Abstract
There are no data available on the effectiveness and safety of isavuconazole (ISA) for treating breakthrough invasive fungal infections (bIFIs). A retrospective and prospective cohort study was conducted between January 2020 and March 2025 in 13 centers in Argentina. Hematologic diseases (HD) and hematopoietic cell transplantation (HCT) patients who received ISA for IFI were included and followed for 12 weeks. Patients with proven and probable bIFIs and non-bIFIs were compared. One hundred and sixty-three patients were included. IFIs were classified as proven (13.5%), probable (26.9%) and possible (59.5%). Among 66 proven and probable IFIs, 53% were bIFIs, with aspergillosis and mucormycosis being the most common. Twenty-three (34.8%) patients had acute myelogenous leukemia, and 40.9% had received HCT. Forty-eight (72.7%) patients experienced neutropenia, with a median duration of 26 days (interquartile range [IQR] 16–44). Fluconazole and posaconazole were the most frequently received antifungal prophylaxis. ISA was prescribed as first-line therapy in 31 (46.9%) patients. The other 35 received ISA as a continuation therapy, mainly as a step-down therapy after liposomal amphotericin B. Four (6.1%) patients developed adverse effects, and one discontinued ISA. The 90-day overall clinical response between patients with bIFI vs. non-bIFI was 91.4% vs. 70.9% (p = 0.052). The 90-day overall and IFI-related mortality rates were, respectively, 11.4% vs. 32.3% (p = 0.068) and 5.7% vs. 9.7% (p = 0.659). The study data evidence ISA effectiveness and safety for the treatment of HD and HCT patients with and without bIFIs.
1. Introduction
Invasive fungal infections (IFIs) are a frequent complication in patients with hematologic diseases (HD) and hematopoietic cell transplantation (HCT), with significant morbidity and mortality rates, as well as high healthcare costs [1,2,3,4].
Patients with acute leukemia and prolonged neutropenia, as well as those with allogeneic HCT with high doses of corticosteroids, have an IFI incidence of 7–13.2% and 8.8–16%, respectively [1,2,3,5]. The epidemiology of IFIs has evolved worldwide, with a significant predominance of mold in recent decades, largely Aspergillus spp. Mucorales and Fusarium spp. [1,2]. Two multicenter studies have addressed this issue. Data from the TRANSNET surveillance study in the United States identified 983 IFIs among 875 HCT recipients, with invasive aspergillosis being the most common (43%) [6]. According to the Prospective Antifungal Therapy (PATH) Alliance registry, invasive aspergillosis was the most common IFI (59.2%) among 234 adult HCT patients [7]. More recently, a study carried out in Switzerland in 515 allogeneic HCT recipients showed that 48 (9.3%) patients developed 51 proven/probable IFI, with invasive aspergillosis (67%) and mucormycosis (18%) being the most frequent [8]. This is largely due to primary antifungal prophylaxis strategies, which are highly active against Candida spp. [9,10]. In this regard, all high-risk patients currently receive antifungal prophylaxis, even before this epidemiology change [11]. IDSA, ESCMID, ASTCT, ECIL, and AGIHO/DGHO guidelines recommend posaconazole (POSA) use as the first choice, followed by voriconazole (VORI). Echinocandins and fluconazole (FLUCO) are recommended as alternative drugs due to their narrow spectrum. Some guidelines consider isavuconazole (ISA) as an alternative antifungal prophylaxis in those cases where POSA and VORI are not appropriate (prolonged QTc, patients who receive QTc-prolonging medications, or drug–drug interaction) [12,13,14,15,16]. Therefore, most of the IFIs generally developed are breakthrough IFIs (bIFIs) [17]. In this sense, a systematic review and meta-analysis that identified 991 patients who received ISA prophylaxis found an incidence of bIFIs of 7% [18]. They pose a significant challenge for diagnosis; furthermore, no randomized studies have been conducted to determine the optimal treatment option [19,20].
In this complex scenario, several antifungal drugs have proved effective for treating IFIs. ISA was approved for the treatment of invasive aspergillosis and mucormycosis, based on the SECURE and VITAL trials [21,22]. After the implementation of these studies ISA was approved by key regulatory agencies, including the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) [23,24]. Given its efficacy and safety, several guidelines recommend ISA as a first-line treatment for invasive aspergillosis and as a step-down therapy or first-line therapy for mucormycosis [12,25]. In addition, many real-life studies are consistent with the data obtained in pivotal studies [26,27,28,29,30].
ISA has several advantages over VORI, including Mucorales coverage, stable drug levels with low intra- and interpatient variability, lower CYP3A4 inhibition, resulting in significantly fewer drug–drug interactions, a highly safe profile, and less drug discontinuation, among others [31,32,33,34,35,36]. In terms of adverse effects, clinical trial data show an overall tolerability profile comparable to voriconazole, with nausea, vomiting, and diarrhea being the most common events. However, ISA is associated with a lower incidence of hepatotoxicity and visual disturbances, with lack of QTc prolongation being a key differentiating safety feature. On the other hand, it causes mild concentration-dependent QTc interval shortening. It is also associated with fewer severe skin reactions compared to other azoles [21,22]. These characteristics are crucial in patients with HD and HCT, as they receive a large number of medications, especially immunosuppressants, which interact with potent CYP3A4 inhibitors, such as VORI. These inhibitors can also have a high rate of adverse effects and are therefore unsuitable for use in these patients.
To the best of our knowledge, no multicenter studies have been published despite all the above mentioned advantages of ISA therapy for bIFIs.
This study aimed to outline the use of ISA for the treatment of IFIs in patients with HD and HCT. We further aimed to describe and compare those who have proven and probable bIFI and non-bIFI in terms of ISA effectiveness and safety, and patients’ outcomes.
2. Materials and Methods
2.1. Setting, Patients and Study Design
A retrospective and prospective observational multicenter study was performed in 13 referral teaching centers (8 private and 5 public) specialized in the management of patients with HD and HCT in Argentina.
Adult patients (≥18 years of age) treated with ISA for IFIs and managed as inpatients or outpatients were included. The retrospective cohort comprised patients included from 1 January 2020 to 31 March 2024; however, all of them were treated and followed up by the Infectious Diseases physicians conducting the study. Patients from the prospective cohort were recruited from 1 April 2024 to 31 March 2025. For the total cohort, the following criteria were met: (a) patients presenting with HD or autologous and allogeneic HCT; (b) those treated with ISA for IFIs for at least 7 days; and (c) those followed until day 90 since the beginning of ISA or until the patient’s death, whichever occurred first.
Patients were excluded in case of missing data that precluded the assessment of baseline, clinical, microbiological, treatment characteristics, and outcomes.
Patients were identified through data files from the Infectious Diseases Services, which treat and follow up all patients with HD and HCT at each center. Data were obtained from direct patient care, medical records, and data from laboratory, microbiology, and pathology databases. Clinical, microbiological, diagnostic, treatment, and outcome variables from the total cohort were evaluated. In addition, these variables were compared between patients with proven and probable bIFIs and non-bIFIs.
Patient data were recorded with RedCap (Research Electronic Data Capture) software (RedCap version 13.7.19) and the server hosting was provided by the Argentine Society of Infectious Diseases.
The study was approved by the Ethics Committees from the different participating institutions, and patient informed consent was waived.
2.2. Definitions
Proven, probable, and possible IFIs were defined according to the revised and updated European Organization for Research and Treatment of Cancer and Mycoses Study Group EORTC/MSG criteria [37]. Proven IFI was defined as histopathologic, cytopathologic, direct microscopic examination, or culture of a biopsy or other specimen obtained by a sterile procedure from a normal sterile site. Probable mold infection was defined as that occurring in patients with (a) one host factor: recent history of neutropenia (<500 neutrophils/mm3) for >10 days, allogeneic HCT, prolonged use of corticosteroids, treatment with other T-cell or B-cell-immunosuppressants; (b) at least one clinical feature: pulmonary CT-scan showing nodules with or without a halo sign, air crescent sign, cavity, consolidation or a reverse halo sign; evidence of tracheobronchitis, sino-nasal or central nervous system infection; and (c) microbiological evidence: microscopic detection or culture of any mold from bronchoalveolar lavage (BAL) or sinus aspirates, galactomannan (GM) antigen test ≥ 1.0 from serum or BAL, or single serum or plasma ≥ 0.7 and BAL fluid ≥ 0.8 or Histoplasma urinary antigen. A possible mold infection was defined as that occurring in patients with one host factor and at least one pulmonary imaging on CT scan.
bIFIs were defined according to the Mycoses Study Group Education and Research Consortium (MSG-ERC) and the European Confederation of Medical Mycology (ECMM) [38]. bIFI was defined to occur during exposure to an antifungal drug, including fungi outside the spectrum of activity of an antifungal. bIFI time point is the first clinical sign or symptom, mycological finding, or radiological feature attributable to it. bIFI time point begins when each antifungal reaches plasma steady state and finishes during the last dose interval upon drug discontinuation.
Risk factors for IFIs were considered and defined as follows: (a) neutropenia < 500 neutrophils/mm3 for >10 days, and profound and prolonged neutropenia < 100 neutrophils/mm3 for >14 days prior to the diagnosis of IFI; (b) high doses of corticosteroids, such as prednisone (or equivalent) at doses ≥ 20 mg/day for a period ≥ 2 weeks prior to IFI, and the use of biological agents and/or anti-lymphocyte therapies within three months prior to IFI; (c) recent chemotherapy, such as the cycle of immunosuppressant drugs within one month prior to the diagnosis of IFI; (d) T-cell depletion, such as antithymocyte globulin or alemtuzumab for conditioning regimen of allogeneic HCT; (e) graft-versus-host disease (GvHD) and grading consistent with consensus guidelines [39]; (f) cytomegalovirus infection or disease occurring within 15 days prior to the diagnosis of IFI; (g) iron overload as ferritin serum level > 2000 ng/mL [40]; and (h) no HEPA filter system in the isolation room for induction chemotherapy in acute leukemia.
The first-line antifungal treatment was selected by the investigator based on the suspicion or diagnosis of IFI according to published guidelines [12,13,14,15,25]. Continuation treatment with ISA was considered either as a step-down therapy, or when the first-line drug could not be prescribed.
Preemptive therapy was given to patients whose diagnosis was based on positive GM and/or pulmonary CT-scan imaging, while targeted therapy implied that the diagnosis was made by microscopic detection or culture of any mold or yeast in clinical samples.
Favorable response to treatment was defined as absence of fever, improved signs and symptoms of the initial infectious source, decrease in GM index, and/or improved results in CT-scan imaging. Partial remission: improved signs and symptoms and imaging, though without resolution. Stable disease: improved signs and symptoms, though with no changes in imaging. Cure: clinical and imaging resolution.
Unfavorable outcome was defined as the patient’s death during the follow-up. Attributable mortality was considered to be the patient’s death with no response to treatment and documented clinical, radiological, microbiological, or histological findings suggestive of active IFI.
2.3. Statistical Analysis
The study population was characterized by descriptive statistics. For continuous variables, centrality (median) and dispersion (IQR) measures were used according to the distribution of variables. Categorical variables were analyzed using absolute frequency and percentage. Groups were compared using the U Mann–Whitney test for continuous variables and the Fisher exact test or the chi-square test for categorical variables. For all tests, a 95% level of statistical significance was used. Analyses were performed with the SPSS (Statistics for Windows, Version 22.0, Armonk, NY, USA) software packages.
3. Results
3.1. Characteristics and Outcomes of Patients’ Cohort
A total of 163 patients (126 retrospective and 37 prospective) diagnosed with IFI were included. Acute myelogenous leukemia (AML) and acute lymphoblastic leukemia (ALL) were the most frequent underlying diseases (96, 58.9%), and were active in 125 (76.6%). Forty-nine (30.1%) had undergone HCT, with allogeneic being the most common type. Thirteen (43.3%) and 7 (23.3%) of allogeneic HCT developed acute and chronic GvHD, respectively. From the total cohort, 63 (38.6%) patients presented with oral mucositis, and 10 (6.1%) HCT patients developed acute or chronic GvHD affecting the gastrointestinal tract. One hundred and thirty-two (80.9%) patients were neutropenic at the onset of IFI. Of them, 115 (87.1%) presented profound and prolonged neutropenia, with a median duration of 27 days (IQR: 16–47). The cohort comprised patients with increased risk factors for IFIs, with a median of 2 (IQR: 2–3). Patients’ baseline characteristics and risk factors are outlined in Table 1 and Figure 1.

Table 1.
Baseline characteristics of patients diagnosed with IFIs.
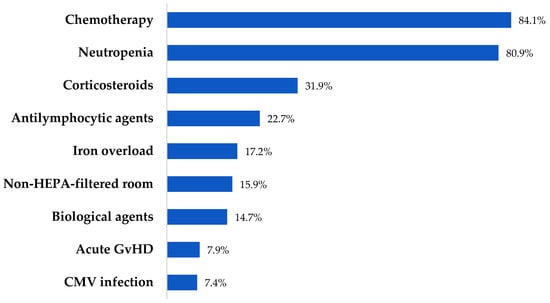
Figure 1.
Risk factors for IFIs.
Proven and probable IFIs were diagnosed in 66 (40.5%) patients and possible IFIs in 97 (59.5%). Ninety-two (56.4%) were bIFIs. The most common locations were lungs (147, 90.2%) and paranasal sinuses (23, 14.1%). Lung CT scan showed nodules in 89 (54.6%) and halo sign in 37 (22.7%). IFIs characteristics are depicted in Table 2.

Table 2.
IFIs classification, location and radiological characteristics.
ISA treatment was prescribed as preemptive therapy in 136 (83.4%) and as targeted therapy in 29 (17.8%) patients. It was used as first-line therapy in 73 (44.8%) of all the patients, and 153 (93.8%) received it as monotherapy. Ten (6.1%) patients received ISA in combination with L-AmB. The other antifungal drugs used as first-line therapy (90 patients) were L-AmB (65, 72.2%), VORI (13, 14.4%), POSA (6, 6.7%), LC-AmB (4, 4.4%), caspofungin (1, 1.1%), and FLUCO (1, 1.1%). ISA administration route was oral (77, 47.2%), intravenous (IV) followed by oral (57, 34.9%), IV (27, 16.6%), and oral followed by IV (2, 1.2%), with a median duration of 90 days (IQR: 59–113). Five patients undergoing treatment with medication that interacts with POSA and VORI (venetoclax 2, sirolimus 2 and ponatinib 1) were therefore administered ISA.
Seven (4.3%) patients developed related adverse effects (nausea 1, hepatobiliary abnormalities 6, rash 1, and shortened QTc 1), and only 1 (0.6%) had to discontinue ISA. One hundred and thirty (79.7%) patients achieved a favorable response at week 12 (cure 54.6%, partial remission 19.6%, and stable disease 5.5%). Overall mortality was 22.7%, and IFI-related mortality was 4.9%. Ninety-day overall mortality in patients with pulmonary vs. non-pulmonary locations was 23.2% vs. 18.4% (p = 0.53).
3.2. Characteristics and Outcomes of Patients with Probable and Proven IFIs
A total of 66 patients were diagnosed with proven and probable IFIs, and 35 (53%) of them were bIFIs. Both bIFIs and non-bIFIs patients had similar baseline characteristics regarding sex, Charlson score comorbidity index, underlying diseases, and disease status. Forty-eight (72.7%) patients had neutropenia, mostly profound and prolonged, with a median duration of 26 days (IQR: 16–44).
The most common primary antifungal prophylaxis administered to bIFIs patients was FLUCO (13, 37.1%), followed by POSA (10, 28.6%). Baseline characteristics and the antifungal prophylaxis prescribed are outlined in Table 3.

Table 3.
Baseline characteristics and primary antifungal prophylaxis of patients with proven and probable non-bIFIs and bIFIs.
Invasive aspergillosis and mucormycosis were the most frequent IFIs in both groups. We compared and contrasted IFI locations in non-bIFI and bIFI patients and found lungs in 26 (83.9%) vs. 27 (77.1%), p = 0.492, and paranasal sinuses in 7 (22.6%) vs. 8 (22.9%), p = 0.97, respectively. A few patients had other locations. The most common CT-scan findings were nodules in 32 (48.5%) patients, halo sign in 17 (25.8%), and a ground glass appearance in 20 (30.3%), with no differences between groups. IFIs were diagnosed by microscopic detection in 16 (24.2%) patients, culture in 29 (43.9%), GM test in 35 (53%), and histopathology in 17 (25.8%).
The etiology and methodology used for the diagnosis of proven and probable IFIs are described in Table 4.

Table 4.
Type of mycoses, etiology, diagnostic methods, and treatment of proven and probable non-bIFIs and bIFIs.
ISA was prescribed as monotherapy in 62 (93.9%) patients and as first-line treatment in 31 (46.9%). In the bIFI group, 15 patients (42.9%) received ISA as first-line treatment. Ten (66.7%) were under prophylaxis with FLUCO, 2 (13.3%) with VORI, 2 (13.3%) with L-AmB, and 1 (6.7%) with LC-AmB. As a continuation treatment, it was used in 20 patients (57.1%). The reasons for prescribing ISA were step-down therapy after L-AmB in 11 (55%), (10 had undergone POSA prophylaxis), and VORI in 1 (5%); related-adverse effects with L-AmB in 2 (10%) or VORI in 2 (10%); and combination treatment with L-AmB in 3 (15%), or caspofungin in 1 (5%). The median duration of treatment in bIFI vs. non-bIFI patients was 90 days (IQR: 62.5–119.5) vs. 85 days (IQR: 61.5–149), p = 0.908. Source control was performed in 18 (27.3%) patients, and mostly consisted of paranasal sinus endoscopic surgery (7 patients in each group). Treatment of proven and probable characteristics is described in Table 5.

Table 5.
Treatment of proven and probable non-bIFIs and bIFIs.
Regarding outcomes, a large number of patients achieved a favorable response, being higher in those with bIFI. Overall and IFI-related mortality in this group were 11.4% and 5.7%, respectively. Outcome variables are shown in Figure 2.
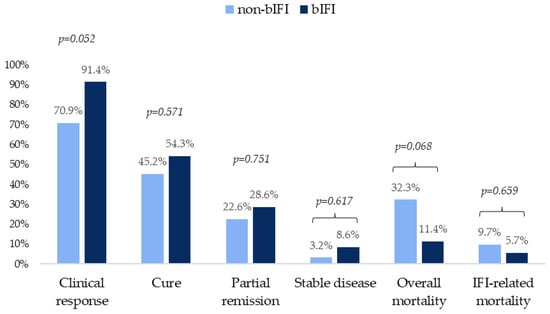
Figure 2.
Clinical outcomes at 12 weeks in patients with probable and proven non-bIFIs and bIFIs.
4. Discussion
The study assessed the effectiveness and safety of ISA for treating HD and HCT patients with IFIs. Patients with several risk factors for IFIs, most of them neutropenic, were included. Patients with proven and probable IFIs, either non-bIFIs or bIFIs, were analyzed separately. Aspergillosis and mucormycoses were the IFIs most commonly diagnosed, largely located in the lungs and paranasal sinuses. Almost half of the cases received ISA as a first-line treatment, mainly as monotherapy. Among bIFI patients, those who received ISA as a first-line therapy were mainly undergoing FLUCO or VORI prophylaxis. Half of the patients treated with ISA as a continuation therapy after L-AmB were receiving POSA prophylaxis. In a large proportion of patients with bIFIs, a clinical response was observed, with low overall and IFI-related 90-day mortality rates.
These are the major findings of the present study: first, in terms of IFI epidemiology, our cohort had similarities and differences with other real-life studies. In a multicenter study including centers from the USA, Europe, and Brazil, aspergillosis was the leading cause of IFI (79%), followed by fusariosis (8%) [41]. In contrast, in our cohort fusariosis only represents 1.5%. Second, approximately 50% of the patients had bIFI. Aspergillosis and mucormycoses were the most frequent molds in those with proven and probable IFI. In this respect, a multicenter study from Spain reported bIFI in 121 cases, with aspergillosis in 59% and mucormycosis in 7% being the most common among 94 patients with proven or probable bIFI [42]. Third, as reported in the SECURE trial and many real-life studies, most IFI locations were the lungs [21,26,27,29].
Fourth, bIFIs are currently and frequently a major concern in clinical practice among HD and HCT patients, as it is difficult to identify the type of fungus involved. The sensitivity of GM in BAL proved to be lower in patients that receive antifungal prophylaxis compared to those that do not: 52% vs. 81%. Likewise, the sensitivity of serum GM and culture in BAL is 31.3% and 18.8%. A combination of these methods can increase diagnostic efficacy [43,44]. Based on these findings, in our cohort bIFI could be diagnosed using several diagnostic methods. In this regard, the ECMM consensus status recommends the use of all available methods to diagnose bIFI [45]. Fifth, almost half of the patients received ISA as a first-line treatment, mostly as monotherapy. This differs from the literature, which recommends using L-AmB as a first-line therapy [19,20,46].
Sixth, 10 patients under continuation ISA therapy after L-AmB were receiving primary prophylaxis with POSA. Interestingly, ISA and POSA have the same antifungal spectrum, and both proved effective for aspergillosis and mucormycosis [21,22,47]. Antifungal cross-resistance between these two azoles does not necessarily occur. Cross-resistance between POSA (used for prophylaxis) and ISA (used for treatment) is complex and not fully deterministic. While a theoretical risk exists due to their shared drug class, several factors mitigate absolute cross-resistance. Thus, ISA could be considered a viable therapeutic option even after POSA prophylaxis failure. In this sense, the binding affinity of ISA to the fungal Cyp51A enzyme target is different from that of POSA. The environmentally driven tandem repeats (TR34/L98H and TR46/Y121F/T289A) are the most relevant resistance mechanisms [48]. These mutations often confer panazole resistance to both POSA and ISA. However, their prevalence is not yet universal, and breakthrough infections in patients on prophylaxis can still be caused by wild-type or susceptible isolates with other resistance mechanisms. Key studies support this lack of absolute cross-resistance. The phase 3 SECURE trial and subsequent analyses have documented successful outcomes with ISA in patients who had received prior azole prophylaxis, including POSA [21].
Moreover, one of the several reasons for developing bIFI is that the antifungal agent does not achieve enough serum levels. This is frequently observed in VORI, but has also been described with POSA, even in tablet formulations. A study in patients with acute leukemia and HCT receiving POSA prophylaxis found that 18% of them had subtherapeutic serum levels (<700 ng/mL). Factors such as having diarrhea, receiving proton pump inhibitors, and weighing more than 90 kg were associated with subtherapeutic serum levels, with the first two being common in HD and HCT patients [49]. Nonetheless, as POSA serum levels were not available, we cannot state that this could occur in our patients. On the other hand, ISA levels are adequate, even in patients with mucositis and gastrointestinal GvHD, as could often be the case with many of our patients [50,51]. Seventh, the cohort had a low rate of related adverse effects and drug discontinuation, which is consistent with the literature [29,30]. Given its safety, ISA is suitable for treating IFIs in severely ill patients.
Eighth, our patients with bIFI had higher clinical response and lower mortality rate than those in other real-life studies, which report a mortality rate of 35% [17]. Apart from the effectiveness of ISA, 62.9% of our patients received preemptive therapy, which means that they were treated early. Several studies have demonstrated that this strategy is consistent with higher survival rates [52,53,54]. We also consider that other factors could have contributed to the high clinical response and low mortality rate, particularly in patients with bIFI. They were younger than non-bIFI patients and had a higher rate of underlying disease in complete or partial remission. Moreover, since all the cohorts were diagnosed with IFI and were followed up by the ID physicians participating in the study, we assume that the diagnostic and therapeutic approach has been timely and appropriate.
Our study has some limitations that should be considered. First, data on the azoles serum levels or susceptibility testing were not available. Patients’ good outcomes could only be partly explained by these factors. However, there are no clinical susceptibility cut-off values for molds, except for Aspergillus fumigatus complex. In addition, the association between exposure to and efficacy of ISA treatment has not been proven [55]. Second, the sample size of patients with bIFI was small. Therefore, a larger population is required to evaluate the outcome variables. Third, several patients were retrospectively included, which may have led to some biased results. Notwithstanding that, all those patients were treated and prospectively followed by the Infectious Diseases physician conducting the study and missing data were not allowed.
The strengths of our study rely on its multicenter design. It was carried out in healthcare facilities specialized in the treatment of patients with HD and HCT. In addition, all of them were evaluated, treated, and followed up by the investigators. Therefore, our results accurately reveal IFIs complex scenario. Moreover, our study comprised the largest cohort from Latin America.
In conclusion, the study data evidenced ISA effectiveness and safety for the treatment of HD and HCT patients with IFI. It further showed a suitable option for treating patients with bIFIs. However, larger studies should be conducted to confirm this finding.
Author Contributions
Conceptualization, F.H., D.T. and J.A.; methodology, F.H., D.T. and J.A.; software, D.T. and J.A.; formal analysis, D.T.; investigation, G.M., N.M., R.J., A.M., M.C. (Myrna Cabral), M.A., N.G.A., J.B., C.S., J.B., M.L.P., H.P., C.N., M.C. (Maximiliano Castro), F.P., S.G.R., J.D., A.R.P., V.F. and R.G.; data curation, D.T. and J.A.; writing: original draft preparation, F.H.; writing: review and editing, D.T. and J.A.; funding acquisition, F.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a research grant from Knight Therapeutics. The Company has not participated in the study design, sample collection, analysis data interpretation, or in the decision to submit the manuscript for publication. Only the authors had full access to the study data files.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by CEMIC Ethics Committee on 27 November 2023 (Approval identification number 1751) and the ethics committees from the different participating institutions.
Informed Consent Statement
Patient consent was waived by the Ethics Committees according to Data Protection Law 25326, Section 7. 2.
Data Availability Statement
Data are available upon request. Contact the corresponding author.
Acknowledgments
We thank Valeria Melia, scientific translator at CEMIC Research Unit, for English edition of the manuscript.
Conflicts of Interest
F.H. has participated in advisory boards and/or received speaker honoraria and grants from Gilead, Knight Therapeutics, Merck, Sharp & Dohme (MSD), SteinCares, Biomerieux, Rochem Biocare, TEVA, TAKEDA, and Pfizer. D.T. has participated in advisory boards and/or received speaker honoraria from Gilead, Knight Therapeutics, MSD, Pfizer and GlaxoSmithKline. G.M. has participated in advisory boards and/or received speaker honoraria from Knight Therapeutics. R.J. has participated in advisory boards and/or received speaker honoraria from Knight Therapeutics and Pfizer. J.A. has participated in advisory boards and/or received speaker honoraria from Knight Therapeutics, Gilead and Pfizer. All other authors report no potential conflicts of interest.
References
- Wasylyshyn, A.I.; Linder, K.A.; Kauffman, C.A.; Richards, B.J.; Maurer, S.M.; Sheffield, V.M.; Benitez Colon, L.; Miceli, M.H. Invasive Fungal Disease in Patients with Newly Diagnosed Acute Myeloid Leukemia. J. Fungi 2021, 7, 761. [Google Scholar] [CrossRef]
- Alkan, A.; Buyukasik, Y.; Uzun, O.; Demir, A.U.; Coplu, L. Invasive fungal infections in patients with acute leukemia: A retrospective cohort study at a tertiary-care hospital. Medicine 2024, 103, 39959. [Google Scholar] [CrossRef]
- Girmenia, C.; Raiola, A.M.; Piciocchi, A.; Algarotti, A.; Stanzani, M.; Cudillo, L.; Pecoraro, C.; Guidi, S.; Iori, A.P.; Montante, B.; et al. Incidence and outcome of invasive fungal diseases after allogeneic stem cell transplantation: A prospective study of the Gruppo Italiano Trapianto Midollo Osseo (GITMO). Biol. Blood Marrow Transplant. 2014, 20, 872–880. [Google Scholar] [CrossRef]
- Menzin, J.; Meyers, J.L.; Friedman, M.; Korn, J.R.; Perfect, J.R.; Langston, A.A.; Danna, R.P.; Papadopoulos, G. The economic costs to United States hospitals of invasive fungal infections in transplant patients. Am. J. Infect. Control 2011, 39, 15–20. [Google Scholar] [CrossRef]
- Busca, A.; Passera, R.; Maffini, E.; Festuccia, M.; Brunello, L.; Dellacasa, C.M.; Aydin, S.; Frairia, C.; Manetta, S.; Butera, S.; et al. Hematopoietic cell transplantation comorbidity index and risk of developing invasive fungal infections after allografting. Bone Marrow Transplant. 2018, 53, 1304–1310. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Marr, K.A.; Park, B.J.; Alexander, B.D.; Anaissie, E.J.; Walsh, T.J.; Ito, J.; Andes, D.R.; Baddley, J.W.; Brown, J.M.; et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: Overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin. Infect. Dis. 2010, 50, 1091–1100. [Google Scholar] [CrossRef]
- Neofytos, D.; Horn, D.; Anaissie, E.; Steinbach, W.; Olyaei, A.; Fishman, J.; Pfaller, M.; Chang, C.; Webster, K.; Marr, K. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: Analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin. Infect. Dis. 2009, 48, 265–273. [Google Scholar] [CrossRef]
- Roth, R.S.; Masouridi-Levrat, S.; Chalandon, Y.; Mamez, A.C.; Giannotti, F.; Riat, A.; Fischer, A.; Poncet, A.; Glampedakis, E.; Van Delden, C.; et al. Invasive Mold Infections in Allogeneic Hematopoietic Cell Transplant Recipients in 2020: Have We Made Enough Progress? Open Forum Infect. Dis. 2021, 9, ofab596. [Google Scholar] [CrossRef]
- Robenshtok, E.; Gafter-Gvili, A.; Goldberg, E.; Weinberger, M.; Yeshurun, M.; Leibovici, L.; Paul, M. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: Systematic review and meta-analysis. J. Clin. Oncol. 2007, 25, 5471–5489. [Google Scholar] [CrossRef]
- Zeng, H.; Wu, Z.; Yu, B.; Wang, B.; Wu, C.; Wu, J.; Lai, J.; Gao, X.; Chen, J. Network meta-analysis of triazole, polyene, and echinocandin antifungal agents in invasive fungal infection prophylaxis in patients with hematological malignancies. BMC Cancer 2021, 21, 404. [Google Scholar] [CrossRef] [PubMed]
- Young, J.H.; Andes, D.R.; Ardura, M.I.; Arrieta, A.; Bow, E.J.; Chandrasekar, P.H.; Chen, S.C.A.; Hammond, S.P.; Husain, S.; Koo, S.; et al. Modeling Invasive Aspergillosis Risk for the Application of Prophylaxis Strategies. Open Forum Infect. Dis. 2024, 11, ofae082. [Google Scholar] [CrossRef]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24, 1–38. [Google Scholar] [CrossRef]
- Dadwal, S.S.; Hohl, T.M.; Fisher, C.E.; Boeckh, M.; Papanicolaou, G.; Carpenter, P.A.; Fisher, B.T.; Slavin, M.A.; Kontoyiannis, D.P. American Society of Transplantation and Cellular Therapy Series, 2: Management and Prevention of Aspergillosis in Hematopoietic Cell Transplantation Recipients. Transplant. Cell. Ther. 2021, 27, 201–211. [Google Scholar] [CrossRef]
- Stemler, J.; Mellinghoff, S.C.; Khodamoradi, Y.; Sprute, R.; Classen, A.Y.; Zapke, S.E.; Hoenigl, M.; Krause, R.; Schmidt-Hieber, M.; Heinz, W.J.; et al. Primary prophylaxis of invasive fungal diseases in patients with haematological malignancies: 2022 update of the recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society for Haematology and Medical Oncology (DGHO). J. Antimicrob. Chemother. 2023, 78, 1813–1826. [Google Scholar] [CrossRef]
- Patterson, T.F.; Thompson, G.R., 3rd; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef]
- Pagano, L.; Maschmeyer, G.; Lamoth, F.; Blennow, O.; Xhaard, A.; Spadea, M.; Busca, A.; Cordonnier, C.; Maertens, J. Primary antifungal prophylaxis in hematological malignancies. Updated clinical practice guidelines by the European Conference on Infections in Leukemia (ECIL). Leukemia 2025, 39, 1547–1557. [Google Scholar] [CrossRef]
- Boutin, C.A.; Durocher, F.; Beauchemin, S.; Ziegler, D.; Abou Chakra, C.N.; Dufresne, S.F. Breakthrough Invasive Fungal Infections in Patients With High-Risk Hematological Disorders Receiving Voriconazole and Posaconazole Prophylaxis: A Systematic Review. Clin. Infect. Dis. 2024, 79, 151–160. [Google Scholar] [CrossRef]
- Ishida, K.; Haraguchi, M.; Kimura, M.; Araoka, H.; Natori, A.; Reynolds, J.M.; Raja, M.; Natori, Y. Incidence of Breakthrough Fungal Infections in Patients With Isavuconazole Prophylaxis: A Systematic Review and Meta-analysis. Open Forum Infect. Dis. 2025, 12, ofaf163. [Google Scholar] [CrossRef] [PubMed]
- Lionakis, M.S.; Lewis, R.E.; Kontoyiannis, D.P. Breakthrough Invasive Mold Infections in the Hematology Patient: Current Concepts and Future Directions. Clin. Infect. Dis. 2018, 67, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Girmenia, C.; Busca, A.; Candoni, A.; Cesaro, S.; Luppi, M.; Nosari, A.M.; Pagano, L.; Rossi, G.; Venditti, A.; Aversa, F. Breakthrough invasive fungal diseases in acute myeloid leukemia patients receiving mould active triazole primary prophylaxis after intensive chemotherapy: An Italian consensus agreement on definitions and management. Med. Mycol. 2019, 57, S127–S137. [Google Scholar] [CrossRef]
- Maertens, J.A.; Raad, I.I.; Marr, K.A.; Patterson, T.F.; Kontoyiannis, D.P.; Cornely, O.A.; Bow, E.J.; Rahav, G.; Neofytos, D.; Aoun, M.; et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): A phase 3, randomised-controlled, non-inferiority trial. Lancet 2016, 387, 760–769. [Google Scholar] [CrossRef]
- Marty, F.M.; Ostrosky-Zeichner, L.; Cornely, O.A.; Mullane, K.M.; Perfect, J.R.; Thompson, G.R., 3rd; Alangaden, G.J.; Brown, J.M.; Fredricks, D.N.; Heinz, W.J.; et al. Isavuconazole treatment for mucormycosis: A single-arm open-label trial and case-control analysis. Lancet Infect. Dis. 2016, 16, 828–837. [Google Scholar] [CrossRef]
- EMA. Cresemba (Isavuconazole). In EPAR Summary for the Public; European Medicines Agency: Amsterdam, The Netherlands, 2015; Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/cresemba (accessed on 22 August 2025).
- FDA. FDA Approves Cresemba (Isavuconazonium sulfate) for Invasive Aspergillosis and Invasive Mucormycosis; Drugs@FDA: FDA-Approved Drugs. Application Number: 207500; FDA: Silver Spring, MD, USA, 2015. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=207500 (accessed on 22 August 2025).
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Hassouna, H.; Athans, V.; Brizendine, K.D. Real-world use-Isavuconazole at a large academic medical center. Mycoses 2019, 62, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Gow-Lee, V.; Abu Saleh, O.M.; Harris, C.E.; Gile, J.J.; Akhiyat, N.; Chesdachai, S. Outcomes of Invasive Fungal Infections Treated with Isavuconazole: A Retrospective Review. Pathogens 2024, 13, 886. [Google Scholar] [CrossRef] [PubMed]
- Gunathilaka, S.S.; Keragala, R.K.; Gunathilaka, K.M.; Wickramage, S.; Bandara, S.R.; Senevirathne, I.S.; Jayaweera, A.S. Use of isavuconazole in mucormycosis: A systematic review. BMC Infect. Dis. 2025, 25, 25. [Google Scholar] [CrossRef]
- Dagher, H.; Hachem, R.; Chaftari, A.M.; Jiang, Y.; Ali, S.; Deeba, R.; Shah, S.; Raad, I. Real-World Use of Isavuconazole as Primary Therapy for Invasive Fungal Infections in High-Risk Patients with Hematologic Malignancy or Stem Cell Transplant. J. Fungi 2022, 8, 74. [Google Scholar] [CrossRef]
- Weng, J.; Du, X.; Fang, B.; Li, Y.; Huang, L.; Ju, Y. Efficacy and safety of isavuconazole versus voriconazole for the treatment of invasive fungal infections: A meta-analysis with trial sequential analysis. BMC Infect. Dis. 2025, 25, 230. [Google Scholar] [CrossRef]
- Ellsworth, M.; Ostrosky-Zeichner, L. Isavuconazole: Mechanism of Action, Clinical Efficacy, and Resistance. J. Fungi 2020, 6, 324. [Google Scholar] [CrossRef]
- Lewis, J.S., 2nd; Wiederhold, N.P.; Hakki, M.; Thompson, G.R., 3rd. New Perspectives on Antimicrobial Agents: Isavuconazole. Antimicrob. Agents Chemother. 2022, 66, 0017722. [Google Scholar] [CrossRef]
- Andes, D.; Kovanda, L.; Desai, A.; Kitt, T.; Zhao, M.; Walsh, T.J. Isavuconazole Concentration in Real-World Practice: Consistency with Results from Clinical Trials. Antimicrob. Agents Chemother. 2018, 62, 10–1128. [Google Scholar] [CrossRef]
- Risum, M.; Vestergaard, M.B.; Weinreich, U.M.; Helleberg, M.; Vissing, N.H.; Jørgensen, R. Therapeutic Drug Monitoring of Isavuconazole: Serum Concentration Variability and Success Rates for Reaching Target in Comparison with Voriconazole. Antibiotics 2021, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.; Niazi-Ali, S.; McIvor, A.; Kanj, S.S.; Maertens, J.; Bassetti, M.; Levine, D.; Groll, A.H.; Denning, D.W. Triazole antifungal drug interactions-practical considerations for excellent prescribing. J. Antimicrob. Chemother. 2024, 79, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- DiPippo, A.J.; Rausch, C.R.; Kontoyiannis, D.P. Tolerability of isavuconazole after posaconazole toxicity in leukaemia patients. Mycoses 2019, 62, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef]
- Cornely, O.A.; Hoenigl, M.; Lass-Flörl, C.; Chen, S.C.; Kontoyiannis, D.P.; Morrissey, C.O.; Thompson, G.R., 3rd; Mycoses Study Group Education and Research Consortium (MSG-ERC) and the European Confederation of Medical Mycology (ECMM). Defining breakthrough invasive fungal infection-Position paper of the mycoses study group education and research consortium and the European Confederation of Medical Mycology. Mycoses 2019, 62, 716–729. [Google Scholar] [CrossRef]
- Przepiorka, D.; Weisdorf, D.; Martin, P.; Klingemann, H.G.; Beatty, P.; Hows, J.; Thomas, E.D. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995, 15, 825–828. [Google Scholar]
- Pagano, L.; Busca, A.; Candoni, A.; Cattaneo, C.; Cesaro, S.; Fanci, R.; Nadali, G.; Potenza, L.; Russo, D.; Tumbarello, M.; et al. Risk stratification for invasive fungal infections in patients with hematological malignancies: SEIFEM recommendations. Blood Rev. 2017, 31, 17–29. [Google Scholar] [CrossRef]
- Batista, M.V.; Ussetti, M.P.; Jiang, Y.; Neofytos, D.; Cortez, A.C.; Feriani, D.; Schmidt-Filho, J.; França-Silva, I.L.A.; Raad, I.; Hachem, R. Comparing the Real-World Use of Isavuconazole to Other Anti-Fungal Therapy for Invasive Fungal Infections in Patients with and without Underlying Disparities: A Multi-Center Retrospective Study. J. Fungi 2023, 9, 166. [Google Scholar] [CrossRef]
- Puerta-Alcalde, P.; Monzó-Gallo, P.; Aguilar-Guisado, M.; Ramos, J.C.; Laporte-Amargós, J.; Machado, M.; Martin-Davila, P.; Franch-Sarto, M.; Sánchez-Romero, I.; Badiola, J.; et al. Breakthrough invasive fungal infection among patients with haematologic malignancies: A national, prospective, and multicentre study. J. Infect. 2023, 87, 46–53. [Google Scholar] [CrossRef]
- Eigl, S.; Prattes, J.; Reinwald, M.; Thornton, C.R.; Reischies, F.; Sess, B.; Neumeister, P.; Zollner-Schwetz, I.; Raggam, R.B.; Flick, H.; et al. Influence of mould-active antifungal treatment on the performance of the Aspergillus-specific bronchoalveolar lavage fluid lateral-flow device test. Int. J. Antimicrob. Agents 2015, 46, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Eigl, S.; Hoenigl, M.; Spiess, B.; Heldt, S.; Prattes, J.; Neumeister, P.; Wolfler, A.; Rabensteiner, J.; Prueller, F.; Krause, R.; et al. Galactomannan testing and Aspergillus PCR in same-day bronchoalveolar lavage and blood samples for diagnosis of invasive aspergillosis. Med. Mycol. 2017, 55, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Jenks, J.D.; Gangneux, J.P.; Schwartz, I.S.; Alastruey-Izquierdo, A.; Lagrou, K.; Thompson Iii, G.R.; Lass-Flörl, C.; Hoenigl, M. European Confederation of Medical Mycology (ECMM) Council Investigators. Diagnosis of Breakthrough Fungal Infections in the Clinical Mycology Laboratory: An ECMM Consensus Statement. J. Fungi 2020, 6, 216. [Google Scholar] [CrossRef] [PubMed]
- Ruhnke, M.; Cornely, O.A.; Schmidt-Hieber, M.; Alakel, N.; Boell, B.; Buchheidt, D.; Christopeit, M.; Hasenkamp, J.; Heinz, W.J.; Hentrich, M.; et al. Treatment of invasive fungal diseases in cancer patients-Revised 2019 Recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Oncology (DGHO). Mycoses 2020, 63, 653–682. [Google Scholar] [CrossRef]
- Maertens, J.A.; Rahav, G.; Lee, D.G.; Ponce-de-León, A.; Ramírez Sánchez, I.C.; Klimko, N.; Sonet, A.; Haider, S.; Diego Vélez, J.; Raad, I.; et al. study investigators. Posaconazole versus voriconazole for primary treatment of invasive aspergillosis: A phase 3, randomised, controlled, non-inferiority trial. Lancet 2021, 397, 499–509. [Google Scholar] [CrossRef]
- Verweij, P.E.; Chowdhary, A.; Melchers, W.J.; Meis, J.F. Azole Resistance in Aspergillus fumigatus: Can We Retain the Clinical Use of Mold-Active Antifungal Azoles? Clin. Infect. Dis. 2016, 62, 362–368. [Google Scholar] [CrossRef]
- Tang, L.A.; Marini, B.L.; Benitez, L.; Nagel, J.L.; Miceli, M.; Berglund, C.; Perissinotti, A.J. Risk factors for subtherapeutic levels of posaconazole tablet. J. Antimicrob. Chemother. 2017, 72, 2902–2905. [Google Scholar] [CrossRef]
- Kovanda, L.L.; Marty, F.M.; Maertens, J.; Desai, A.V.; Lademacher, C.; Engelhardt, M.; Lu, Q.; Hope, W.W. Impact of Mucositis on Absorption and Systemic Drug Exposure of Isavuconazole. Antimicrob. Agents Chemother. 2017, 61, e00101-17. [Google Scholar] [CrossRef]
- Stern, A.; Su, Y.; Lee, Y.J.; Seo, S.; Shaffer, B.; Tamari, R.; Gyurkocza, B.; Barker, J.; Bogler, Y.; Giralt, S.; et al. A Single-Center, Open-Label Trial of Isavuconazole Prophylaxis against Invasive Fungal Infection in Patients Undergoing Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2020, 26, 1195–1202. [Google Scholar] [CrossRef]
- Greene, R.E.; Schlamm, H.T.; Oestmann, J.W.; Stark, P.; Durand, C.; Lortholary, O.; Wingard, J.R.; Herbrecht, R.; Ribaud, P.; Patterson, T.F.; et al. Imaging findings in acute invasive pulmonary aspergillosis: Clinical significance of the halo sign. Clin. Infect. Dis. 2007, 44, 373–379. [Google Scholar] [CrossRef]
- Aguado, J.M.; Vázquez, L.; Fernández-Ruiz, M.; Villaescusa, T.; Ruiz-Camps, I.; Barba, P.; Silva, J.T.; Batlle, M.; Solano, C.; Gallardo, D.; et al. Serum galactomannan versus a combination of galactomannan and polymerase chain reaction-based Aspergillus DNA detection for early therapy of invasive aspergillosis in high-risk hematological patients: A randomized controlled trial. Clin. Infect. Dis. 2015, 60, 405–414. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Azie, N.; Franks, B.; Horn, D.L. Prospective antifungal therapy (PATH) alliance(®): Focus on mucormycosis. Mycoses 2014, 57, 240–246. [Google Scholar] [CrossRef]
- Desai, A.V.; Kovanda, L.L.; Hope, W.W.; Andes, D.; Mouton, J.W.; Kowalski, D.L.; Townsend, R.W.; Mujais, S.; Bonate, P.L. Exposure-Response Relationships for Isavuconazole in Patients with Invasive Aspergillosis and Other Filamentous Fungi. Antimicrob. Agents Chemother. 2017, 61, e01034-17. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).