Metagenomic Insights into Disease-Induced Microbial Dysbiosis and Elemental Cycling Alterations in Morchella Cultivation Soils: Evidence from Two Distinct Regions
Abstract
1. Introduction
2. Materials and Methods
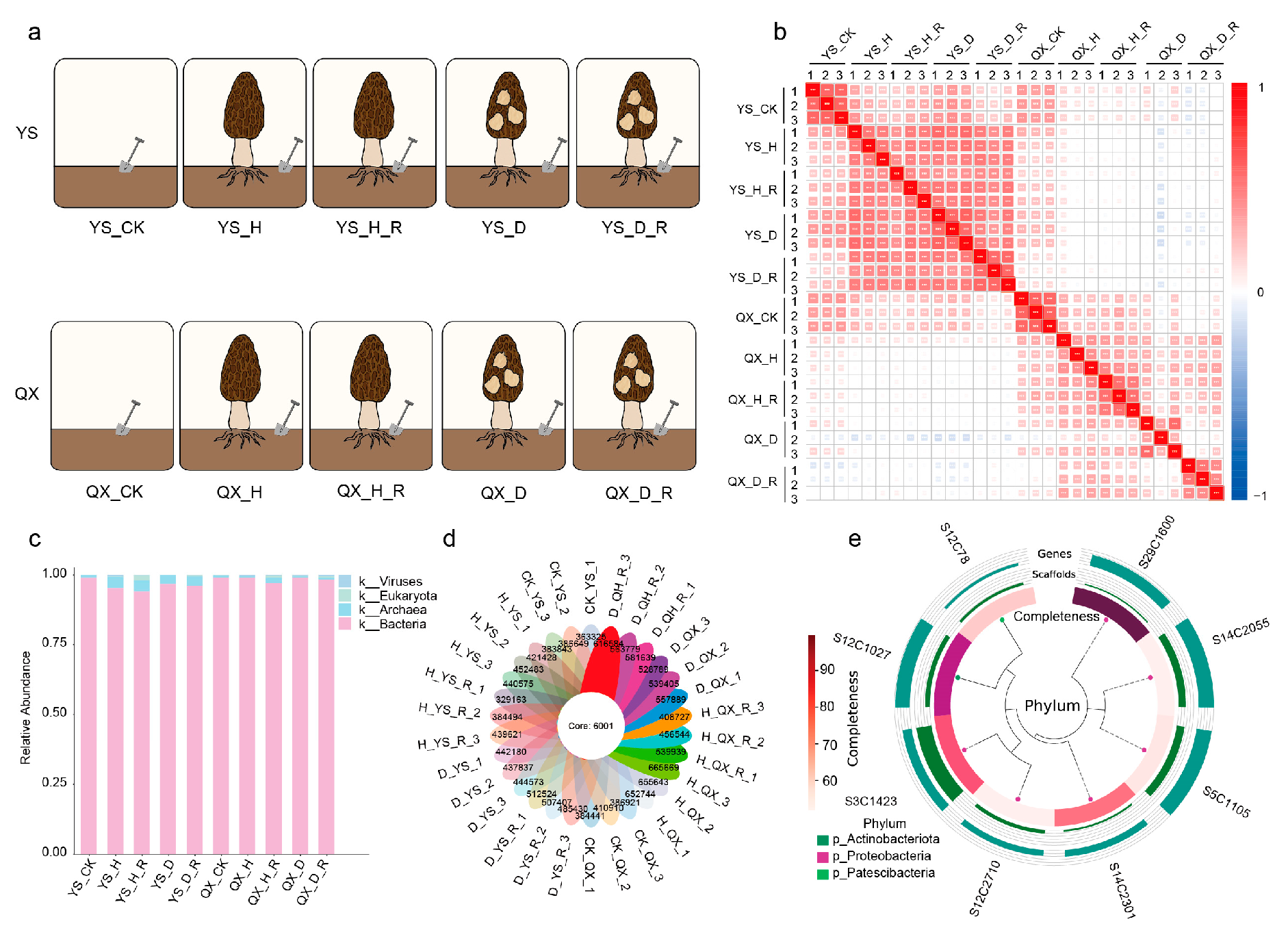
2.1. Soil Sampling and Experimental Design
2.2. DNA Extraction and Metagenomic Sequencing
2.3. Quality Control and Metagenomic Assembly
2.4. Gene Prediction and Functional Annotation
2.5. Taxonomic Profiling and Abundance Estimation
2.6. Diversity Analysis
2.7. Differential Abundance and Functional Pathway Analysis
2.8. Co-Occurrence Network Analysis
2.9. Statistical Analysis and Visualization
3. Results
3.1. Experimental Design and Metagenomic Data Summary
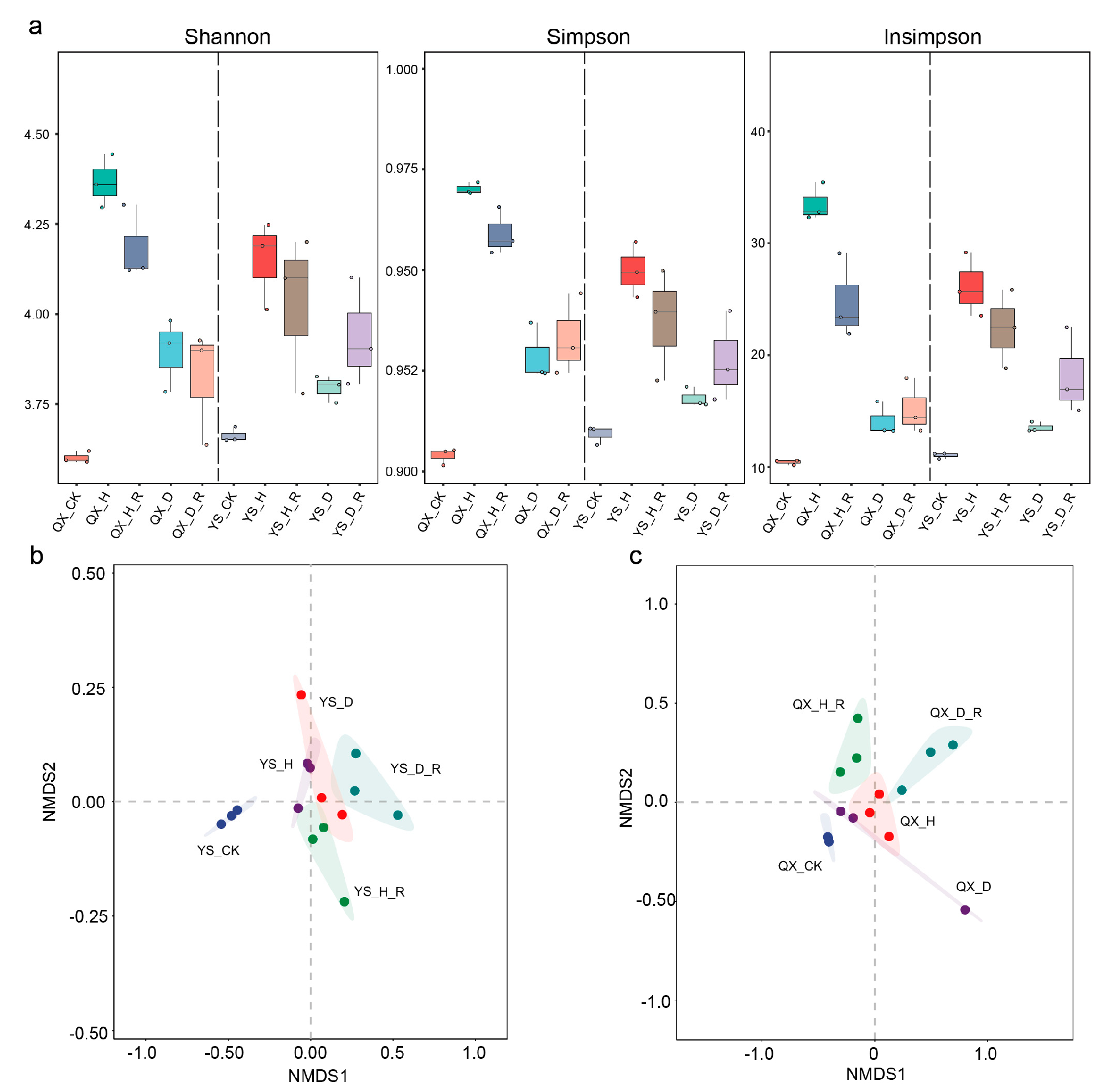
3.2. Soil Microbial Alpha and Beta Diversity Reveal Community Disruption and Stability Loss Under Disease-Induced Dysbiosis
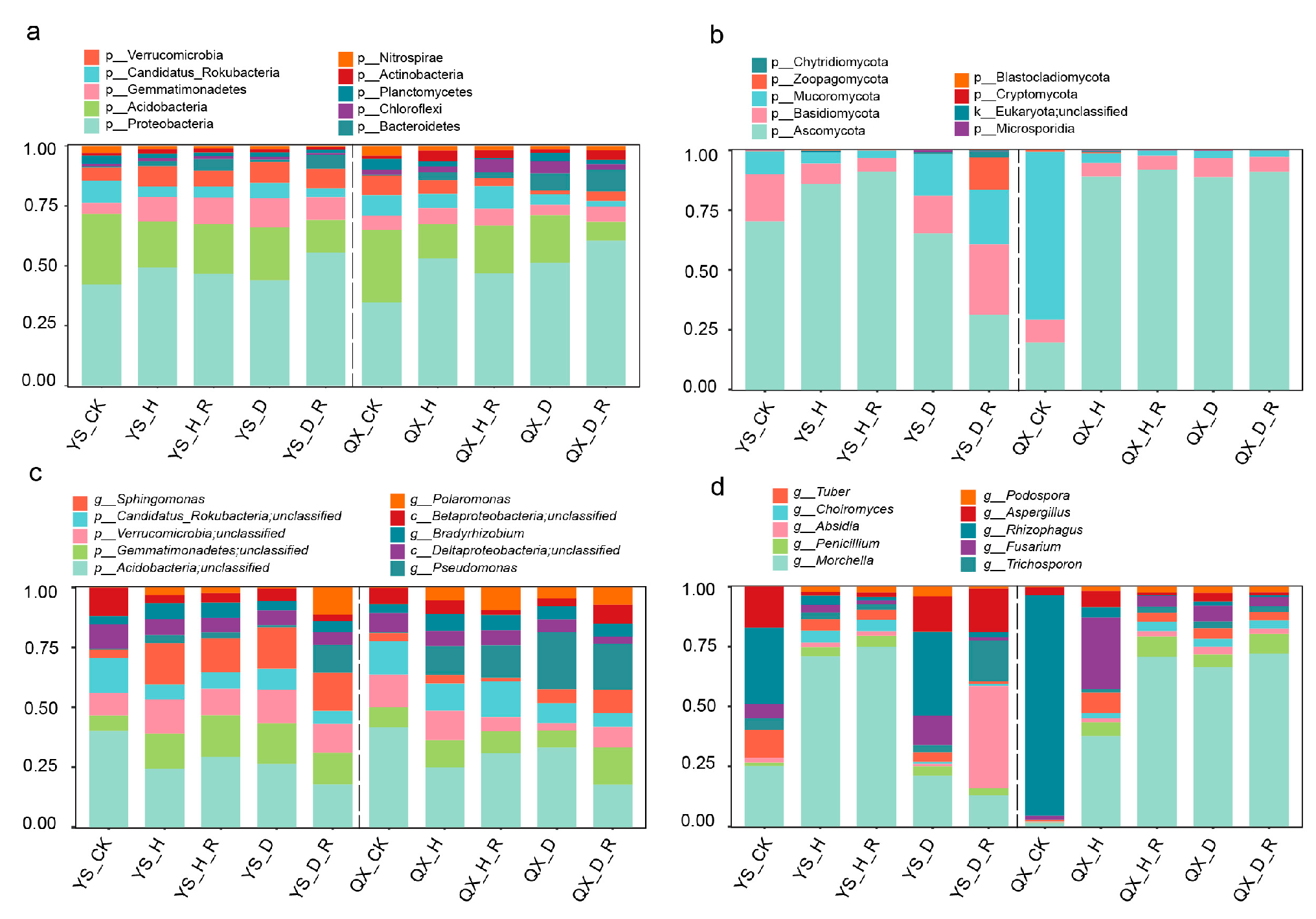
3.3. Taxonomic Composition of Bacterial and Fungal Communities Across Soil Types
3.4. Region-Specific Taxonomic Biomarkers Identified by LEfSe and Differential Abundance Analyses
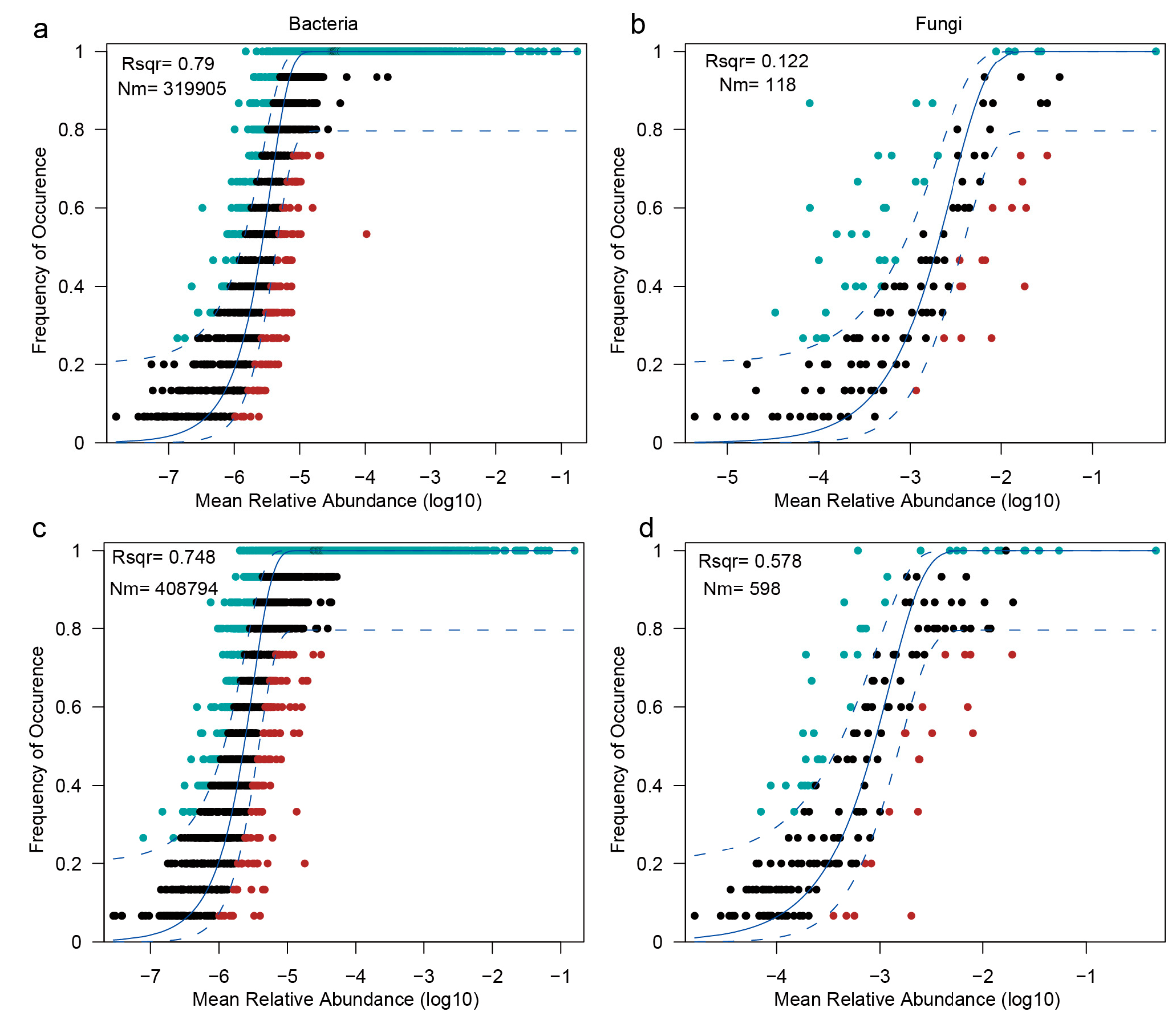
3.5. Contrasting Assembly Dynamics of Bacterial and Fungal Communities Revealed by Neutral Model Fitting
3.6. Network Topology Reveals Disease-Induced Destabilization of Fungal Communities
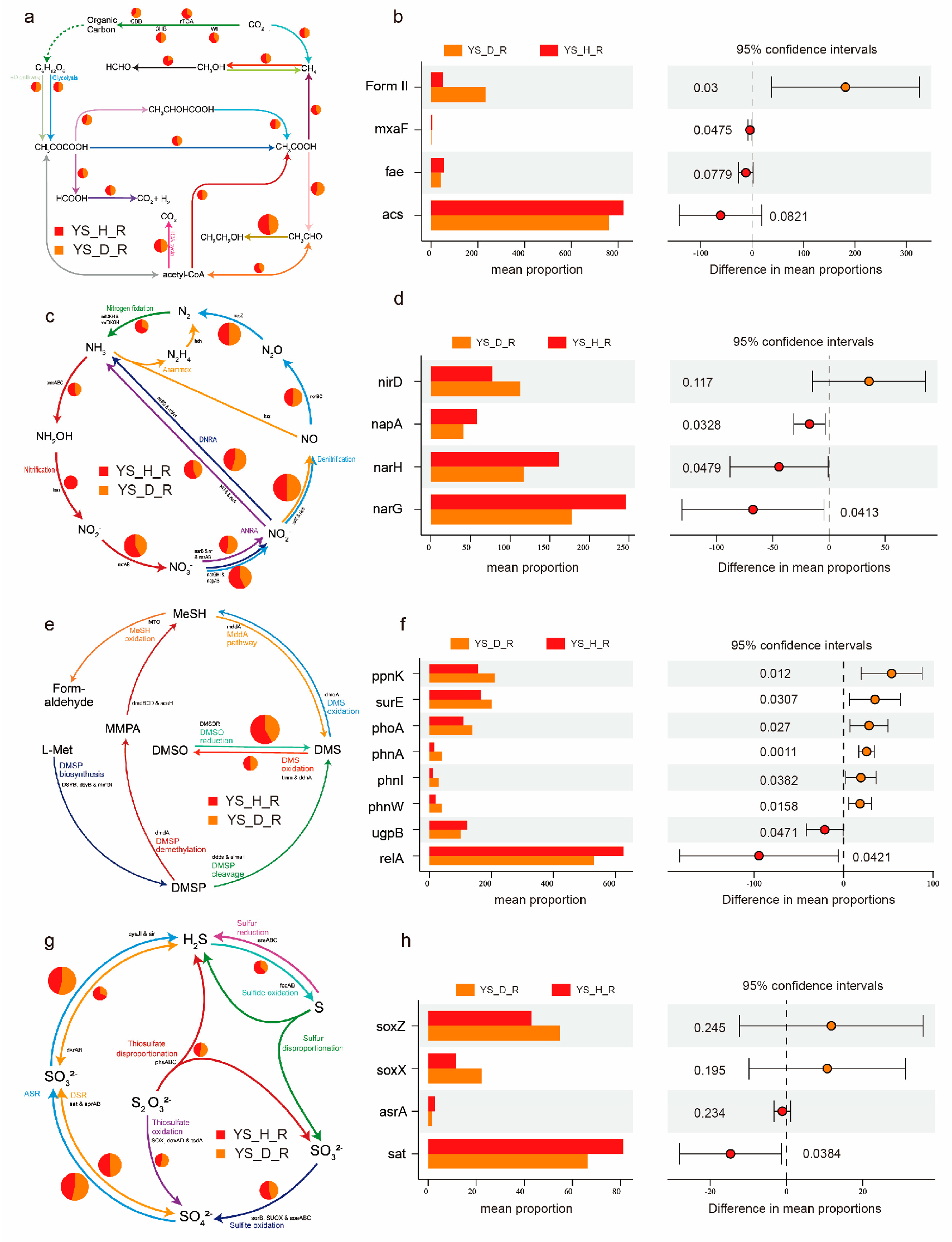
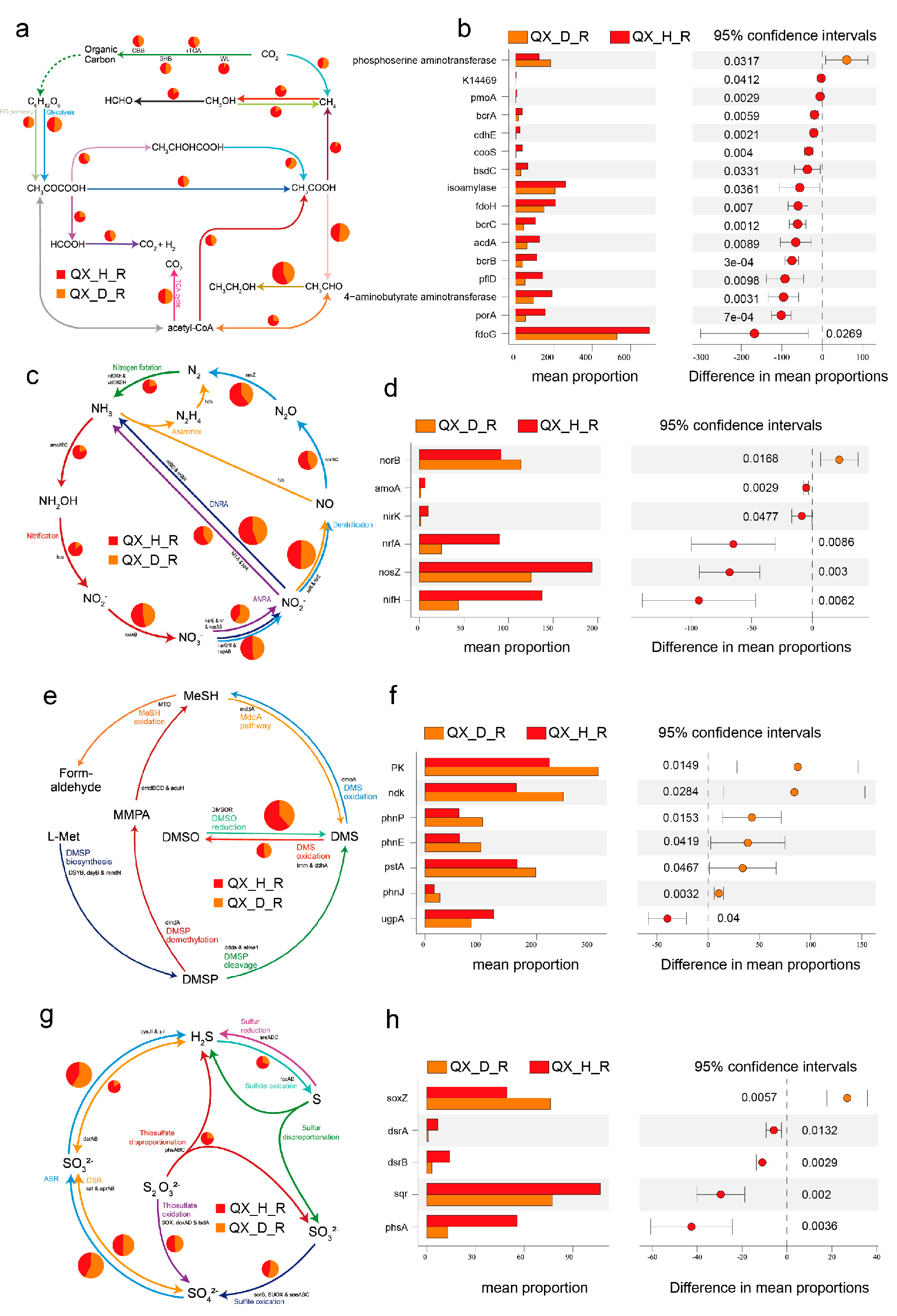
3.7. Functional Differentiation of Microbial Communities Highlights Health-Dependent Metabolic and Elemental Cycling Potentials
3.8. Integrative Remodeling of Elemental Cycles Driven by Disease Pressure Functional Attenuation of Carbon Cycling in Diseased Soils
4. Discussion
4.1. Microbial Community Shifts Reveal Disease-Associated Disruption of Microbial Homeostasis
4.2. Deterministic Assembly and Network Destabilization Reveal Microbial Vulnerability Under Disease Pressure
4.3. Disrupted Elemental Cycling Reflects Microbial Dysbiosis and Ecosystem Instability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tietel, Z.; Masaphy, S. True morels (Morchella)—Nutritional and phytochemical composition, health benefits and flavor: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1888–1901. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, Z.; Shang, Y.; Zheng, Y. Extraction, structure and antioxidant activity of the polysaccharides from morels (Morchella spp.): A review. Int. J. Biol. Macromol. 2024, 264, 130656. [Google Scholar] [CrossRef]
- Sunil, C.; Xu, B. Mycochemical profile and health-Promoting effects of morel mushroom Morchella esculenta (L.): A review. Food Res. Int. 2022, 159, 111571. [Google Scholar] [CrossRef]
- Liu, W.; He, P.; Shi, X.; Zhang, Y.; Perez-Moreno, J.; Yu, F. Large-scale field cultivation of Morchella and relevance of basic knowledge for its steady production. J. Fungi 2023, 9, 855. [Google Scholar] [CrossRef]
- Xu, Y.; Tang, J.; Wang, Y.; He, X.; Tan, H.; Yu, Y.; Chen, Y.; Peng, W. Large-scale commercial cultivation of morels: Current state and perspectives. Appl. Microbiol. Biotechnol. 2022, 106, 4401–4412. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Hao, H.; Wang, Q.; Xiao, T.; Zhang, Y.; Chen, Q.; Chen, H.; Zhang, J. Dynamics of the soil microbial community associated with Morchella cultivation: Diversity, assembly mechanism and yield prediction. Front. Microbiol. 2024, 15, 1345231. [Google Scholar] [CrossRef] [PubMed]
- Pion, M.; Spangenberg, J.E.; Simon, A.; Bindschedler, S.; Flury, C.; Chatelain, A.; Bshary, R.; Job, D.; Junier, P. Bacterial farming by the fungus Morchella crassipes. Proc. R. Soc. B-Biol. Sci. 2013, 280, 20132242. [Google Scholar] [CrossRef]
- Orlofsky, E.; Zabari, L.; Bonito, G.; Masaphy, S. Changes in soil bacteria functional ecology associated with Morchella rufobrunnea fruiting in a natural habitat. Environ. Microbiol. 2021, 23, 6651–6662. [Google Scholar] [CrossRef]
- Longley, R.; Benucci, G.M.N.; Mills, G.; Bonito, G. Fungal and bacterial community dynamics in substrates during the cultivation of morels (Morchella rufobrunnea) indoors. FEMS Microbiol. Lett. 2019, 366, fnz215. [Google Scholar] [CrossRef]
- Guo, M.-C.; Wu, B.-C.; Luo, C.-Y.; Sa, W.; Wang, L.; Li, Z.-H.; Shang, Q.-H. The effects of fungal pathogen infestation on soil microbial communities for Morchella sextelata cultivation on the Qinghai-Xizang Plateau. J. Fungi 2025, 11, 264. [Google Scholar] [CrossRef]
- Benucci, G.M.N.; Longley, R.; Zhang, P.; Zhao, Q.; Bonito, G.; Yu, F. Microbial communities associated with the black morel Morchella sextelata cultivated in greenhouses. PeerJ 2019, 7, e7744. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, X.; Zhang, J.; Zhang, Y.; Wang, W. Dynamics of soil microbiome throughout the cultivation life cycle of morel (Morchella sextelata). Front. Microbiol. 2023, 14, 979835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, Q.; Uroz, S.; Gao, T.; Li, J.; He, F.; Rosazlina, R.; Martin, F.; Xu, L. The cultivation regimes of Morchella sextelata trigger shifts in the community assemblage and ecological traits of soil bacteria. Front. Microbiol. 2023, 14, 1257905. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, X.; Zhang, K.; Liu, J.; Wei, M.; Yang, Z.; Peng, Y.; Zhang, B. Continuous cropping obstacles in fungal production: A review of mechanisms and remedial strategies. Soil Use Manag. 2025, 41, e70014. [Google Scholar] [CrossRef]
- Huang, K.; Li, L.; Wu, W.; Pu, K.; Qi, W.; Qi, J.; Li, M. Enhancing Morchella mushroom yield and quality through the amendment of soil physicochemical properties and microbial community with wood ash. Microorganisms 2024, 12, 2406. [Google Scholar] [CrossRef]
- Feng, W.; Wang, J.; Jin, Q.; Guo, Z.; Cai, W. Analysis of soil fungal community characteristics of Morchella sextelata under different rotations and intercropping patterns and influencing factors. Agriculture 2025, 15, 823. [Google Scholar] [CrossRef]
- Yan, Q.; Wang, P.; Liu, Z.; Yu, Y.; Tan, X.; Huang, X.; Wen, J.; Zhang, W. Analysis of soil microbial community structure and function in Morchella esculenta habitats in Jilin Province. Agronomy 2025, 15, 15. [Google Scholar] [CrossRef]
- Tan, H.; Yu, Y.; Tang, J.; Liu, T.; Miao, R.; Huang, Z.; Martin, F.M.; Peng, W. Build your own mushroom soil: Microbiota succession and nutritional accumulation in semi-synthetic substratum drive the fructification of a soil-saprotrophic morel. Front. Microbiol. 2021, 12, 656656. [Google Scholar] [CrossRef]
- Tan, H.; Liu, T.; Yu, Y.; Tang, J.; Jiang, L.; Martin, F.M.; Peng, W. Morel production related to soil microbial diversity and evenness. Microbiol. Spectr. 2021, 9, e00229-21. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.-Z.; Liu, J.; Zhang, J.; Wang, C.-L. Determination of the effects of pear-Morchella intercropping mode on M. sextelata quality, yield, and soil microbial community. J. Fungi 2024, 10, 759. [Google Scholar] [CrossRef]
- Li, Y.; Lin, W.; Chen, J.; Lin, J.; Feng, R.; Yan, J.; Miao, R.; Gan, B. Nitrates and microbiome components engaged in denitrification within soil regulate Morchella spp. growth. Horticulturae 2024, 10, 905. [Google Scholar] [CrossRef]
- Chen, B.; Shao, G.; Zhou, T.; Fan, Q.; Yang, N.; Cui, M.; Zhang, J.; Wu, X.; Zhang, B.; Zhang, R. Dazomet changes microbial communities and improves morel mushroom yield under continuous cropping. Front. Microbiol. 2023, 14, 1200226. [Google Scholar] [CrossRef]
- Yan, Q.; Wang, P.; Liu, Z.; Yu, Y.; Tan, X.; Huang, X.; Wen, J.; Zhang, W. High-throughput sequencing uncovers fungal community succession during Morchella sextelata development. J. Fungi 2025, 11, 364. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Singh, P.; Sharma, A.; Guo, D.J.; Upadhyay, S.K.; Song, Q.Q.; Verma, K.K.; Li, D.P.; Malviya, M.K.; Song, X.P.; et al. Unraveling nitrogen fixing potential of endophytic diazotrophs of different Saccharum species for sustainable sugarcane growth. Int. J. Mol. Sci. 2022, 23, 6242. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Li, D.H.; Liu, C.M.; Luo, R.B.; Sadakane, K.; Lam, T.W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Fu, L.M.; Niu, B.F.; Zhu, Z.W.; Wu, S.T.; Li, W.Z. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Li, D.; Wang, H.; Chen, N.; Jiang, H.; Chen, N. Metagenomic analysis of soil microbial communities associated with Poa alpigena Lindm in Haixin Mountain, Qinghai Lake. Braz. J. Microbiol. 2024, 55, 4219. [Google Scholar] [CrossRef]
- Luo, T.; He, J.; Shi, Z.; Shi, Y.; Zhang, S.; Liu, Y.; Luo, G. Metagenomic binning revealed microbial shifts in anaerobic degradation of phenol with hydrochar and pyrochar. Fermentation 2023, 9, 387. [Google Scholar] [CrossRef]
- Fierer, N.; Barberan, A.; Laughlin, D.C. Seeing the forest for the genes: Using metagenomics to infer the aggregated traits of microbial communities. Front. Microbiol. 2014, 5, 614. [Google Scholar] [CrossRef] [PubMed]
- Ofaim, S.; Ofek-Lalzar, M.; Sela, N.; Jinag, J.; Kashi, Y.; Minz, D.; Freilich, S. Analysis of microbial functions in the rhizosphere using a metabolic-network based framework for metagenomics interpretation. Front. Microbiol. 2017, 8, 1606. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Song, Y.; Mackelprang, R.; Zhang, D.; Qin, S.; Chen, L.; Wu, L.; Peng, Y.; Yang, Y. Metagenomic insights into microbial community structure and metabolism in alpine permafrost on the Tibetan Plateau. Nat. Commun. 2024, 15, 5920. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Chen, Z.; Feng, X.; Wang, M.; Luo, Y.; Wang, Y.; Xu, P. L-theanine exuded from Camellia sinensis roots regulates element cycling in soil by shaping the rhizosphere microbiome assembly. Sci. Total Environ. 2022, 837, 155801. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, Q.; Wang, J.; Khan, M.A.; Guo, G.; Liu, Y.; Hu, S.; Jin, F.; Wang, J.; Yu, Y. Dissolved organic carbon drives nutrient cycling via microbial community in paddy soil. Chemosphere 2021, 285, 131472. [Google Scholar] [CrossRef]
- Zhang, F.; Jonas, L.; Lin, H.; Hill, R.T. Microbially mediated nutrient cycles in marine sponges. FEMS Microbiol. Ecol. 2019, 95, fiz155. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Jiang, L.; Li, E.; Yang, X.; Yang, J. Salinity affects microbial function genes related to nutrient cycling in arid regions. Front. Microbiol. 2024, 15, 1407760. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, S.F.; Luo, D.D.; Mao, P.; Rosazlina, R.; Martin, F.; Xu, L.L. Decline in morel production upon continuous cropping is related to changes in soil mycobiome. J. Fungi 2023, 9, 492. [Google Scholar] [CrossRef]
- He, P.; Li, C.; Cai, Y.; Zhang, Y.; Bian, Y.; Liu, W. First report of pileus rot disease on cultivated Morchella importuna caused by Diploospora longispora in China. J. Gen. Plant Pathol. 2018, 84, 65–69. [Google Scholar] [CrossRef]
- Sun, J.; Yu, S.; Lu, Y.; Liu, H.; Liu, X. Proposal of a new family Pseudodiploosporeaceae fam. nov. (Hypocreales) based on phylogeny of Diploospora longispora and Paecilomyces penicillatus. Mycology 2023, 14, 60–73. [Google Scholar] [CrossRef]
- He, X.L.; Peng, W.H.; Miao, R.Y.; Tang, J.; Chen, Y.; Liu, L.X.; Wang, D.; Gan, B.C. White mold on cultivated morels caused by Paecilomyces penicillatus. FEMS Microbiol. Lett. 2017, 364, fnx037. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.P.; Chen, K.; Wang, G.Z.; Bian, Y.B. First report of stipe rot disease on Morchella importuna caused by Fusarium incarnatum–F. equiseti species complex in China. Plant Dis. 2016, 100, 2530. [Google Scholar] [CrossRef]
- Liu, Z.; Cong, Y.; Sossah, F.L.; Lu, Y.; Kang, J.; Li, Y. Characterization and genome analysis of Cladobotryum mycophilum, the causal agent of cobweb disease of Morchella sextelata in China. J. Fungi 2023, 9, 411. [Google Scholar] [CrossRef]
- Lan, Y.F.; Cong, Q.Q.; Wang, Q.W.; Tang, L.N.; Li, X.M.; Yu, Q.W.; Cui, X.; An, X.R.; Yu, C.X.; Kong, F.H.; et al. First report of Cladobotryum protrusum causing cobweb disease on cultivated Morchella importuna. Plant Dis. 2020, 104, 977–978. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, L.J.; Li, Q.; Zou, J.; Tan, H.; Tan, W.; Peng, W.H.; Li, X.L.; Zhang, X.P. Dynamic succession of substrate-associated bacterial composition and function during Ganoderma lucidum growth. PeerJ 2018, 6, e4975. [Google Scholar] [CrossRef]
- Pugliese, M. Effect of compost against soil-borne plant pathogens and its impact on rhizosphere microbiota. Environ. Eng. Manag. J. 2021, 20, 1655–1659. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, Y.; Zhang, P.; Xue, K.; Liang, Y.; Van Nostrand, J.D.; Yang, Y.; He, Z.; Wu, L.; Stahl, D.A.; et al. Stochasticity, succession, and environmental perturbations in a fluidic ecosystem. Proc. Natl. Acad. Sci. USA 2014, 111, E836–E845. [Google Scholar] [CrossRef]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G.A. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef]
- Zhong, Y.; Sorensen, P.O.; Zhu, G.; Jia, X.; Liu, J.; Shangguan, Z.; Wang, R.; Yan, W. Differential microbial assembly processes and co-occurrence networks in the soil-root continuum along an environmental gradient. iMeta 2022, 1, e18. [Google Scholar] [CrossRef]
- Hernandez, D.J.; David, A.S.; Menges, E.S.; Searcy, C.A.; Afkhami, M.E. Environmental stress destabilizes microbial networks. ISME J. 2021, 15, 1722–1734. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Brettell, L.E.; Qiu, Z.; Singh, B.K. Microbiome-Mediated Stress Resistance in Plants. Trends Plant Sci. 2020, 25, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Xiong, C.; Gao, C.; Tsui, C.K.M.; Wang, M.-M.; Zhou, X.; Zhang, A.-M.; Cai, L. Disease-Induced Changes in Plant Microbiome Assembly and Functional Adaptation. Microbiome 2021, 9, 187. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial Diversity Drives Multifunctionality in Terrestrial Ecosystems. Nat. Commun. 2016, 7, 10541. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-H.; Aslam, M.M.; Gao, Y.-Y.; Dai, L.; Hao, G.-F.; Wei, Z.; Chen, M.-X.; Dini-Andreote, F. Microbiome-Mediated Signal Transduction within the Plant Holobiont. Trends Microbiol. 2023, 31, 616–628. [Google Scholar] [CrossRef]
- Ren, H.; Hong, H.; Zha, B.; Lamlom, S.F.; Qiu, H.; Cao, Y.; Sun, R.; Wang, H.; Ma, J.; Zhang, H.; et al. Soybean Productivity Can Be Enhanced by Understanding Rhizosphere Microbiota: Evidence from Metagenomics Analysis from Diverse Agroecosystems. Microbiome 2025, 13, 105. [Google Scholar] [CrossRef]
- Sun, R.; Li, W.; Dong, W.; Tian, Y.; Hu, C.; Liu, B. Tillage Changes Vertical Distribution of Soil Bacterial and Fungal Communities. Front. Microbiol. 2018, 9, 699. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Luo, Y. Meta-Analysis of the Impacts of Global Change Factors on Soil Microbial Diversity and Functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef]
- Anantharaman, K.; Hausmann, B.; Jungbluth, S.P.; Kantor, R.S.; Lavy, A.; Warren, L.A.; Rappe, M.S.; Pester, M.; Loy, A.; Thomas, B.C.; et al. Expanded Diversity of Microbial Groups That Shape the Dissimilatory Sulfur Cycle. ISME J. 2018, 12, 1715–1728. [Google Scholar] [CrossRef]
- Louca, S.; Polz, M.F.; Mazel, F.; Albright, M.B.N.; Huber, J.A.; O’Connor, M.I.; Ackermann, M.; Hahn, A.S.; Srivastava, D.S.; Crowe, S.A.; et al. Function and Functional Redundancy in Microbial Systems. Nat. Ecol. Evol. 2018, 2, 936–943. [Google Scholar] [CrossRef]
- Nelson, M.B.; Martiny, A.C.; Martiny, J.B.H. Global Biogeography of Microbial Nitrogen-Cycling Traits in Soil. Proc. Natl. Acad. Sci. USA 2016, 113, 8033–8040. [Google Scholar] [CrossRef] [PubMed]
- Jansson, J.K.; Hofmockel, K.S. The Soil Microbiome—From Metagenomics to Metaphenomics. Curr. Opin. Microbiol. 2018, 43, 162–168. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Z.-L.; Yu, F.-M.; Ma, X.; Zhao, Q.; Liu, J.-K. Metagenomic Insights into Disease-Induced Microbial Dysbiosis and Elemental Cycling Alterations in Morchella Cultivation Soils: Evidence from Two Distinct Regions. J. Fungi 2025, 11, 663. https://doi.org/10.3390/jof11090663
Deng Z-L, Yu F-M, Ma X, Zhao Q, Liu J-K. Metagenomic Insights into Disease-Induced Microbial Dysbiosis and Elemental Cycling Alterations in Morchella Cultivation Soils: Evidence from Two Distinct Regions. Journal of Fungi. 2025; 11(9):663. https://doi.org/10.3390/jof11090663
Chicago/Turabian StyleDeng, Zong-Lin, Feng-Ming Yu, Xiang Ma, Qi Zhao, and Jian-Kui Liu. 2025. "Metagenomic Insights into Disease-Induced Microbial Dysbiosis and Elemental Cycling Alterations in Morchella Cultivation Soils: Evidence from Two Distinct Regions" Journal of Fungi 11, no. 9: 663. https://doi.org/10.3390/jof11090663
APA StyleDeng, Z.-L., Yu, F.-M., Ma, X., Zhao, Q., & Liu, J.-K. (2025). Metagenomic Insights into Disease-Induced Microbial Dysbiosis and Elemental Cycling Alterations in Morchella Cultivation Soils: Evidence from Two Distinct Regions. Journal of Fungi, 11(9), 663. https://doi.org/10.3390/jof11090663






