Virulence of Candida Isolates in Patients with Tuberculosis and Oral/Oesophageal Candidiasis: Co-Infection Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Population
2.3. Samples and Strains
2.4. Molecular Identification: DNA Isolation and Sequencing
2.5. Inoculum Preparation
2.6. Antifungal Susceptibility Testing
2.7. Assessment of Virulence Factors
2.8. Phagocytosis Assay and Quantification of Reactive Oxygen and Nitrogen Species
2.9. Statistical Analysis
3. Results
3.1. Clinical and Laboratory Characterisation of TB/Candida/HIV Co-Infection
3.2. Sequencing of the Partial 18S-5.8S-28S Ribosomal DNA (rDNA) Loci
3.3. Antifungal Susceptibility Profile
3.4. Virulence Factors
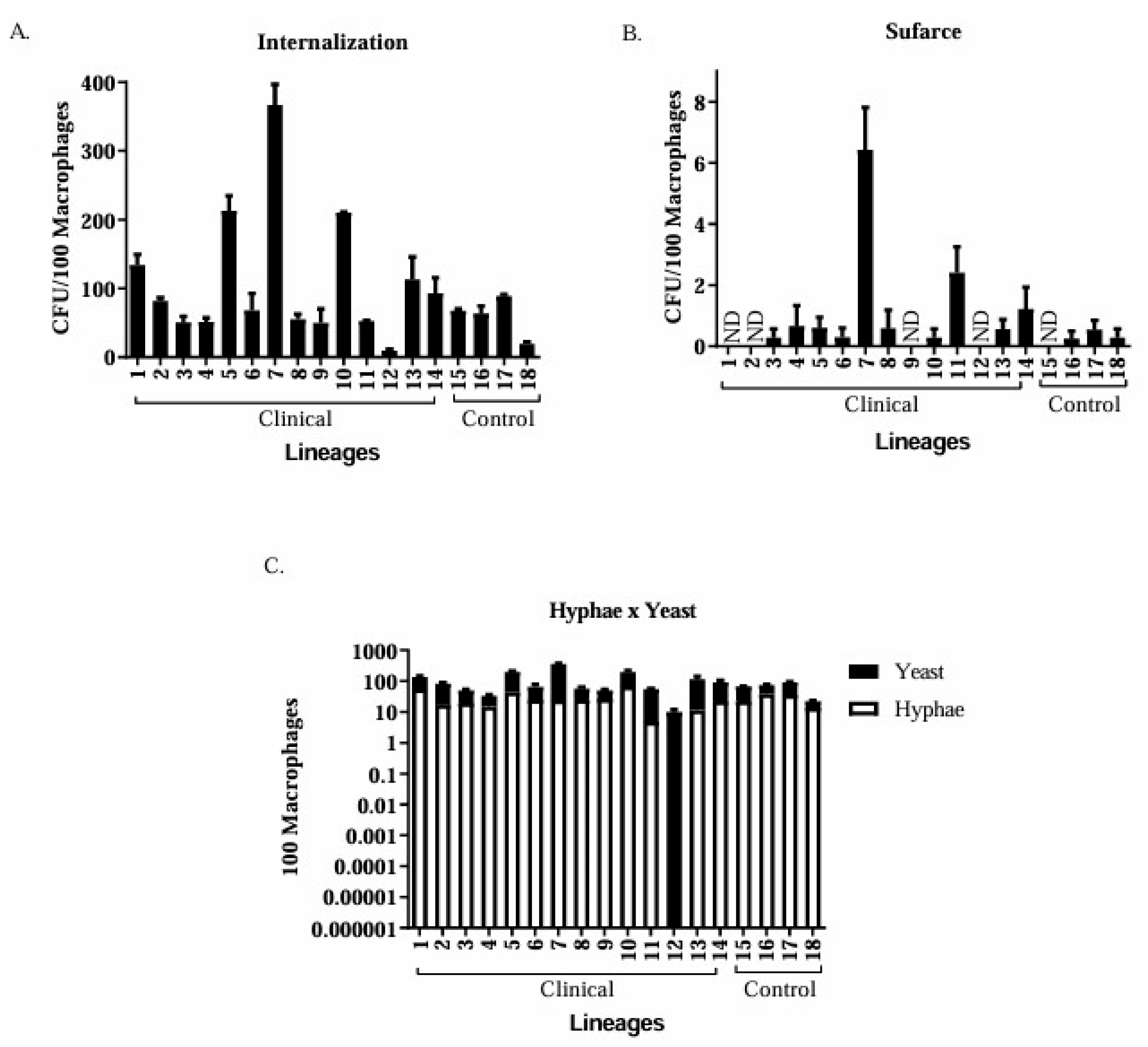
3.5. Phagocytosis Assay
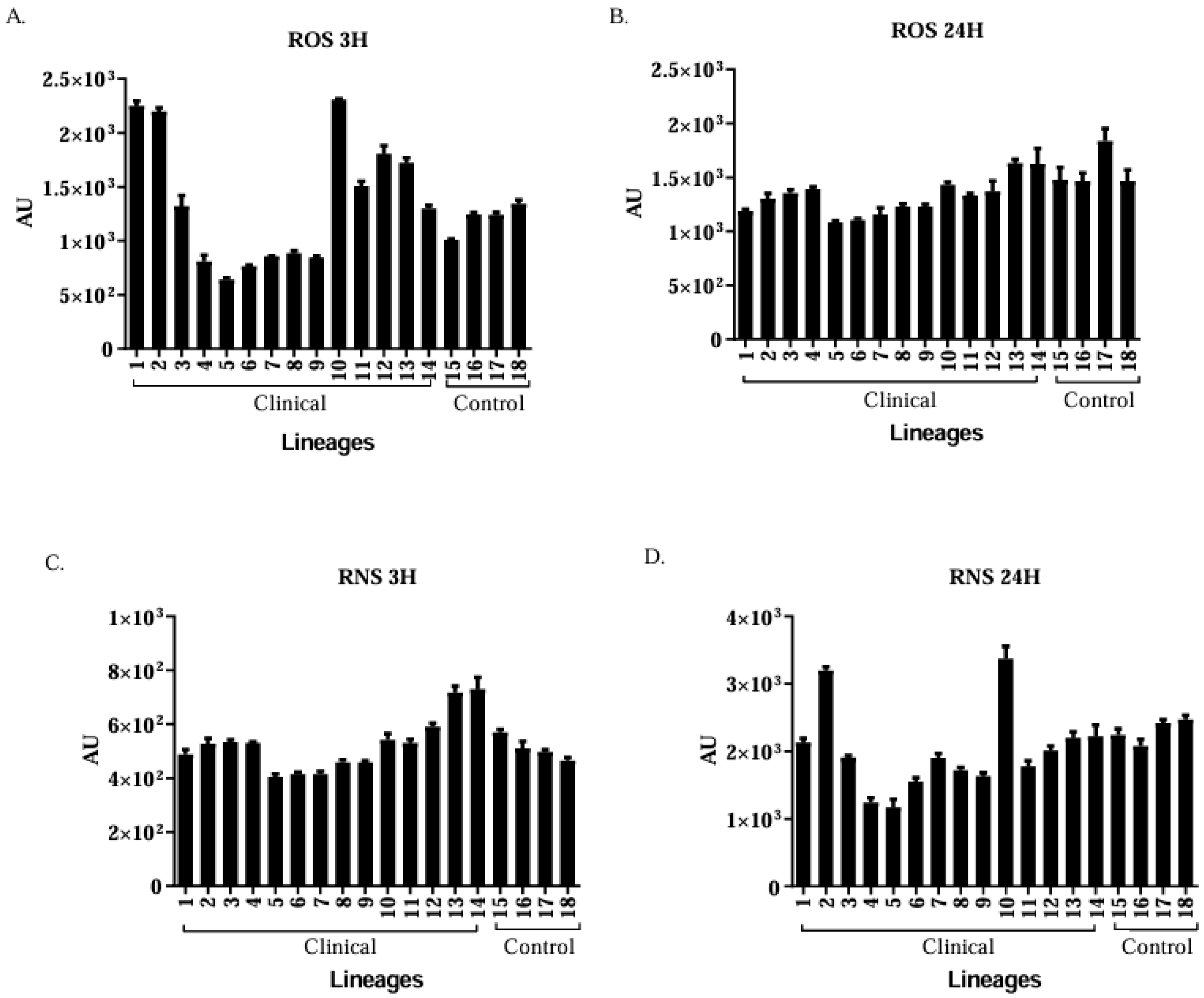
3.6. Production of ROS and RNS by Murine Macrophages
Clinical Isolates of Candida spp. Are More Virulent than the Control Group Isolates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brasil, Ministério da Saúde. Secretaria de Vigilância em Saúde. Implantação do Plano Nacional pelo Fim da Tuberculose como Problema de Saúde Pública no Brasil: Primeiros passos rumo ao alcance das metas. Bol. Epidemiológico 2018, 49, 1–18. [Google Scholar]
- Zagmignan, A.; Alves, M.S.; Sousa, E.M.; Lima Neto, L.G.; Sabbadini, P.S.; Monteiro, S.G. Caracterização epidemiológica e demográfica de pacientes com tuberculose pulmonar no estado Maranhão. Rev. Investig. Biomédica 2014, 6, 2–9. [Google Scholar]
- Astekar, M.; Bhatiya, P.S.; Sowmya, G.V. Prevalence and characterization of opportunistic Candidal infections among patients with pulmonary tuberculosis. J. Oral. Maxillofac. Pathol. 2016, 20, 183–189. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nittayananta, W.; Chanowanna, N.; Winn, T.; Silpapojakul, K.; Rodklai, A.; Jaruratanasirikul, S.; Liewchanpatana, K. Co-existence between oral lesions and opportunistic systemic diseases among HIV-infected subjects in Thailand. J. Oral. Pathol. Med. 2002, 31, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Sudbery, P.E. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 2011, 9, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Perlin, D.S.; Muldoon, E.G.; Colombo, A.L.; Chakrabarti, A.; Richardson, M.D.; Sorrell, T.C. Delivering on Antimicrobial Resistance Agenda Not Possible without Improving Fungal Diagnostic Capabilities. Emerg. Infect. Dis. 2017, 23, 177–183. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, X.H.; Gao, Y.C.; Zhang, Y.; Tang, Z.H.; Yu, Y.S.; Zang, G.Q. Tuberculosis infection might increase the risk of invasive candidiasis in an immunocompetent patient. Rev. Inst. Med. Trop. 2015, 57, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.D.; Tagliaferri, T.L.; de Carvalho, M.A.R.; de Resende-Stoianoff, M.A.; Holanda, R.A.; de Magalhães, T.F.F.; Magalhães, P.P.; Santos, S.G.D.; de Macêdo Farias, L. Investigating cross-contamination by yeast strains from dental solid waste to waste-handling workers by DNA sequencing. Microbiologyopen 2018, 7, e00554. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brilhante, R.S.N.; Rocha, M.F.G.; Cordeiro, R.A.; Rabenhorst, S.H.B.; Granjeiro, T.B.; Monteiro, A.J.; Sidrim, J.J.C. Phenotypical and molecular characterization of Microsporum canis strains in north-east Brazil. J. Appl. Microbiol. 2005, 99, 776–782. [Google Scholar] [CrossRef]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of 304 Yeasts, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Price, M.F.; Wilkinson, I.D.; Gentry, L.O. Plate method for detection of phospholipase activity in Candida albicans. Sabouraudia 1982, 20, 7–14. [Google Scholar] [CrossRef] [PubMed]
- D'Eça Júnior, A.; Silva, A.F.; Rosa, F.C.; Monteiro, S.G.; de Maria Silva Figueiredo, P.; de Andrade Monteiro, C. In vitro differential activity of phospholipases and acid proteinases of clinical isolates of Candida. Rev. Soc. Bras. Med. Trop. 2011, 44, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Ito-Kuwa, S.; Nakamura, Y.; Masuhara, T. Comparative pathogenicity of a wild-type strain and respiratory mutants of Candida albicans in mice. Zentralbl. Bakteriol. 1990, 273, 332–343. [Google Scholar] [CrossRef]
- Shin, J.H.; Kee, S.J.; Shin, M.G.; Kim, S.H.; Shin, D.H.; Lee, S.K.; Suh, S.P.; Ryang, D.W. Biofilm production by isolates of Candida species recovered from nonneutropenic patients: Comparison of bloodstream isolates with isolates from other sources. J. Clin. Microbiol. 2002, 40, 1244–1248. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Goncalves, R.; Mosser, D.M. The isolation and characterization of murine macrophages. Curr. Protoc. Immunol. 2008, 83, 14.1.1–14.1.14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soares, B.M.; Alves, O.A.; Ferreira, M.V.; Amorim, J.C.; Sousa, G.R.; de Barros Silveira, L.; Prates, R.A.; Ávila, T.V.; de Matos Baltazar, L.; da Glória de Souza, D.; et al. Cryptococcus gattii: In vitro susceptibility to photodynamic inactivation. Photochem. Photobiol. 2011, 87, 357–364. [Google Scholar] [CrossRef] [PubMed]
- WHO (Ed.) The End TB Srategy. In Document Production Services; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Sánchez, M.S.; Lloyd-Smith, J.O.; Getz, W.M. Monitoring linked epidemics: The case of tuberculosis and HIV. PLoS ONE 2010, 5, e8796. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Van Tongeren, L.; Shaipanich, T.; Fleetham, J.A. Coinfection with Cryptococcus gattii and Mycobacterium tuberculosis in an otherwise healthy 18-year-old woman. Can. Respir. J. 2011, 18, e62–e63. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shiboski, C.H.; Chen, H.; Ghannoum, M.A.; Komarow, L.; Evans, S.; Mukherjee, P.K.; Isham, N.; Katzenstein, D.; Asmelash, A.; Omozoarhe, A.E.; et al. Role of oral candidiasis in TB and HIV co-infection: AIDS Clinical Trial Group Protocol A5253. Int. J. Tuberc. Lung Dis. 2014, 18, 682–688. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bansod, S.; Rai, M. Emerging of mycotic. infection in patients infected with Mycobacterium tuberculosis. World J. Med. Sci. 2008, 3, 74–80. [Google Scholar]
- Amiri, M.R.J.; Siami, R.; Khaledi, A. Tuberculosis Status and Coinfection of Pulmonary Fungal Infections in Patients Referred to Reference Laboratory of Health Centers Ghaemshahr City during 2007–2017. Ethiop. J. Health Sci. 2018, 28, 683–690. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peixoto, J.V.; Rocha, M.G.; Nascimento, R.T.L.; Moreira, V.V.; Kashiwabará, T.G.B. Candidiasis—A literature review. Braz. J. Surg. Clin. Res. 2014, 8, 75–82. [Google Scholar]
- Das, P.P.; Saikia, L.; Nath, R.; Phukan, S.K. Species distribution & antifungal susceptibility pattern of oropharyngeal Candida isolates from human immunodeficiency virus infected individuals. Indian. J. Med. Res. 2016, 143, 495–501. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kali, A.; Charles, M.P.; Noyal, M.J.; Sivaraman, U.; Kumar, S.; Easow, J.M. Prevalence of Candida co-infection in patients with pulmonary tuberculosis. Australas. Med. J. 2013, 6, 387–391. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Latha, R.; Sasikala, R.; Muruganandam, N.; Venkatesh Babu, R. Study on the patterns of change in Candida albicans. Candida Infection in Lower Respiratory Tract Infections and Evaluation of CHROMagar in the Identification of Candida species. J. Microbiol. Biotechnol. Res. 2011, 1, 113–119. [Google Scholar]
- Hasan, F.; Xess, I.; Wang, X.; Jain, N.; Fries, B.C. Biofilm formation in clinical Candida isolates and its association with virulence. Microbes Infect. 2009, 11, 753–761. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- May, R.C.; Casadevall, A. In Fungal Intracellular Pathogenesis, Form Determines Fate. mBio 2018, 9, e02092-18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- da Silva Dantas, A.; Day, A.; Ikeh, M.; Kos, I.; Achan, B.; Quinn, J. Oxidative stress responses in the human fungal pathogen, Candida albicans. Biomolecules 2015, 5, 142–165. [Google Scholar] [CrossRef]
- Kaur, R.; Ma, B.; Cormack, B.P. A family of glycosylphosphatidylinositol-linked aspartyl proteases is required for virulence of Candida glabrata. Proc. Natl. Acad. Sci. USA 2007, 104, 7628–7633. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Isolated | Gender | Age | Tuberculosis | Fungi Isolation | Sample | Identification by MALDI-TOF | HIV |
|---|---|---|---|---|---|---|---|
| 1 | F | 38 | + | + | Sputum | C. albicans | - |
| 2 | M | 20 | + | + | Sputum | C. albicans | - |
| 3 | F | 28 | + | + | Sputum | C. albicans | - |
| 4 | F | 43 | + | + | Sputum | C. albicans | - |
| 5 | M | 43 | + | + | Sputum | C. albicans | - |
| 6 | M | 37 | + | + | Sputum | C. tropicalis | - |
| 7 | M | 77 | + | + | Sputum | C. tropicalis | - |
| 8 | M | 24 | + | + | Tracheal Secretion | C. albicans | - |
| 9 | M | 47 | + | + | Tracheal Secretion | C. albicans | - |
| 10 | M | 54 | + | + | Sputum | C. albicans | + |
| 11 | F | 23 | + | + | Sputum | C. albicans | + |
| 12 | M | 37 | + | + | Sputum | C. tropicalis | + |
| 13 | M | 44 | + | + | Sputum | C. albicans | + |
| 14 | M | 41 | + | + | Sputum | C. albicans | + |
| Isolated | FLU50 | AmB100 |
|---|---|---|
| 1 (Cl) | 4 (S) | 0.5 (S) |
| 2 (Cl) | 2 (S) | 0.5 (S) |
| 3 (Cl) | 4 (S) | 0.5 (S) |
| 4 (Cl) | 4 (S) | 0.5 (S) |
| 5 (Cl) | 1 (S) | 1 (S) |
| 6 (Cl) | 16 (SDD) | 1 (S) |
| 7 (Cl) | 2 (S) | 1 (S) |
| 8 (Cl) | 4 (S) | 1 (S) |
| 9 (Cl) | 1 (S) | 1 (S) |
| 10 (Cl) | 4 (S) | 0.5 (S) |
| 11 (Cl) | 4 (S) | 1 (S) |
| 12 (Cl) | 4 (S) | 2 (R) |
| 13 (Cl) | 4 (S) | 1 (S) |
| 14 (Cl) | 2 (S) | 0.5 (S) |
| 15 (Co) | 8 (S) | 0.5 (S) |
| 16 (Co) | 1 (S) | 1 (S) |
| 17 (Co) | 4 (S) | 1 (S) |
| 18 (Co) | 4 (S) | 1 (S) |
| 19 (Co) | 4 (S) | 1 (S) |
| 20 (Co) | 4 (S) | 1 (S) |
| 21 (Co) | 4 (S) | 2 (S) |
| 22 (Co) | 8 (S) | 0.5 (S) |
| 23 (Co) | 0.5 (S) | 2 (R) |
| 24 (Co) | 2 (S) | 1(S) |
| 25 (Co) | 2 (S) | 1 (S) |
| 26 (Co) | 2 (S) | 1 (S) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, R.L.P.S.; Macedo, A.T.; Azevedo, C.d.M.P.e.S.d.; Marques, S.G.; Costa, M.C.; Oliveira, J.C.M.D.d.; Carmo, P.H.F.d.; Diniz, Y.C.M.; Cutrim, H.F.; Monteiro, C.A.; et al. Virulence of Candida Isolates in Patients with Tuberculosis and Oral/Oesophageal Candidiasis: Co-Infection Evaluation. J. Fungi 2025, 11, 665. https://doi.org/10.3390/jof11090665
Ferreira RLPS, Macedo AT, Azevedo CdMPeSd, Marques SG, Costa MC, Oliveira JCMDd, Carmo PHFd, Diniz YCM, Cutrim HF, Monteiro CA, et al. Virulence of Candida Isolates in Patients with Tuberculosis and Oral/Oesophageal Candidiasis: Co-Infection Evaluation. Journal of Fungi. 2025; 11(9):665. https://doi.org/10.3390/jof11090665
Chicago/Turabian StyleFerreira, Rayana Larissa Pinheiro Soares, Alessandra Teixeira Macedo, Conceição de Maria Pedrozo e Silva de Azevedo, Sirlei Garcia Marques, Marliete Carvalho Costa, João Carlos Maia Dornelas de Oliveira, Paulo Henrique Fonseca do Carmo, Yankee Costa Magalhães Diniz, Heylane Ferreira Cutrim, Cristina Andrade Monteiro, and et al. 2025. "Virulence of Candida Isolates in Patients with Tuberculosis and Oral/Oesophageal Candidiasis: Co-Infection Evaluation" Journal of Fungi 11, no. 9: 665. https://doi.org/10.3390/jof11090665
APA StyleFerreira, R. L. P. S., Macedo, A. T., Azevedo, C. d. M. P. e. S. d., Marques, S. G., Costa, M. C., Oliveira, J. C. M. D. d., Carmo, P. H. F. d., Diniz, Y. C. M., Cutrim, H. F., Monteiro, C. A., Bomfim, M. R. Q., Santos, D. A., Holanda, R. A., & Santos, J. R. A. (2025). Virulence of Candida Isolates in Patients with Tuberculosis and Oral/Oesophageal Candidiasis: Co-Infection Evaluation. Journal of Fungi, 11(9), 665. https://doi.org/10.3390/jof11090665








