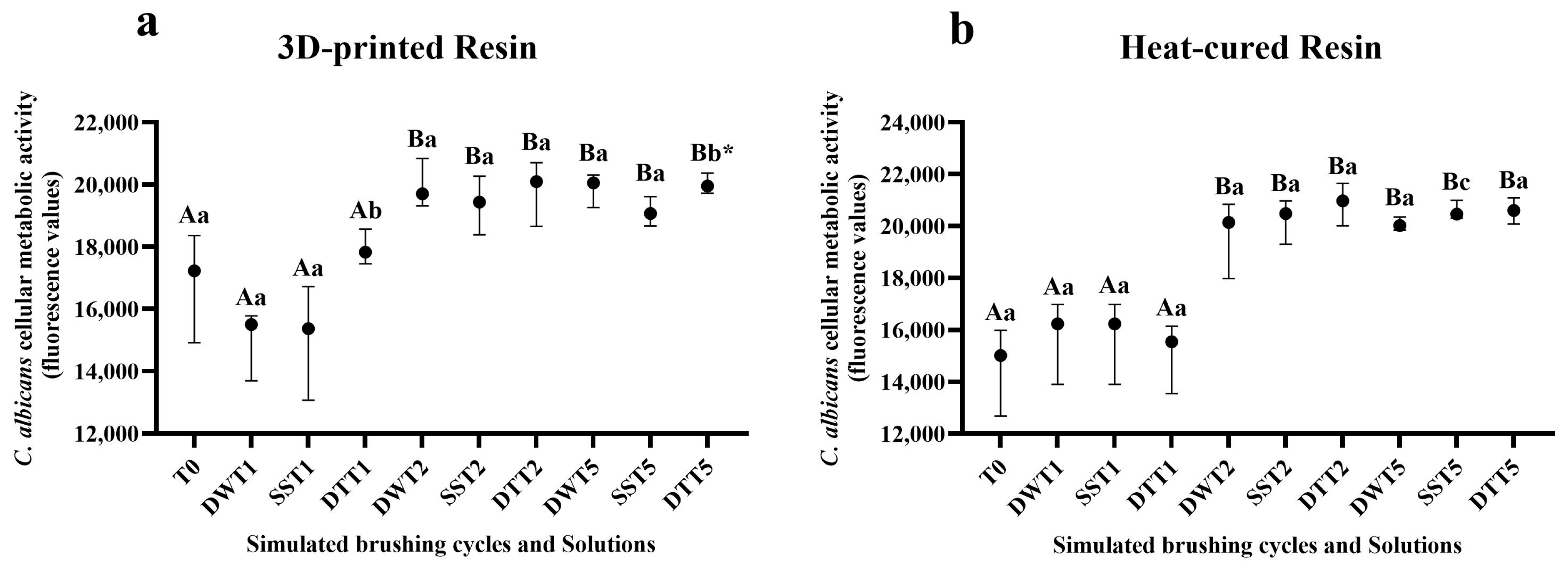
3.3. Assessment of Cellular Metabolic Activity Using the alamarBlue® Assay
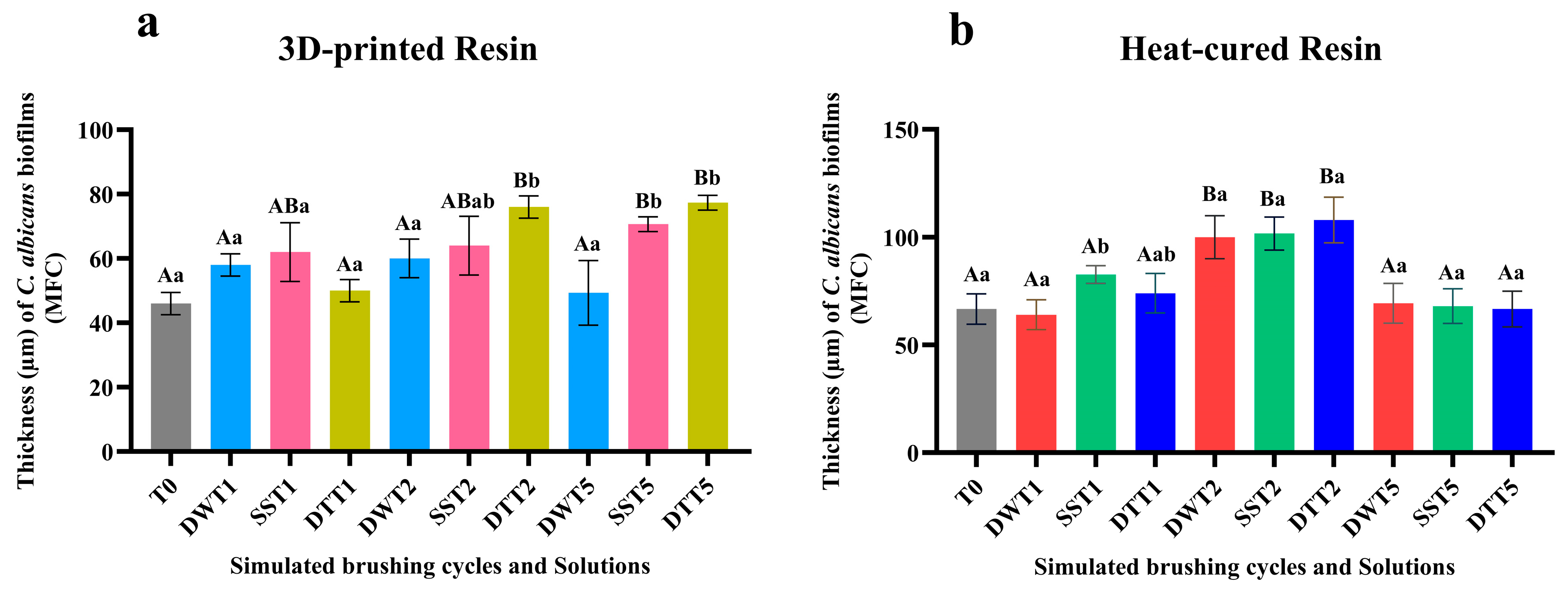
3.4. Qualitative and Quantitative Analysis of Cell Viability Using CFM
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jorge, J.H.; Giampolo, E.T.; Vergani, C.E.; Machado, A.L.; Pavarina, A.C.; Carlos, I.Z. Effect of post-polymerization heat treatments on the cytotoxicity of two denture base acrylic resins. J. Appl. Oral Sci. 2006, 14, 203–207. [Google Scholar] [CrossRef]
- Goiato, M.C.; Freitas, E.; Santos, D.; Medeiros, R.; Sonego, M. Acrylic resin cytotoxicity for denture base-literature review. Adv. Clin. Exp. Med. 2015, 24, 679–686. [Google Scholar] [CrossRef]
- Friel, T.; Waia, S. Removable partial dentures for older adults. Prim. Dent. J. 2020, 9, 34–39. [Google Scholar] [CrossRef]
- Rashid, H.; Sheikh, Z.; Vohra, F. Allergic effects of the residual monomer used in denture base acrylic resins. Eur. J. Dent. 2015, 9, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.L.; Giampolo, E.T.; Vergani, C.E.; Souza, J.F.; Jorge, J.H. Changes in roughness of denture base and reline materials by chemical disinfection or microwave irradiation: Surface roughness of denture base and reline materials. J. Appl. Oral Sci. 2011, 19, 521–528. [Google Scholar] [CrossRef]
- Alghazzawi, T.F. Advancements in CAD/CAM technology: Options for practical implementation. J. Prosthodont. Res. 2016, 60, 72–84. [Google Scholar] [CrossRef]
- Lin, L.; Fang, Y.; Liao, Y.; Chen, G.; Gao, C.; Zhu, P. 3D printing and digital processing techniques in Dentistry: A review of literature. Adv. Eng. Mater. 2019, 21, 2–28. [Google Scholar] [CrossRef]
- Revilla-León, M.; Ozcan, M. Additive manufacturing technologies used for processing polymers: Current status and potential application in prosthetic dentistry. J. Prosthodont. 2019, 28, 146–158. [Google Scholar] [CrossRef]
- Stansbury, J.W.; Idacavage, M.J. 3D printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Brida, A.S.; Taylor, T.D.; Agar, J.R. Computer-aided technology for fabricating complete dentures: Systematic review of historical background, current status, and future perspectives. J. Prosthet. Dent. 2013, 109, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.S.; Rizk, A.M.; Vila, T.; Ji, Y.; Masri, R.; Jabra-Rizk, M.A. Digital design of a universal rat intraoral device for therapeutic evaluation of a topical formulation against Candida-associated denture stomatitis. Infect. Immun. 2019, 87, e00617-19. [Google Scholar] [CrossRef] [PubMed]
- Meirowitz, A.; Rahmanov, A.; Shlomo, E.; Zelikman, H.; Dolev, E.; Sterer, N. Effect of denture base fabrication technique on Candida albicans adhesion in vitro. Materials 2021, 14, 221. [Google Scholar] [CrossRef]
- Gendreau, L.; Loewy, Z.G. Epidemiology and etiology of denture stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Mayahara, M.; Kataoka, R.; Arimoto, T.; Tamaki, Y.; Yamaguchi, N.; Watanabe, Y.; Yamasaki, Y.; Miyazaki, T. Effects of surface roughness and dimorphism on the adhesion of Candida albicans to the surface of resins: Scanning electron microscope analyses of mode and number of adhesions. J. Investig. Clin. Dent. 2014, 5, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Aslanimehr, M.; Mojarad, N.; Ranjbar, S.; Aalaei, S. In vitro comparison of the effects of microwave irradiation and chemical and mechanical methods on the disinfection of complete dentures contaminated with Candida albicans. Dent. Res. J. 2018, 15, 340–346. [Google Scholar] [CrossRef]
- Gad, M.M.; Fouda, S.M. Current perspectives and the future of Candida albicans-associated denture stomatitis treatment. Dent. Med. Probl. 2020, 57, 95–102. [Google Scholar] [CrossRef]
- Paranhos, H.F.O.; Silva, C.H.L.; Venezian, G.C.; Macedo, L.D.; Souza, R.F. Distribution of biofilm on internal and external surfaces of upper complete dentures: The effect of hygiene instruction. Gerodontology 2007, 24, 162–168. [Google Scholar] [CrossRef]
- Takamiya, A.S.; Monteiro, D.R.; Barão, V.A.R.; Pero, A.C.; Compagnoni, M.A.; Barbosa, D.B. Complete denture hygiene and nocturnal wearing habits among patients attending the Prosthodontic Department in a Dental University in Brazil. Gerodontology 2011, 28, 91–96. [Google Scholar] [CrossRef]
- Wang, W.; Hou, Y.; Li, J.; Chu, Y.; Tang, X.; Ai, H. Effect of different denture cleaning methods on roughness in resin denture base. J. Cent. South Univ. (Med. Sci.) 2013, 38, 1065–1069. [Google Scholar] [CrossRef]
- Verran, J.; Jackson, S.; Coulthwaite, L.; Scallan, A.; Loewy, Z.; Whitehead, K. The effect of dentifrice abrasion on denture topography and the subsequent retention of microorganisms on abraded surfaces. J. Prosthet. Dent. 2014, 112, 1513–1522. [Google Scholar] [CrossRef]
- Zoccolotti, J.O.; Tasso, C.O.; Arbeláez, M.I.A.; Malavolta, I.F.; Pereira, E.C.S.; Esteves, C.S.G.; Jorge, J.H. Properties of an acrylic resin after immersion in antiseptic soaps: Low-cost, easy-acess procedure for the prevention of denture stomatitis. PLoS ONE 2018, 13, e0203187. [Google Scholar] [CrossRef]
- Tasso, C.O.; Zoccolotti, J.O.; Ferrise, T.M.; Malavolta, I.F.; Jorge, J.H. Effectiveness of disinfectant liquid soaps in the reduction of Candida spp present in complete dentures: A crossover randomized clinical trial. Int. J. Prosthodont. 2020, 33, 620–628. [Google Scholar] [CrossRef]
- Ribas, B.R.; Tasso, C.O.; Ferrise, T.M.; Jorge, J.H. Influence of brushing with antiseptic soap solution on the surface and biological properties of an acrylic denture base resin. Am. J. Dent. 2022, 35, 238–244. [Google Scholar] [PubMed]
- Andrade, I.M.; Silva-Lovato, C.H.; Souza, R.F.; Pisani, M.X.; Andrade, K.M.; Paranhos, H.F.O. Trial of experimental toothpastes regarding quality for cleaning dentures. Int. J. Prosthodont. 2012, 25, 157–159. [Google Scholar] [PubMed]
- Salles, M.M.; Oliveira, V.C.; Souza, R.F.; Silva, C.H.L.; Paranhos, H.F.O. Antimicrobial action of sodium hypochlorite and castor oil solutions for denture cleaning—In vitro evaluation. Braz. Oral Res. 2015, 29, 1–6. [Google Scholar] [CrossRef]
- Izumida, F.E.; Moffa, E.B.; Vergani, C.E.; Machado, A.L.; Jorge, J.H.; Giampolo, E.T. In vitro evaluation of adherence of Candida albicans, Candida glabrata, and Streptococcus mutans to an acrylic resin modified by experimental coatings. Biofouling 2014, 30, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Kim, J.E.; Jeong, S.H.; Choi, Y.J.; Ryu, J.J. Printing accuracy, mechanical properties, surface characteristics, and microbial adhesion of 3D-printed resins with various printing orientations. J. Prosthet. Dent. 2020, 12, 468–475. [Google Scholar] [CrossRef]
- De Foggi, C.C.; Machado, A.L.; Zamperini, C.A.; Fernandes, D.; Wady, A.D.; Vergani, C.E. Effect of surface roughness on the hydrophobicity of a denture-base acrylic resin and Candida albicans colonization. J. Investig. Clin. Dent. 2016, 7, 141–148. [Google Scholar] [CrossRef]
- ISO 20795-1:2013; Dentistry—Base polymers Part 1: Denture Base Polymers. International Standards Organization: Geneva, Switzerland. Available online: https://www.iso.org/standard/62277.html (accessed on 15 May 2025).
- Machado, A.L.; Giampaolo, E.T.; Vergani, C.E.; Pavarina, A.C.; Salles, D.S.L.; Jorge, J.H. Weight loss and changes in surface roughness of denture base and reline materials after simulated toothbrushing in vitro. Gerodontology 2012, 29, e121–e127. [Google Scholar] [CrossRef]
- Tribst, J.P.M.; Maria de Oliveira Dal Piva, A.; Werner, A.; Sampaio Silva, L.T.; Anami, L.C.; Bottino, M.A.; Kleverlaan, C.J. Effect of surface treatment and glaze application on shade characterized resin-modified ceramic after toothbrushing. J. Prosthet. Dent. 2021, 125, 691.e1–691.e7. [Google Scholar] [CrossRef] [PubMed]
- Alfouzan, A.F.; Alotiabi, H.M.; Labban, N.; Al-Otaibi, H.N.; Taweel, S.M.A.; AlShehri, H.Á. Color stability of 3D-printed denture resins: Effect of aging, mechanical brushing and immersion in staining medium. J. Adv. Prosthodont. 2022, 14, 334. [Google Scholar] [CrossRef]
- Alfouzan, A.F.; Alotiabi, H.M.; Labban, N.; Al-Otaibi, H.N.; Taweel, S.M.A.; AlShehri, H.A. Effect of aging and mechanical brushing on surface roughness of 3D printed denture resins: A profilometer and scanning electron microscopy analysis. Technol. Health Care 2022, 30, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, P.J.; Koka, S.; Ewoldsen, N.O.; Lefebvre, C.A.; Lavin, M.T. Cytotoxicity of denture base resins. Int. J. Prosthodont. 1997, 10, 73–77. [Google Scholar] [PubMed]
- Oliveira, J.S.; Ribas, B.R.; Ferro, A.C.; Tasso, C.O.; Camargo, R.; Cavalheiro, A.J.; Jorge, J.H. Cryptocarya moschata fractions decrease planktonic cells and biofilms of Candida albicans and Streptococcus mutans. Biofouling 2024, 40, 831–846. [Google Scholar] [CrossRef]
- Chang, Y.H.; Lee, C.Y.; Hsu, M.S.; DU, J.K.; Chen, K.K.; Wu, J.H. Effect of toothbrush/dentifrice abrasion on weight variation, surface roughness, surface morphology and hardness of conventional and CAD/CAM denture base materials. Dent. Mater. J. 2021, 40, 220–227. [Google Scholar] [CrossRef]
- Çakmak, G.; Donmez, M.B.; Akay, C.; Atalay, S.; Paula, M.S.; Schimmel, M.; Yilmaz, B. Effect of simulated brushing and disinfection on the surface roughness and color stability of CAD-CAM denture base materials. J. Mech. Behav. Biomed. Mater. 2022, 134, 105390. [Google Scholar] [CrossRef]
- Freitas-Pontes, K.M.; Silva-Lovato, C.H.; Paranhos, H.F.O. Mass loss of four commercially available heat-polymerized acrylic resins after toothbrushing with three different dentifrices. J. Appl. Oral Sci. 2009, 17, 116–121. [Google Scholar] [CrossRef]
- Campos, D.E.S.; Muniz, I.A.F.; Costa, T.K.V.L.; Lima, R.B.W.; Neppelenbroek, K.H.; Batista, A.U.D. Effect of simulated brushing with dentifrices on surface roughness and the massa loss of acrylic resin: A systematic review and meta-analysis of in vitro studies. J. Prosthet. Dent. 2025, 133, 1209–1220. [Google Scholar] [CrossRef]
- Meyer, F.; Wiesche, E.S.Z.; Amechi, B.T.; Limeback, H.; Enax, J. Caries etiology and preventive measures. Eur. J. Dent. 2024, 18, 766–776. [Google Scholar] [CrossRef]
- Enax, J.; Meyer, F.; Wiesche, E.S.Z.; Fuhrmann, I.C.; Fabritius, H.O. Toothpaste abrasion and abrasive particle content: Correlating high-resolution profilometric analysis with relative dentin abrasivity (RDA). Dent. J. 2023, 11, 79. [Google Scholar] [CrossRef]
- Carvalho, L.F.; Alves, L.M.M.; Bergamo, E.T.P.; Jalkh, E.B.B.; Campos, T.M.B.; Zahoui, A.; Fermino, E.S.; Magalhães, A.C.; Silva, T.L.; Coelho, P.G.; et al. Influence of abrasive dentifrices on polymeric reconstructive material properties after simulated toothbrushing. Biomater. Investig. Dent. 2023, 10, 2268670. [Google Scholar] [CrossRef]
- Nunes, T.S.B.S.; Silva, M.D.D.; Coelho, S.R.G.; Viotto, H.E.C.; Pero, A.C. Effectiveness of disinfectant solution associated or not with brushing on the biofilm control of a 3D printed-denture base resin. J. Appl. Oral Sci. 2023, 31, e20230104. [Google Scholar] [CrossRef]
- Compagnoni, M.A.; Barbosa, D.B.; Souza, R.F.; Pero, A.C. The effect of polymerization cycles on porosity of microwave-processed denture base resin. J. Prosthet. Dent. 2004, 91, 281–285. [Google Scholar] [CrossRef]
- Jose, A.; Coco, B.J.; Milligan, S.; Young, B.; Lappin, D.F.; Bagg, J.; Murray, C.; Ramage, G. Reducing the incidence of denture stomatitis: Are denture cleansers sufficient? J. Prosthodont. 2010, 19, 252–257. [Google Scholar] [CrossRef]
- Ramage, G.; Zalewska, A.Z.; Cameron, D.A.; Sherry, S.; Murray, C.; Finnegan, M.B.; Loewy, Z.G.; Jagger, D.C. A comparative in vitro study of two denture cleaning techniques as an effective strategy for inhibiting Candida albicans biofilms on denture surfaces and reducing inflammation. J. Prosthodont. 2012, 21, 516–522. [Google Scholar] [CrossRef]
- Richmond, R.; Macfarlane, T.V.; McCord, J.F. An evaluation of the surface changes in PMMA biomaterial formulations as a result of toothbrush/dentifrice abrasion. Dent. Mater. 2004, 20, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Longhin, E.M.; Yamani, N.E.; Rundén-Pran, E.; Dusinska, M. The alamar blue assay in the context of safety testing of nanomaterials. Front. Toxicol. 2022, 4, 981701. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.B.; de Albuquerque, M.C.; Rosa, L.M.; Klein, M.I.; Pavarina, A.C.; Barbugli, P.A.; Dovigo, L.N.; Mima, E.G.O. Quantification methods of Candida albicans are independent irrespective of fungal morphology. Microb. Cell 2024, 26, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Alhajj, M.N.; Halboub, E.; Yacob, N.; Al-Maweri, S.A.; Ahmad, S.F.; Celebic, A.; Al-Mekhlafi, H.M.; Salleh, N.M. Adhesion of Candida albicans to digital versus conventional acrylic resins: A systematic review and meta-analysis. BMC Oral Health 2024, 24, 303. [Google Scholar] [CrossRef]
- Dorocka-Bokowska, B.; Budtz-Jorgensen, E.; Wloch, S. Non-insulin-dependent diabetes mellitus as a risk factor for denture stomatitis. J. Oral Pathol. Med. 1996, 25, 411–415. [Google Scholar] [CrossRef]
- Budtz-Jorgensen, E.; Mojon, P.; Rentsch, A.; Deslauriers, N. Effects of an oral health program on the occurrence of oral candidosis in a long-term care facility. Community Dent. Oral Epidemiol. 2000, 28, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.C.; Thomas, C.J.; Willcox, M.D.; Harty, D.W.; Knox, K.W. Candida-associated denture stomatitis. Aetiology and management: A review. Part 2. Oral diseases caused by Candida species. Aust. Dent. J. 1998, 43, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.C.; Thomas, C.J.; Willcox, M.D.; Harty, D.W.; Knox, K.W. Candida-associated denture stomatitis. Aetiology and management: A review. Part 1. Factors influencing distribution of Candida species in the oral cavity. Aust. Dent. J. 1998, 43, 45–50. [Google Scholar] [CrossRef]
- Webb, B.C.; Thomas, C.J.; Willcox, M.D.; Harty, D.W.; Knox, K.W. Candida-associated denture stomatitis. Aetiology and management: A review. Part 3. Treatment of oral candidosis. Aust. Dent. J. 1998, 43, 244–249. [Google Scholar] [CrossRef]
- Pisani, M.X.; Bruhn, J.P.; Paranhos, H.F.O.; Silva-Lovato, C.H.; Souza, R.F.; Panzeri, H. Evaluation of the abrasiveness of dentifrices for complete dentures. J. Prosthodont. 2010, 19, 369–373. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camargo, R.; Oliveira, J.S.d.; Ferro, A.C.; Ribas, B.R.; Alves, A.A.V.; Jorge, J.H. Surface Changes Induced by Brushing Increase Candida albicans Biofilms on 3D-Printed Denture Base Resin. J. Fungi 2025, 11, 668. https://doi.org/10.3390/jof11090668
Camargo R, Oliveira JSd, Ferro AC, Ribas BR, Alves AAV, Jorge JH. Surface Changes Induced by Brushing Increase Candida albicans Biofilms on 3D-Printed Denture Base Resin. Journal of Fungi. 2025; 11(9):668. https://doi.org/10.3390/jof11090668
Chicago/Turabian StyleCamargo, Rafaelly, Jonatas Silva de Oliveira, Amanda Costa Ferro, Beatriz Ribeiro Ribas, Alan Augusto Valério Alves, and Janaina Habib Jorge. 2025. "Surface Changes Induced by Brushing Increase Candida albicans Biofilms on 3D-Printed Denture Base Resin" Journal of Fungi 11, no. 9: 668. https://doi.org/10.3390/jof11090668
APA StyleCamargo, R., Oliveira, J. S. d., Ferro, A. C., Ribas, B. R., Alves, A. A. V., & Jorge, J. H. (2025). Surface Changes Induced by Brushing Increase Candida albicans Biofilms on 3D-Printed Denture Base Resin. Journal of Fungi, 11(9), 668. https://doi.org/10.3390/jof11090668





