Potential Protective Role of Amphibian Skin Bacteria Against Water Mold Saprolegnia spp.
Abstract
1. Introduction
2. Materials and Methods
2.1. Oomycete Isolation
2.2. Molecular Identification of Oomycetes and Phylogenetic Analysis
2.3. Culture Conditions of Saprolegnia spp.
2.4. Bacterial Strains
2.5. Screening of Antagonistic Bacteria
3. Results
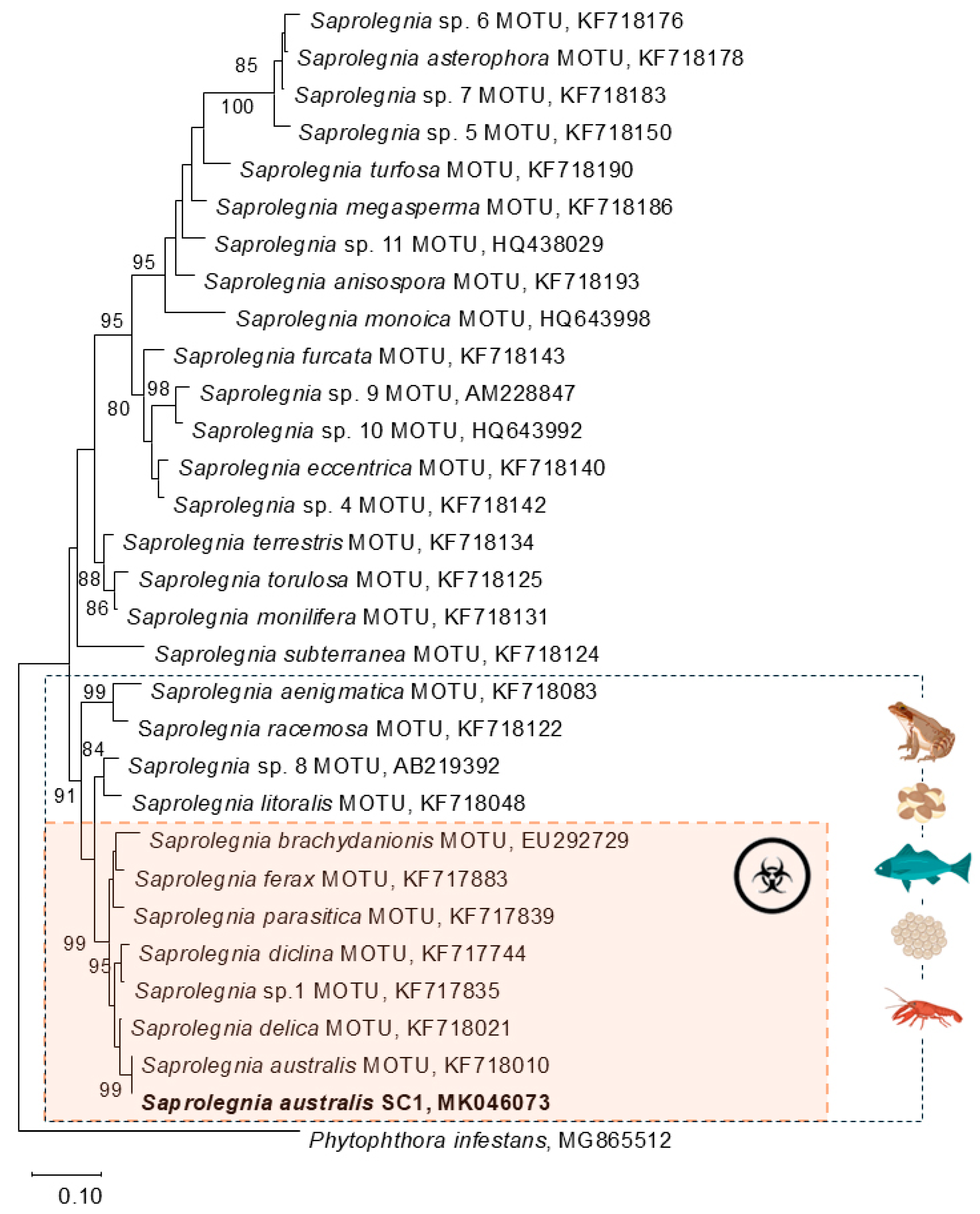
3.1. Molecular Identification of Oomycetes
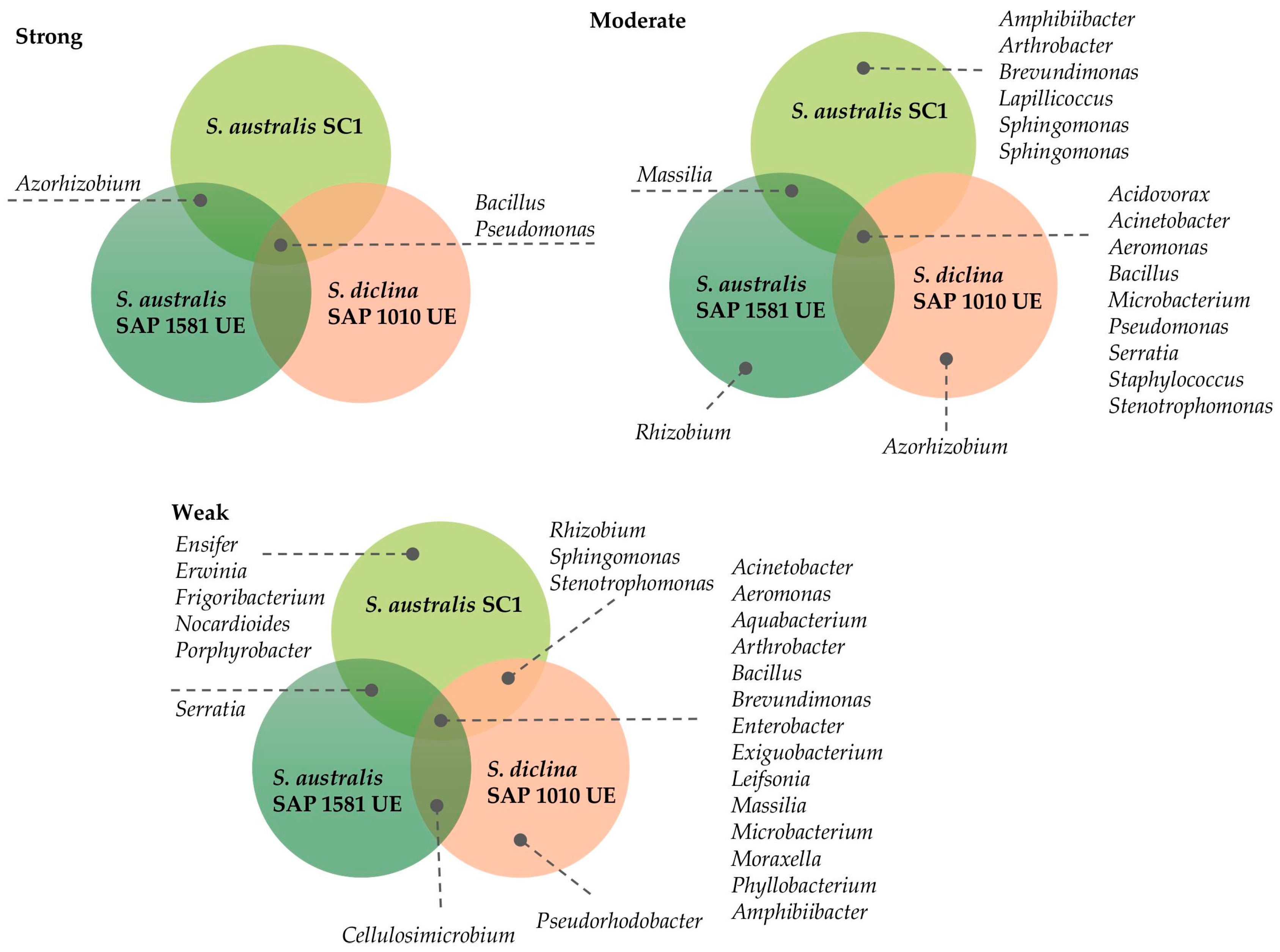
3.2. Bacterial Screening
4. Discussion
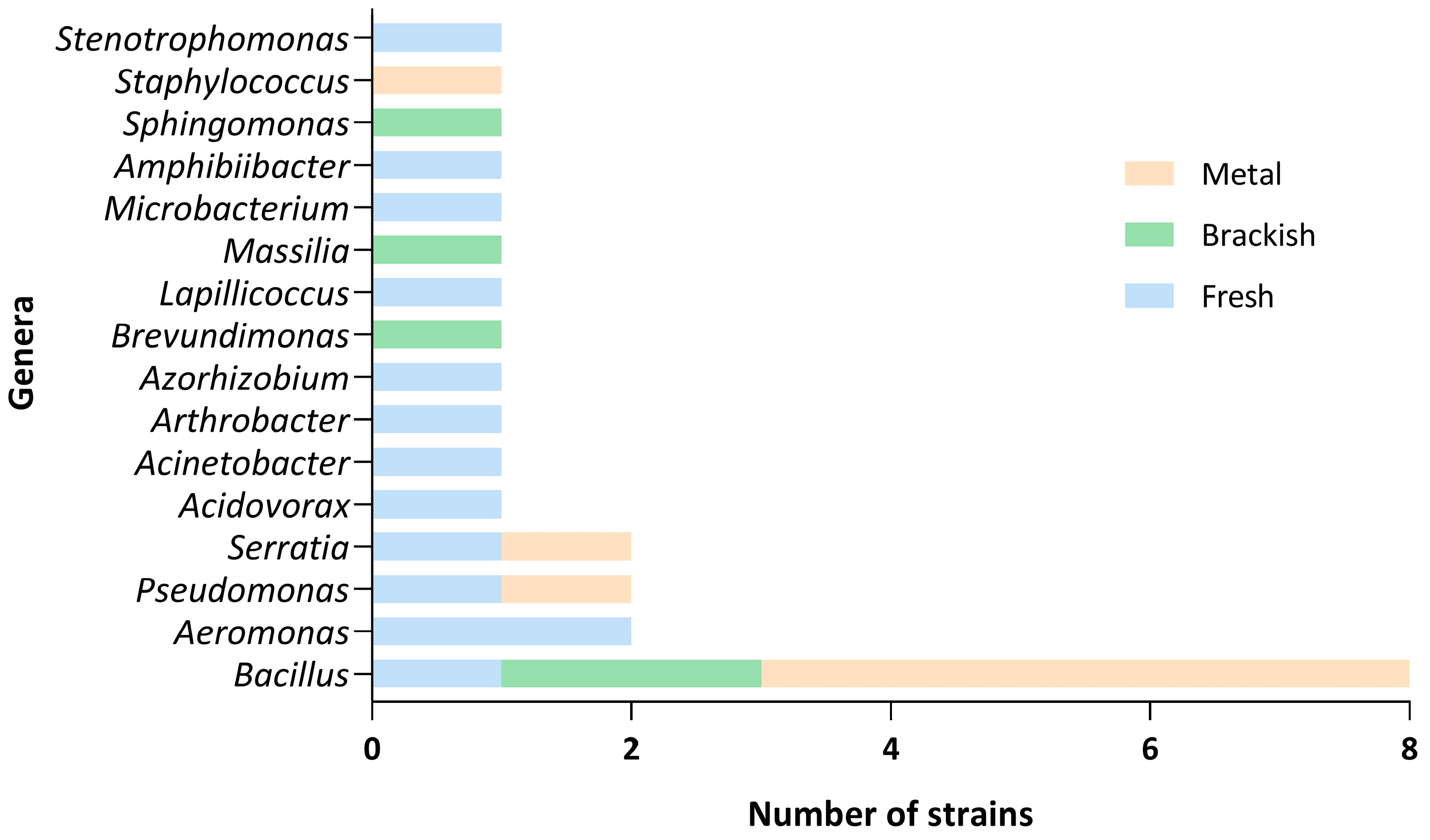
4.1. Bacteria’s Antagonistic Growth Effects Against Saprolegnia spp.
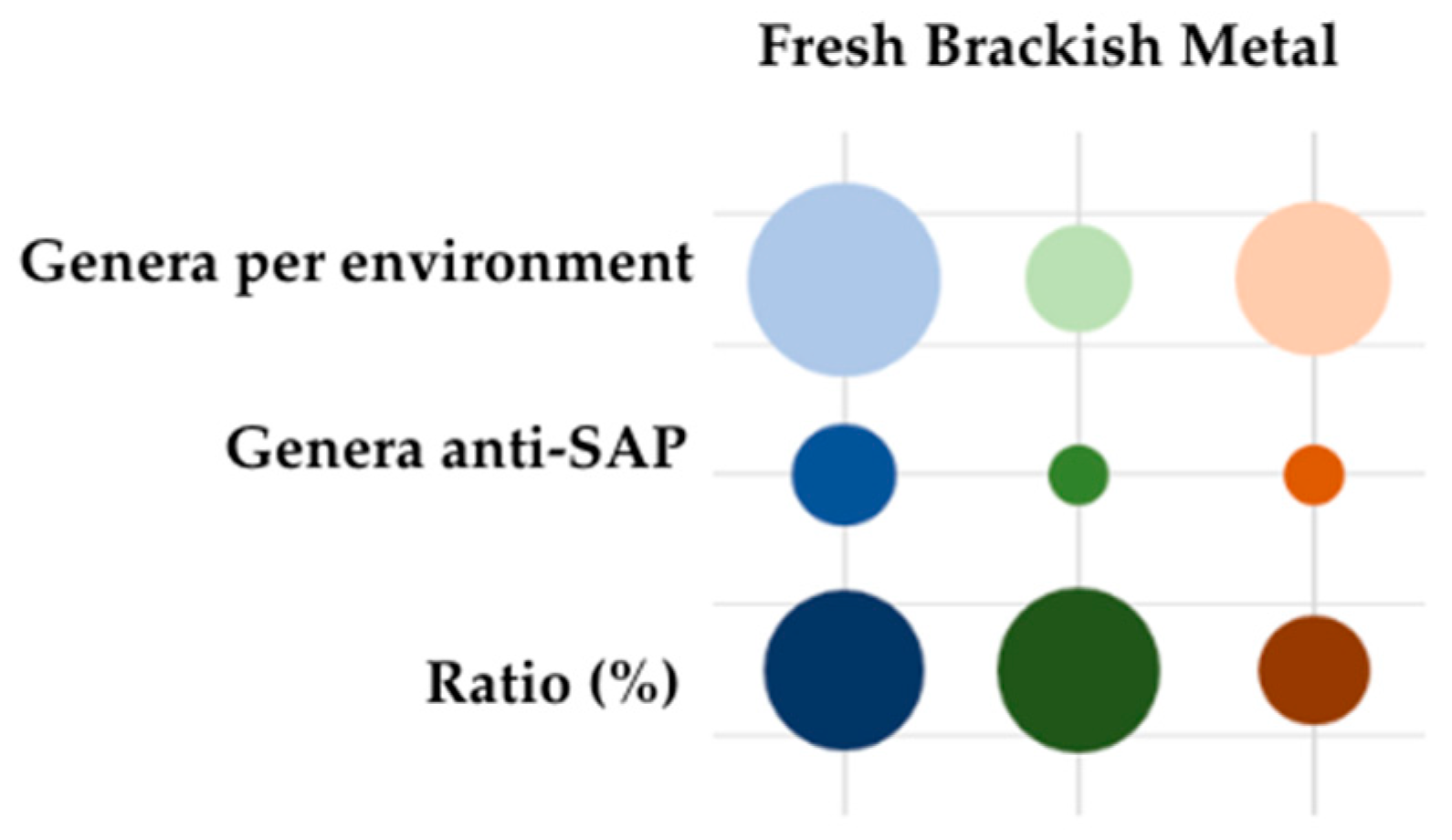
4.2. Origins of the Isolate Populations and Environmental Conditions
4.3. Substrate Impacts on the Antagonistic Effects of Bacteria Against Saprolegnia spp.
4.4. Pathogen Identification as Saprolegnia australis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- van den Berg, A.H.; McLaggan, D.; Diéguez-Uribeondo, J.; van West, P. The impact of the water moulds Saprolegnia diclina and Saprolegnia parasitica on natural ecosystems and the aquaculture industry. Fungal Biol. Rev. 2013, 27, 33–42. [Google Scholar] [CrossRef]
- Costa, S.; Lopes, I. Saprolegniosis in amphibians: An integrated overview of a fluffy killer disease. J. Fungi 2022, 8, 537. [Google Scholar] [CrossRef]
- Sarowar, M.N.; van den Berg, A.H.; McLaggan, D.; Young, M.R.; Van West, P. Saprolegnia strains isolated from river insects and amphipods are broad spectrum pathogens. Fungal Biol. 2013, 117, 752–763. [Google Scholar] [CrossRef]
- Fernández-Benéitez, M.J.; Ortiz-Santaliestra, M.E.; Lizana, M.; Diéguez-Uribeondo, J. Saprolegnia diclina: Another species responsible for the emergent disease “Saprolegnia infections” in amphibians. FEMS Microbiol. Lett. 2008, 279, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Groffen, J.; Oh, S.Y.; Kwon, S.; Jang, Y.; Borzee, A. High mortality in Bufo gargarizans eggs associated with an undescribed Saprolegnia ferax strain in the Republic of Korea. Dis. Aquat. Organ. 2019, 137, 89–99. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Grant Hokit, D.; O’Hara, R.K.; Holt, R.A. Pathogenic fungus contributes to amphibian losses in the pacific northwest. Biol. Conserv. 1994, 67, 251–254. [Google Scholar] [CrossRef]
- van West, P. Saprolegnia parasitica, an oomycete pathogen with a fishy appetite: New challenges for an old problem. Mycologist 2006, 20, 99–104. [Google Scholar] [CrossRef]
- Kiesecker, J.M.; Blaustein, A.R. Influences of Egg Laying Behavior on Pathogenic Infection of Amphibian Eggs. Conserv. Biol. 1997, 11, 214–220. [Google Scholar] [CrossRef]
- Romansic, J.; Diez, K.; Higashi, E.; Blaustein, A. Effects of nitrate and the pathogenic water mold Saprolegnia on survival of amphibian larvae. Dis. Aquat. Organ. 2006, 68, 235–243. [Google Scholar] [CrossRef]
- Coughlan, M.P.; Waters, T.R.; Touchon, J.C. Salinity increases growth and pathogenicity of water mold to cause mortality and early hatching in Rana sylvatica embryos. FEMS Microbiol. Ecol. 2021, 97, 257. [Google Scholar] [CrossRef] [PubMed]
- Pavić, D.; Grbin, D.; Gregov, M.; Ćurko, J.; Vladušić, T.; Šver, L.; Miljanović, A.; Bielen, A. Variations in the Sporulation Efficiency of Pathogenic Freshwater Oomycetes in Relation to the Physico-Chemical Properties of Natural Waters. Microorganisms 2022, 10, 520. [Google Scholar] [CrossRef]
- Tedesco, P.; Saraiva, M.; Sandoval-Sierra, J.V.; Alves, M.T.; Galuppi, R.; Dieguez-Uribeondo, J.; van West, P.; Cook, A.; Posen, P.; Oidtmann, B.; et al. Impact of abiotic factors and husbandry on saprolegniosis in salmonid farms. Aquaculture 2022, 561, 738679. [Google Scholar] [CrossRef]
- Carbajal-González, M.T.; Fregeneda-Grandes, J.M.; Suárez-Ramos, S.; Rodríguez Cadenas, F.; Aller-Gancedo, J.M. Bacterial skin flora variation and in vitro inhibitory activity against Saprolegnia parasitica in brown and rainbow trout. Dis. Aquat. Organ. 2011, 96, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Walke, J.B.; Belden, L.K. Harnessing the Microbiome to Prevent Fungal Infections: Lessons from Amphibians. PLoS Pathog. 2016, 12, e1005796. [Google Scholar] [CrossRef]
- Woodhams, D.C.; Rollins-Smith, L.A.; Alford, R.A.; Simon, M.A.; Harris, R.N. Innate immune defenses of amphibian skin: Antimicrobial peptides and more. Anim. Conserv. 2007, 10, 425–428. [Google Scholar] [CrossRef]
- Woodhams, D.C.; Vredenburg, V.T.; Simon, M.-A.; Billheimer, D.; Shakhtour, B.; Shyr, Y.; Briggs, C.J.; Rollins-Smith, L.A.; Harris, R.N. Symbiotic bacteria contribute to innate immune defenses of the threatened mountain yellow-legged frog, Rana muscosa. Biol. Conserv. 2007, 138, 390–398. [Google Scholar] [CrossRef]
- Jani, A.J.; Briggs, C.J. Host and aquatic environment shape the amphibian skin microbiome but effects on downstream resistance to the pathogen Batrachochytrium dendrobatidis are variable. Front. Microbiol. 2018, 9, 487. [Google Scholar] [CrossRef]
- Bletz, M.C.; Loudon, A.H.; Becker, M.H.; Bell, S.C.; Woodhams, D.C.; Minbiole, K.P.C.; Harris, R.N. Mitigating amphibian chytridiomycosis with bioaugmentation: Characteristics of effective probiotics and strategies for their selection and use. Ecol. Lett. 2013, 16, 807–820. [Google Scholar] [CrossRef]
- Harris, R.N.; Brucker, R.M.; Walke, J.B.; Becker, M.H.; Schwantes, C.R.; Flaherty, D.C.; Lam, B.A.; Woodhams, D.C.; Briggs, C.J.; Vredenburg, V.T.; et al. Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. ISME J. 2009, 3, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Woodhams, D.C.; Bletz, M.; Kueneman, J.; McKenzie, V. Managing Amphibian Disease with Skin Microbiota. Trends Microbiol. 2016, 24, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Flores, G.; Pickard, J.M.; Núñez, G. Microbiota-mediated colonization resistance: Mechanisms and regulation. Nat. Rev. Microbiol. 2022, 21, 347. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.H.; Harris, R.N. Cutaneous bacteria of the redback salamander prevent morbidity associated with a lethal disease. PLoS ONE 2010, 5, e10957. [Google Scholar] [CrossRef]
- Bletz, M.C.; Kelly, M.; Sabino-Pinto, J.; Bales, E.; Van Praet, S.; Bert, W.; Boyen, F.; Vences, M.; Steinfartz, S.; Pasmans, F.; et al. Disruption of skin microbiota contributes to salamander disease. Proc. R. Soc. B Biol. Sci. 2018, 285, 20180758. [Google Scholar] [CrossRef] [PubMed]
- Rollins-Smith, L.A. Amphibian immunity–stress, disease, and climate change. Dev. Comp. Immunol. 2017, 66, 111–119. [Google Scholar] [CrossRef]
- Costa, S.; Lopes, I.; Proença, D.N.; Ribeiro, R.; Morais, P.V. Diversity of cutaneous microbiome of Pelophylax perezi populations inhabiting different environments. Sci. Total Environ. 2016, 572, 995–1004. [Google Scholar] [CrossRef]
- Buttimer, S.; Moura-Campos, D.; Greenspan, S.E.; Neely, W.J.; Ferrante, L.; Toledo, L.F.; Becker, C.G. Skin microbiome disturbance linked to drought-associated amphibian disease. Ecol. Lett. 2024, 27, e14372. [Google Scholar] [CrossRef]
- Jiménez, R.R.; Alvarado, G.; Sandoval, J.; Sommer, S. Habitat disturbance influences the skin microbiome of a rediscovered neotropical-montane frog. BMC Microbiol. 2020, 20, 292. [Google Scholar] [CrossRef] [PubMed]
- Schuck, L.K.; Neely, W.J.; Buttimer, S.M.; Moser, C.F.; Barth, P.C.; Liskoski, P.E.; Caberlon, C.d.A.; Valiati, V.H.; Tozetti, A.M.; Becker, C.G. Effects of grassland controlled burning on symbiotic skin microbes in Neotropical amphibians. Sci. Rep. 2024, 14, 959. [Google Scholar] [CrossRef]
- Antwis, R.E.; Haworth, R.L.; Engelmoer, D.J.P.; Ogilvy, V.; Fidgett, A.L.; Preziosi, R.F. Ex situ Diet Influences the Bacterial Community Associated with the Skin of Red-Eyed Tree Frogs (Agalychnis callidryas). PLoS ONE 2014, 9, e85563. [Google Scholar] [CrossRef]
- Xu, L.; Xiang, M.; Zhu, W.; Zhang, M.; Chen, H.; Huang, J.; Chen, Y.; Chang, Q.; Jiang, J.; Zhu, L. The Behavior of Amphibians Shapes Their Symbiotic Microbiomes. mSystems 2020, 5. [Google Scholar] [CrossRef]
- Kueneman, J.G.; Parfrey, L.W.; Woodhams, D.C.; Archer, H.M.; Knight, R.; McKenzie, V.J. The amphibian skin-associated microbiome across species, space and life history stages. Mol. Ecol. 2014, 23, 1238–1250. [Google Scholar] [CrossRef]
- Coelho, L.; Afonso, M.; Jesus, F.; Campos, I.; Abrantes, N.; Gonçalves, F.J.M.; Serpa, D.; Marques, S.M. Effects of Eucalypt ashes from moderate and high severity wildfires on the skin microbiome of the Iberian frog (Rana iberica). Environ. Pollut. 2022, 313, 120065. [Google Scholar] [CrossRef]
- Afonso, M.; Coelho, L.; Jesus, F.; Campos, I.; Abrantes, N.; Gonçalves, F.J.M.; Marques, S.; Serpa, D. Effects of Pine and Eucalypt ashes on bacterial isolates from the skin microbiome of the fire salamander (Salamandra salamandra). Sci. Total Environ. 2022, 841, 156677. [Google Scholar] [CrossRef]
- Becker, M.H.; Walke, J.B.; Cikanek, S.; Savage, A.E.; Mattheus, N.; Santiago, C.N.; Minbiole, K.P.C.; Harris, R.N.; Belden, L.K.; Gratwicke, B. Composition of symbiotic bacteria predicts survival in Panamanian golden frogs infected with a lethal fungus. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142881. [Google Scholar] [CrossRef]
- Daskin, J.H.; Bell, S.C.; Schwarzkopf, L.; Alford, R.A. Cool Temperatures Reduce Antifungal Activity of Symbiotic Bacteria of Threatened Amphibians—Implications for Disease Management and Patterns of Decline. PLoS ONE 2014, 9, e100378. [Google Scholar] [CrossRef] [PubMed]
- Muletz-Wolz, C.R.; Almario, J.G.; Barnett, S.E.; DiRenzo, G.V.; Martel, A.; Pasmans, F.; Zamudio, K.R.; Toledo, L.F.; Lips, K.R. Inhibition of fungal pathogens across genotypes and temperatures by amphibian skin bacteria. Front. Microbiol. 2017, 8, 1551. [Google Scholar] [CrossRef]
- Engblom, C.; Landor, L.; Sjöqvist, C.; Korkea-aho, T.; Viljamaa-Dirks, S.; Paulin, L.; Wiklund, T. Identification and genetic characterization of Saprolegnia parasitica, isolated from farmed and wild fish in Finland. J. Fish Dis. 2023, 46, 849–860. [Google Scholar] [CrossRef]
- Alves, A.; Crous, P.W.; Correia, A.; Phillips, A.J.L. Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Divers. 2008, 28, 1–13. [Google Scholar]
- Sandoval-Sierra, J.V.; Martín, M.P.; Diéguez-Uribeondo, J. Species identification in the genus Saprolegnia (Oomycetes): Defining DNA-based molecular operational taxonomic units. Fungal Biol. 2014, 118, 559–578. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Page, R.D.M. Visualizing phylogenetic trees using TreeView. Curr. Protoc. Bioinform. 2003, 6.2.1–6.2.15. [Google Scholar] [CrossRef]
- Proença, D.N.; Fasola, E.; Lopes, I.; Morais, P.V. Characterization of the skin cultivable microbiota composition of the frog Pelophylax perezi inhabiting different environments. Int. J. Environ. Res. Public Health 2021, 18, 2585. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.H.; Brucker, R.M.; Schwantes, C.R.; Harris, R.N.; Minbiole, K.P.C. The bacterially produced metabolite violacein is associated with survival of amphibians infected with a lethal fungus. Appl. Environ. Microbiol. 2009, 75, 6635–6638. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.M.; Ramsey, J.P.; Blackman, A.L.; Nichols, A.E.; Minbiole, K.P.C.; Harris, R.N. Synergistic Inhibition of the Lethal Fungal Pathogen Batrachochytrium dendrobatidis: The Combined Effect of Symbiotic Bacterial Metabolites and Antimicrobial Peptides of the Frog Rana muscosa. J. Chem. Ecol. 2012, 38, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Brucker, R.M.; Baylor, C.M.; Walters, R.L.; Lauer, A.; Harris, R.N.; Minbiole, K.P.C. The identification of 2,4-diacetylphloroglucinol as an antifungal metabolite produced by cutaneous bacteria of the salamander Plethodon cinereus. J. Chem. Ecol. 2008, 34, 39–43. [Google Scholar] [CrossRef]
- Loudon, A.H.; Holland, J.A.; Umile, T.P.; Burzynski, E.A.; Minbiole, K.P.C.C.; Harris, R.N. Interactions between amphibians’ symbiotic bacteria cause the production of emergent anti-fungal metabolites. Front. Microbiol. 2014, 5, 441. [Google Scholar] [CrossRef]
- Woodhams, D.C.; LaBumbard, B.C.; Barnhart, K.L.; Becker, M.H.; Bletz, M.C.; Escobar, L.A.; Flechas, S.V.; Forman, M.E.; Iannetta, A.A.; Joyce, M.D.; et al. Prodigiosin, violacein, and volatile organic compounds produced by widespread cutaneous bacteria of amphibians can inhibit two Batrachochytrium fungal pathogens. Microb. Ecol. 2018, 75, 1049–1062. [Google Scholar] [CrossRef]
- Becker, M.H.; Walke, J.B.; Murrill, L.; Woodhams, D.C.; Reinert, L.K.; Rollins-Smith, L.A.; Burzynski, E.A.; Umile, T.P.; Minbiole, K.P.C.; Belden, L.K. Phylogenetic distribution of symbiotic bacteria from Panamanian amphibians that inhibit growth of the lethal fungal pathogen Batrachochytrium dendrobatidis. Mol. Ecol. 2015, 24, 1628–1641. [Google Scholar] [CrossRef]
- Assis, A.B.d.; Barreto, C.C.; Navas, C.A. Skin microbiota in frogs from the Brazilian Atlantic Forest: Species, forest type, and potential against pathogens. PLoS ONE 2017, 12, e0179628. [Google Scholar] [CrossRef]
- Albecker, M.A.; Belden, L.K.; McCoy, M.W. Comparative Analysis of Anuran Amphibian Skin Microbiomes Across Inland and Coastal Wetlands. Microb. Ecol. 2019, 78, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; You, G.; Yin, J.; Zhang, M.; Peng, D.; Xu, J.; Yang, S.; Hou, J. Salt tolerance evolution facilitates antibiotic resistome in soil microbiota: Evidences from dissemination evaluation, hosts identification and co-occurrence exploration. Environ. Pollut. 2023, 317, 120830. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.; Walke, J.B.; Gajewski, Z.; Becker, M.H.; Swartwout, M.C.; Belden, L.K. Culture Media and Individual Hosts Affect the Recovery of Culturable Bacterial Diversity from Amphibian Skin. Front. Microbiol. 2017, 8, 1574. [Google Scholar] [CrossRef]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2010, 8, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Frediansyah, A.; Manuhara, Y.S.W.; Kristanti, A.N.; Luqman, A.; Wibowo, A.T. Fermentation in Minimal Media and Fungal Elicitation Enhance Violacein and Deoxyviolacein Production in Two Janthinobacterium Strains. Fermentation 2022, 8, 714. [Google Scholar] [CrossRef]
- Kim, S.; Eom, A.-H.; Park, D.; Ra, N.-Y. Detection of infectious fungal diseases of frogs inhabiting in Korea. Mycobiology 2008, 36, 10. [Google Scholar] [CrossRef]
- Sandoval-Sierra, J.V.; Latif-Eugenin, F.; Martín, M.P.; Zaror, L.; Diéguez-Uribeondo, J. Saprolegnia species affecting the salmonid aquaculture in Chile and their associations with fish developmental stage. Aquaculture 2014, 434, 462–469. [Google Scholar] [CrossRef]
- Rezinciuc, S.; Sandoval-Sierra, J.-V.; Diéguez-Uribeondo, J. Molecular identification of a bronopol tolerant strain of Saprolegnia australis causing egg and fry mortality in farmed brown trout, Salmo trutta. Fungal Biol. 2014, 118, 591–600. [Google Scholar] [CrossRef]
- Chang, P.H.; Wu, T.P.; Chung, H.Y.; Chien, C.Y. Aeromonas hydrophila and Saprolegnia australis isolated from Ayu, Plecoglossus altivelis with ulcerative skin disease in Taiwan. Bull. Eur. Assoc. Fish Pathol. 2002, 22, 393–399. [Google Scholar]
- Kozubikova-Balcarova, E.; Koukol, O.; Martín, M.P.; Svoboda, J.; Petrusek, A.; Diéguez-Uribeondo, J. The diversity of oomycetes on crayfish: Morphological vs. molecular identification of cultures obtained while isolating the crayfish plague pathogen. Fungal Biol. 2013, 117, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, P.E.; Nechwatal, J.; Fischer, P. A previously undescribed set of Saprolegnia spp. in the invasive spiny-cheek crayfish (Orconectes limosus, Rafinesque). Fundam. Appl. Limnol. Arch. Hydrobiol. 2008, 172, 161–165. [Google Scholar] [CrossRef]
- Krugner-Higby, L.; Haak, D.; Johnson, P.; Shields, J.; Jones, W.; Reece, K.; Meinke, T.; Gendron, A.; Rusak, J. Ulcerative disease outbreak in crayfish Orconectes propinquus linked to Saprolegnia australis in Big Muskellunge Lake, Wisconsin. Dis. Aquat. Organ. 2010, 91, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Kiesecker, J.M.; Blaustein, A.R.; Miller, C.L. Transfer of a pathogen from fish to amphibians. Conserv. Biol. 2001, 15, 1064–1070. [Google Scholar] [CrossRef]
- Masigol, H.; Woodhouse, J.N.; van West, P.; Mostowfizadeh-Ghalamfarsa, R.; Rojas-Jimenez, K.; Goldhammer, T.; Khodaparast, S.A.; Grossart, H.P. Phylogenetic and Functional Diversity of Saprolegniales and Fungi Isolated from Temperate Lakes in Northeast Germany. J. Fungi 2021, 7, 968. [Google Scholar] [CrossRef]
- Masigol, H.; Khodaparast, S.A.; Mostowfizadeh-Ghalamfarsa, R.; Rojas-Jimenez, K.; Woodhouse, J.N.; Neubauer, D.; Grossart, H.P. Taxonomical and functional diversity of Saprolegniales in Anzali lagoon, Iran. Aquat. Ecol. 2020, 54, 323–336. [Google Scholar] [CrossRef]

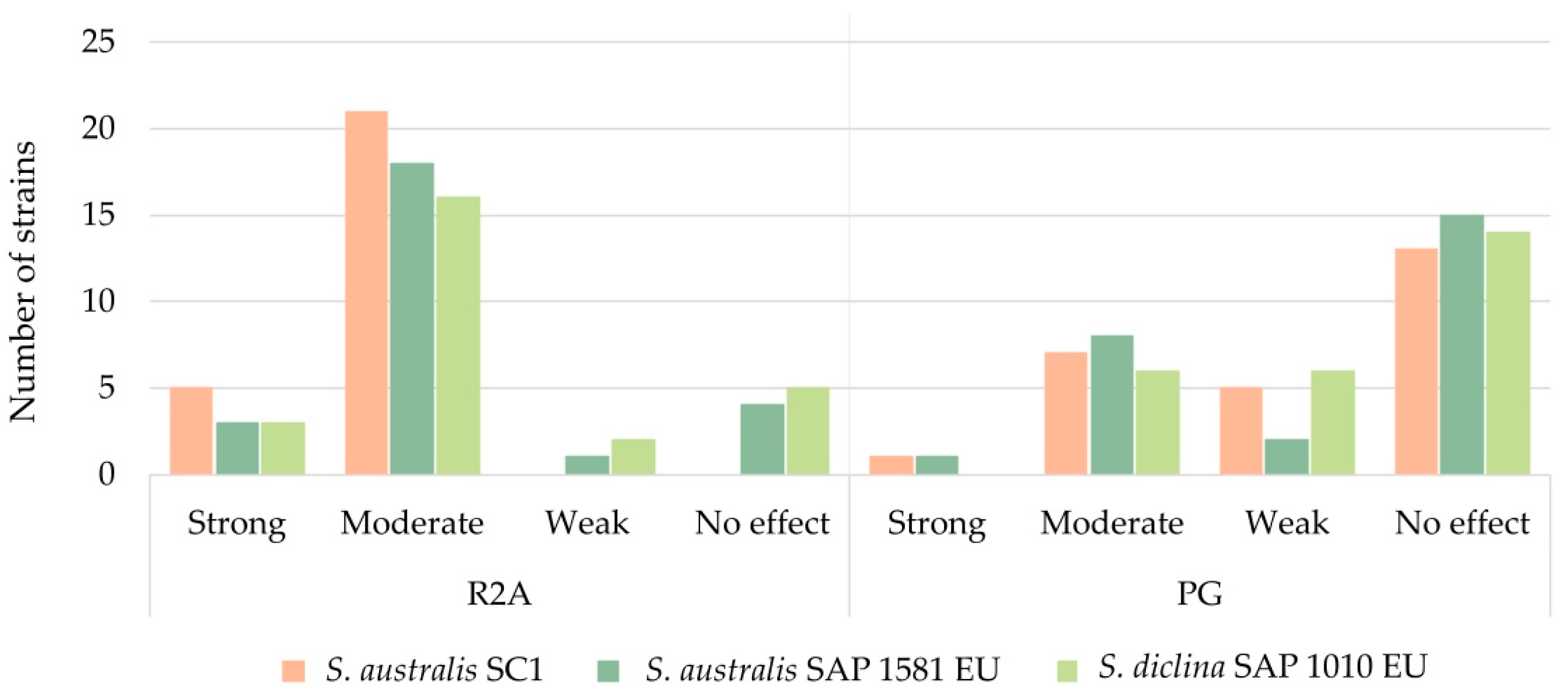
| Saprolegnia Species | Type of Bacterial Antagonistic Response | Number of Strains Per Response | Ratio of Bacterial Strains According to Isolation Site Environment (%) | ||
|---|---|---|---|---|---|
| Fresh | Brackish | Metal | |||
| S. australis SC1 | Strong | 5 (3%) | 1.9 | 4.5 | 2.9 |
| Moderate | 20 (10%) | 9.4 | 18.2 | 8.8 | |
| Weak | 43 (22%) | 20.8 | 22.7 | 23.5 | |
| No effect | 128 (65%) | 67.9 | 54.5 | 64.7 | |
| S. australis SAP 1581 UE | Strong | 3 (2%) | 1.9 | 4.5 | 0.0 |
| Moderate | 20 (10%) | 8.5 | 9.1 | 13.2 | |
| Weak | 21 (11%) | 9.4 | 13.6 | 11.8 | |
| No effect | 152 (78%) | 80.2 | 72.7 | 75.0 | |
| S. diclina SAP 1010 UE | Strong | 3 (2%) | 0.9 | 9.1 | 0.0 |
| Moderate | 17 (9%) | 8.5 | 0.0 | 11.8 | |
| Weak | 29 (15%) | 12.3 | 31.8 | 13.2 | |
| No effect | 147 (75%) | 78.3 | 59.1 | 75.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, S.; Proença, D.N.; Alves, A.; Morais, P.V.; Lopes, I. Potential Protective Role of Amphibian Skin Bacteria Against Water Mold Saprolegnia spp. J. Fungi 2025, 11, 649. https://doi.org/10.3390/jof11090649
Costa S, Proença DN, Alves A, Morais PV, Lopes I. Potential Protective Role of Amphibian Skin Bacteria Against Water Mold Saprolegnia spp. Journal of Fungi. 2025; 11(9):649. https://doi.org/10.3390/jof11090649
Chicago/Turabian StyleCosta, Sara, Diogo Neves Proença, Artur Alves, Paula V. Morais, and Isabel Lopes. 2025. "Potential Protective Role of Amphibian Skin Bacteria Against Water Mold Saprolegnia spp." Journal of Fungi 11, no. 9: 649. https://doi.org/10.3390/jof11090649
APA StyleCosta, S., Proença, D. N., Alves, A., Morais, P. V., & Lopes, I. (2025). Potential Protective Role of Amphibian Skin Bacteria Against Water Mold Saprolegnia spp. Journal of Fungi, 11(9), 649. https://doi.org/10.3390/jof11090649









