Malassezia Folliculitis: An Underdiagnosed Mimicker of Acneiform Eruptions
Abstract
1. Introduction
2. Epidemiology
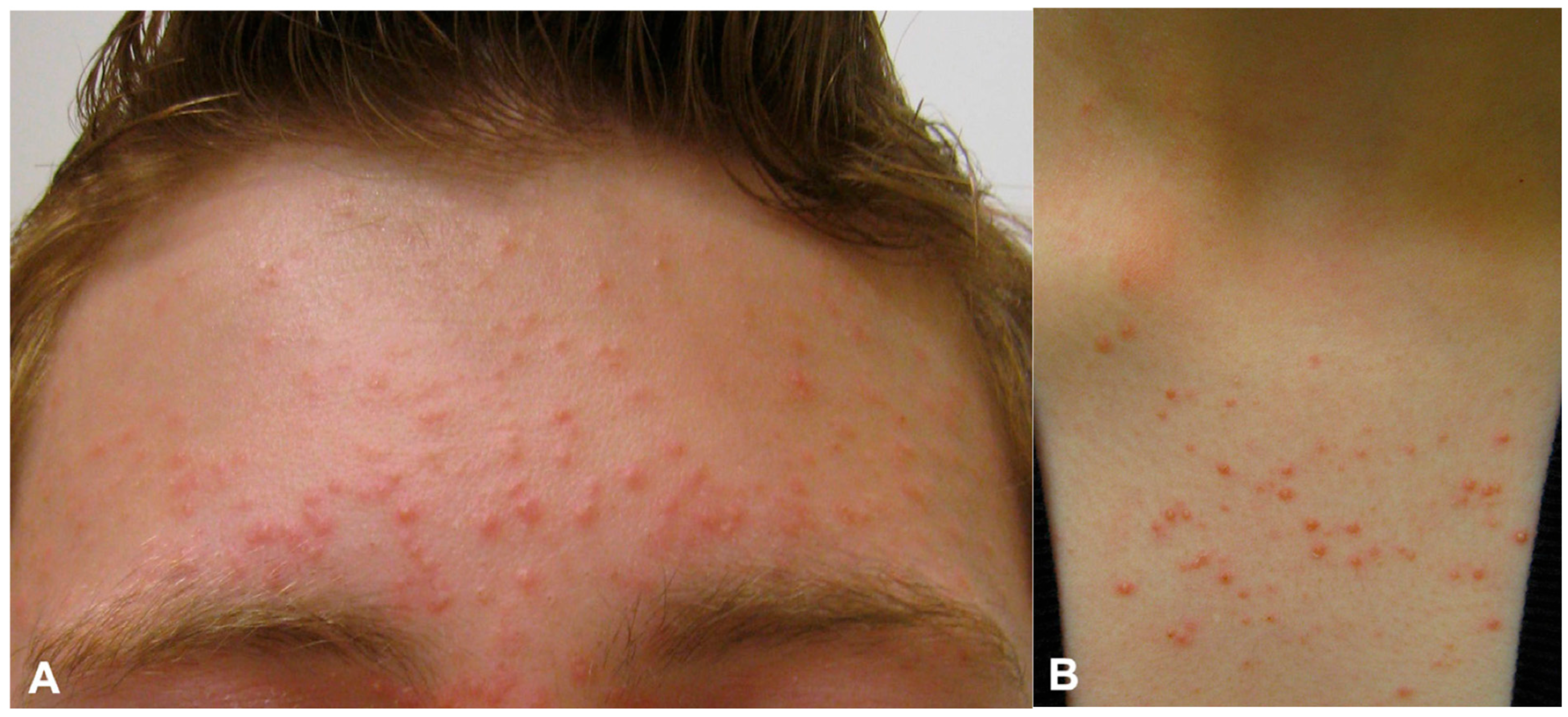
3. Clinical Presentation and History
4. Diagnostic Workup
5. Differential Diagnosis
6. Management
Treatment Challenged and Considerations
7. Special Considerations
7.1. Recurrence and Maintenance Strategies
7.2. Skin of Color Patients
7.3. Immunosuppressed Populations
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MF | Malassezia folliculitis |
| AV | Acne vulgaris |
| KOH | Potassium hydroxide |
| PDT | Photodynamic therapy |
| CAP | Cold atmospheric plasma |
| PCR | Polymerase chain reaction |
| UV | Ultraviolet |
| RCT | Randomized controlled trial |
| PAS | Periodic acid–Schiff |
References
- Weary, P.E.; Russell, C.M.; Butler, H.K.; Hsu, Y.T. Acneform Eruption Resulting from Antibiotic Administration. Arch. Dermatol. 1969, 100, 179–183. [Google Scholar] [CrossRef]
- Rubenstein, R.M.; Malerich, S.A. Malassezia (pityrosporum) Folliculitis. J. Clin. Aesthet. Dermatol. 2014, 7, 37–41. [Google Scholar]
- Green, M.; Feschuk, A.M.; Kashetsky, N.; Maibach, H.I. Clinical Characteristics and Treatment Outcomes of Pityrosporum Folliculitis in Immunocompetent Patients. Arch. Derm. Res. 2023, 315, 1497–1509. [Google Scholar] [CrossRef]
- Martínez-Ortega, J.I.; Mut Quej, J.E.; Franco González, S. Malassezia Folliculitis: Pathogenesis and Diagnostic Challenges. Cureus 2024, 16, e73429. [Google Scholar] [CrossRef]
- Ayers, K.; Sweeney, S.M.; Wiss, K. Pityrosporum Folliculitis: Diagnosis and Management in Six Female Adolescents with Acne Vulgaris. Arch. Pediatr. Adolesc. Med. 2005, 159, 64–67. [Google Scholar] [CrossRef]
- Ljubojević, S.; Skerlev, M.; Lipozencić, J.; Basta-Juzbasić, A. The Role of Malassezia Furfur in Dermatology. Clin. Dermatol. 2002, 20, 179–182. [Google Scholar] [CrossRef]
- Marcon, M.J.; Powell, D.A. Human Infections due to Malassezia spp. Clin. Microbiol. Rev. 1992, 5, 101–119. [Google Scholar] [CrossRef]
- Prindaville, B.; Belazarian, L.; Levin, N.A. Pityrosporum Folliculitis: A Retrospective Review of 110 Cases. J. Am. Acad. Dermatol. 2018, 78, 511–551. [Google Scholar] [CrossRef]
- Poli, F. Differential Diagnosis of Facial Acne on Black Skin. Int. J. Dermatol. 2012, 51 (Suppl. S1), 24–29. [Google Scholar] [CrossRef]
- Lim, K.B.; Giam, Y.C.; Tan, T. The Epidemiology of Malassezia (Pityrosporon) Folliculitis in Singapore. Int. J. Dermatol. 1987, 26, 438–441. [Google Scholar] [CrossRef]
- Green, M.; Kashetsky, N.; Feschuk, A.; Maibach, H. Pityrosporum Folliculitis in Immunocompromised Populations: A Systematic Review. J. Am. Acad. Dermatol. 2023, 88, 690–692. [Google Scholar] [CrossRef]
- Kaviarasan, P.K.; Jaisankar, T.; Thappa, D.; Sujatha, S. Pityrosporum Infection In HIV Infected Patients. Indian J. Dermatol. 1990, 35, 215. [Google Scholar]
- Krzyściak, P.; Bakuła, Z.; Gniadek, A.; Garlicki, A.; Tarnowski, M.; Wichowski, M.; Jagielski, T. Prevalence of Malassezia Species on the Skin of HIV-Seropositive Patients. Sci. Rep. 2020, 10, 17779. [Google Scholar] [CrossRef]
- Potter, B.S.; Burgoon, C.F., Jr.; Johnson, W.C. Pityrosporum Folliculitis. Report of Seven Cases and Review of the Pityrosporum Organism Relative to Cutaneous Disease. Arch. Dermatol. 1973, 107, 388–391. [Google Scholar] [CrossRef]
- Durdu, M.; Güran, M.; Ilkit, M. Epidemiological Characteristics of Malassezia Folliculitis and Use of the May-Grünwald-Giemsa Stain to Diagnose the Infection. Diagn. Microbiol. Infect. Dis. 2013, 76, 450–457. [Google Scholar] [CrossRef]
- Sun, K.-L.; Chang, J.-M. Special Types of Folliculitis Which Should Be Differentiated from Acne. Dermatoendocrinology 2017, 9, e1356519. [Google Scholar] [CrossRef]
- Gatselis, N.K.; Zachou, K.; Loza, A.J.M.; Cançado, E.L.R.; Arinaga-Hino, T.; Muratori, P.; Efe, C.; Floreani, A.; Invernizzi, P.; Takahashi, A.; et al. Prevalence and Significance of Antimitochondrial Antibodies in Autoimmune Hepatitis (AIH): Results from a Large Multicentre Study of the International AIH Group. Eur. J. Intern. Med. 2023, 116, 43–50. [Google Scholar] [CrossRef]
- Cohen, P.R.; Erickson, C.; Calame, A. Malassezia (Pityrosporum) Folliculitis Incognito: Malessezia-Associated Folliculitis Masked by Topical Corticosteroid Therapy. Cureus 2020, 12, e6531. [Google Scholar] [CrossRef]
- Jakhar, D.; Kaur, I.; Chaudhary, R. Dermoscopy of Pityrosporum Folliculitis. J. Am. Acad. Dermatol. 2019, 80, e43–e44. [Google Scholar] [CrossRef]
- Silva, A.M.G.; Michalany, A.O.; de Sá Menezes Carvalho, G. UV Dermoscopy for the Diagnosis of Pityrosporum Folliculitis. Dermatol. Pract. Concept. 2024, 14, e2024092. [Google Scholar] [CrossRef]
- Errichetti, E.; Pietkiewicz, P.; Bhat, Y.J.; Salwowska, N.; Szlązak, P.; Stinco, G. Diagnostic Accuracy of Ultraviolet-Induced Fluorescence Dermoscopy in Non-Neoplastic Dermatoses (general Dermatology): A Multicentric Retrospective Comparative Study. J. Eur. Acad. Dermatol. Venereol. 2025, 39, 97–108. [Google Scholar] [CrossRef]
- Vlachos, C.; Henning, M.A.S.; Gaitanis, G.; Faergemann, J.; Saunte, D.M. Critical Synthesis of Available Data in Malassezia Folliculitis and a Systematic Review of Treatments. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1672–1683. [Google Scholar] [CrossRef]
- Jacinto-Jamora, S.; Tamesis, J.; Katigbak, M.L. Pityrosporum Folliculitis in the Philippines: Diagnosis, Prevalence, and Management. J. Am. Acad. Dermatol. 1991, 24, 693–696. [Google Scholar] [CrossRef]
- Nagata, R.; Nagano, H.; Ogishima, D.; Nakamura, Y.; Hiruma, M.; Sugita, T. Transmission of the Major Skin Microbiota, Malassezia, from Mother to Neonate: Transmission of Malassezia. Pediatr. Int. 2012, 54, 350–355. [Google Scholar] [CrossRef]
- Cafarchia, C.; Gasser, R.B.; Figueredo, L.A.; Latrofa, M.S.; Otranto, D. Advances in the Identification of Malassezia. Mol. Cell. Probes 2011, 25, 1–7. [Google Scholar] [CrossRef]
- Akaza, N.; Akamatsu, H.; Sasaki, Y.; Kishi, M.; Mizutani, H.; Sano, A.; Hirokawa, K.; Nakata, S.; Nishijima, S.; Matsunaga, K. Malassezia Folliculitis Is Caused by Cutaneous Resident Malassezia Species. Med. Mycol. 2009, 47, 618–624. [Google Scholar] [CrossRef]
- Ilahi, A.; Hadrich, I.; Neji, S.; Trabelsi, H.; Makni, F.; Ayadi, A. Real-Time PCR Identification of Six Malassezia Species. Curr. Microbiol. 2017, 74, 671–677. [Google Scholar] [CrossRef]
- Park, H.R.; Oh, J.H.; Lee, Y.J.; Park, S.H.; Lee, Y.W.; Lee, S.; Kang, H.; Kim, J.E. Inflammasome-Mediated Inflammation by Malassezia in Human Keratinocytes: A Comparative Analysis with Different Strains. Mycoses 2021, 64, 292–299. [Google Scholar] [CrossRef]
- An, M.K.; Hong, E.H.; Cho, E.B.; Park, E.J.; Kim, K.H.; Kim, K.J. Clinicopathological Differentiation between Pityrosporum Folliculitis and Acneiform Eruption. J. Dermatol. 2019, 46, 978–984. [Google Scholar] [CrossRef]
- Henning, M.A.S.; Hay, R.; Rodriguez-Cerdeira, C.; Szepietowski, J.C.; Piraccini, B.M.; Ferreirós, M.P.; Arabatzis, M.; Sergeev, A.; Nenoff, P.; Kotrekhova, L.; et al. Position Statement: Recommendations on the Diagnosis and Treatment of Malassezia Folliculitis. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 1268–1275. [Google Scholar] [CrossRef]
- Malgotra, V.; Singh, H. Malassezia (Pityrosporum) Folliculitis Masquerading as Recalcitrant Acne. Cureus 2021, 13, e13534. [Google Scholar] [CrossRef]
- Lévy, A.; Feuilhade de Chauvin, M.; Dubertret, L.; Morel, P.; Flageul, B. Malassezia folliculitis: Characteristics and therapeutic response in 26 patients. Ann. Dermatol. Venereol. 2007, 134, 823–828. [Google Scholar] [CrossRef]
- Song, H.S.; Kim, S.K.; Kim, Y.C. Comparison between Malassezia Folliculitis and Non-Malassezia Folliculitis. Ann. Dermatol. 2014, 26, 598–602. [Google Scholar] [CrossRef]
- Neubert, U.; Jansen, T.; Plewig, G. Bacteriologic and Immunologic Aspects of Gram-Negative Folliculitis: A Study of 46 Patients. Int. J. Dermatol. 1999, 38, 270–274. [Google Scholar] [CrossRef]
- Yu, H.J.; Lee, S.K.; Son, S.J.; Kim, Y.S.; Yang, H.Y.; Kim, J.H. Steroid Acne vs. Pityrosporum Folliculitis: The Incidence of Pityrosporum Ovale and the Effect of Antifungal Drugs in Steroid Acne. Int. J. Dermatol. 1998, 37, 772–777. [Google Scholar] [CrossRef]
- Gupta, A.K.; Batra, R.; Bluhm, R.; Boekhout, T.; Dawson, T.L., Jr. Skin Diseases Associated with Malassezia Species. J. Am. Acad. Dermatol. 2004, 51, 785–798. [Google Scholar] [CrossRef]
- Bäck, O.; Faergemann, J.; Hörnqvist, R. Pityrosporum Folliculitis: A Common Disease of the Young and Middle-Aged. J. Am. Acad. Dermatol. 1985, 12, 56–61. [Google Scholar] [CrossRef]
- Suzuki, C.; Hase, M.; Shimoyama, H.; Sei, Y. Treatment Outcomes for Malassezia Folliculitis in theDermatology Department of a University Hospital in Japan. Med. Mycol. J. 2016, 57, E63–E66. [Google Scholar] [CrossRef][Green Version]
- Rhie, S.; Turcios, R.; Buckley, H.; Suh, B. Clinical Features and Treatment of Malassezia Folliculitis with Fluconazole in Orthotopic Heart Transplant Recipients. J. Heart Lung Transplant. 2000, 19, 215–219. [Google Scholar] [CrossRef]
- Parsad, D.; Saini, R.; Negi, K.S. Short-Term Treatment of Pityrosporum Folliculitis: A Double Blind Placebo-Controlled Study. J. Eur. Acad. Dermatol. Venereol. 1998, 11, 188–190. [Google Scholar] [CrossRef]
- Abdel-Razek, M.; Fadaly, G.; Abdel-Raheim, M.; Morsy, F. Pityrosporum (Malassezia) Folliculitis in Saudi Arabia—Diagnosis and Therapeutic Trials. Clin. Exp. Dermatol. 1995, 20, 406–409. [Google Scholar] [CrossRef]
- Friedman, S.J. Pityrosporum Folliculitis: Treatment with Isotretinoin. J. Am. Acad. Dermatol. 1987, 16, 632–633. [Google Scholar] [CrossRef]
- Chung, C.S.; Tapia-Centola, B.; Loo, D.S. Successful Treatment of Pityrosporum Folliculitis with Isotretinoin. J. Dermatol. Res. 2024, 5, 1–4. [Google Scholar]
- Goodfield, M.J.; Saihan, E.M. Failure of Isotretinoin Therapy in Pityrosporum Folliculitis. J. Am. Acad. Dermatol. 1988, 18, 143–144. [Google Scholar] [CrossRef]
- Kamamoto, C.S.L.; Nishikaku, A.S.; Gompertz, O.F.; Melo, A.S.; Hassun, K.M.; Bagatin, E. Cutaneous Fungal Microbiome: Malassezia Yeasts in Seborrheic Dermatitis Scalp in a Randomized, Comparative and Therapeutic Trial. Dermatoendocrinol. 2017, 9, e1361573. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Kim, B.J.; Kim, M.N. Photodynamic Therapy: New Treatment for Recalcitrant Malassezia Folliculitis. Lasers Surg. Med. 2010, 42, 192–196. [Google Scholar] [CrossRef]
- Wang, N.; Yan, T.; Mei, X.; Liu, J.; Lei, Y.; Yang, C. Cold Atmospheric Plasma Therapy for Malassezia Folliculitis: Laboratory Investigations and a Randomized Clinical Trial. Skin. Res. Technol. 2024, 30, e13850. [Google Scholar] [CrossRef]
- Sharquie, K.E.; Al-Hamdi, K.I.; Al-Haroon, S.S.; Al-Mohammadi, A. Malassezia Folliculitis versus Truncal Acne Vulgaris (Clinical and Histopathological Study). J. Cosmet. Dermatol. Sci. Appl. 2012, 2, 277–282. [Google Scholar] [CrossRef]
- Vest, B.E.; Krauland, K. Malassezia Furfur. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Korman, N.J. Macular, Papular, Vesiculobullous, and Pustular Diseases. In Goldman’s Cecil Medicine; Elsevier: Amsterdam, The Netherlands, 2012; pp. 2522–2532. ISBN 9781437716047. [Google Scholar]
- Davis, E.C.; Callender, V.D. Postinflammatory Hyperpigmentation: A Review of the Epidemiology, Clinical Features, and Treatment Options in Skin of Color. J. Clin. Aesthet. Dermatol. 2010, 3, 20–31. [Google Scholar]
- Kupper, T.S.; Fuhlbrigge, R.C. Immune Surveillance in the Skin: Mechanisms and Clinical Consequences. Nat. Rev. Immunol. 2004, 4, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, V.; Ruocco, E.; Piccolo, V.; Brunetti, G.; Guerrera, L.P.; Wolf, R. The Immunocompromised District in Dermatology: A Unifying Pathogenic View of the Regional Immune Dysregulation. Clin. Dermatol. 2014, 32, 569–576. [Google Scholar] [CrossRef] [PubMed]


| Feature | Malassezia Folliculitis | Acne Vulgaris | Bacterial Folliculitis |
|---|---|---|---|
| Lesion Morphology | Monomorphic papules/pustules | Polymorphic; includes comedones | Erythematous pustules |
| Pruritus | Common and often intense | Mild or absent | Mild to moderate |
| Comedones | Absent | Present | Absent |
| Response to Treatment | Antifungals effective; antibiotics ineffective | Improves with standard acne therapy | Rapid response to antibiotics |
| KOH Prep | Positive for yeast and short hyphae | Negative | Negative |
| Wood’s Lamp | May fluoresce yellow-green (non-specific) | No fluorescence | No fluorescence |
| Distribution | Upper trunk, shoulders, face (seborrheic areas) | Face, chest, back | Often localized (scalp, legs) |
| Histology | Yeast in follicles on PAS/GMS stains | Follicular plugging, inflammation | Neutrophilic infiltrate in follicles |
| Therapy Type | Agent/Example | Dose and Duration | Level of Evidence | Notes |
|---|---|---|---|---|
| Oral antifungals | Itraconazole; fluconazole | 200 mg/day for 7–14 days; 200 mg/day for 1–2 weeks | Ib–IIb; III | RCTs and controlled studies support use in moderate-to-severe MF |
| Topical antifungals | Econazole cream; 2% ketoconazole cream | Daily for 1 week followed by once-weekly application; twice daily for 1–1.5 months | IIb; III | Effective in mild cases, slower onset, adjunct use |
| Keratolytics | Selenium disulfide; 50% propylene glycol | 2% shampoo once weekly for 3 weeks; twice daily for 3 weeks | IIb | Often adjunctive, limited studies |
| Oral retinoids | Isotretinoin | 0.65–1.00 mg/kg/day for 3–4 months | III | Used for refractory disease, not antifungal |
| Photodynamic therapy (PDT) | MAL-PDT | Three sessions at 2-week intervals for 1 month | III | Limited case reports suggest benefit |
| Cold atmospheric plasma (CAP) | CAP device | Daily for 2 weeks | IIb | One comparative study, novel anti-biofilm mechanism |
| Maintenance | Ketoconazole/ciclopirox shampoo | 1–2x/week indefinitely | IV | Based on clinical experience and recurrence patterns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalupczak, N.V.; Lipner, S.R. Malassezia Folliculitis: An Underdiagnosed Mimicker of Acneiform Eruptions. J. Fungi 2025, 11, 662. https://doi.org/10.3390/jof11090662
Chalupczak NV, Lipner SR. Malassezia Folliculitis: An Underdiagnosed Mimicker of Acneiform Eruptions. Journal of Fungi. 2025; 11(9):662. https://doi.org/10.3390/jof11090662
Chicago/Turabian StyleChalupczak, Natalia V., and Shari R. Lipner. 2025. "Malassezia Folliculitis: An Underdiagnosed Mimicker of Acneiform Eruptions" Journal of Fungi 11, no. 9: 662. https://doi.org/10.3390/jof11090662
APA StyleChalupczak, N. V., & Lipner, S. R. (2025). Malassezia Folliculitis: An Underdiagnosed Mimicker of Acneiform Eruptions. Journal of Fungi, 11(9), 662. https://doi.org/10.3390/jof11090662






