Predicting the Structural Effects of CUG Codon Translation on Uncharacterized Proteins in Candida albicans
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Proteins with Insignificant Ser-Leu Exchange Effect
3.2. Proteins with Predicted Serine Phosphorylation
3.3. Proteins Affected by Ser-Leu Exchange
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Amino Acid |
| ORF | Open Reading Frame |
| P | Phosphorylation |
References
- Chen, W.; Geng, Y.; Zhang, B.; Yan, Y.; Zhao, F.; Miao, M. Stop or Not: Genome-Wide Profiling of Reassigned Stop Codons in Ciliates. Mol. Biol. Evol. 2023, 40, msad064. [Google Scholar] [CrossRef]
- Yamao, F.; Muto, A.; Kawauchi, Y.; Iwami, M.; Iwagami, S.; Azumi, Y.; Osawa, S. UGA Is Read as Tryptophan in Mycoplasma Capricolum. Proc. Natl. Acad. Sci. USA 1985, 82, 2306–2309. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.D.; Landweber, L.F.; Yarus, M. How Mitochondria Redefine the Code. J. Mol. Evol. 2001, 53, 299–313. [Google Scholar] [CrossRef]
- Miranda, I.; Silva, R.; Santos, M.A.S. Evolution of the Genetic Code in Yeasts. Yeast 2006, 23, 203–213. [Google Scholar] [CrossRef]
- Záhonová, K.; Kostygov, A.Y.; Ševčíková, T.; Yurchenko, V.; Eliáš, M. An Unprecedented Non-Canonical Nuclear Genetic Code with All Three Termination Codons Reassigned as Sense Codons. Curr. Biol. 2016, 26, 2364–2369. [Google Scholar] [CrossRef] [PubMed]
- Swart, E.C.; Serra, V.; Petroni, G.; Nowacki, M. Genetic Codes with No Dedicated Stop Codon: Context-Dependent Translation Termination. Cell 2016, 166, 691–702. [Google Scholar] [CrossRef]
- Kachale, A.; Pavlíková, Z.; Nenarokova, A.; Roithová, A.; Durante, I.M.; Miletínová, P.; Záhonová, K.; Nenarokov, S.; Votýpka, J.; Horáková, E.; et al. Short tRNA Anticodon Stem and Mutant eRF1 Allow Stop Codon Reassignment. Nature 2023, 613, 751–758. [Google Scholar] [CrossRef]
- Kollmar, M.; Mühlhausen, S. Nuclear Codon Reassignments in the Genomics Era and Mechanisms behind Their Evolution. BioEssays 2017, 39, 1600221. [Google Scholar] [CrossRef]
- Ohama, T.; Suzuki, T.; Mori, M.; Osawa, S.; Ueda, T.; Watanabe, K.; Nakase, T. Non-Universal Decoding of the Leucine Codon CUG in Several Candida Species. Nucleic Acids Res. 1993, 21, 4039–4045. [Google Scholar] [CrossRef]
- Ueda, T.; Suzuki, T.; Tokogawa, T.; Nishikawa, K.; Watanabe, K. Unique Structure of New Serine tRNAs Responsible for Decoding Leucine Codon CUG in Various Candida Species and Their Putative Ancestral tRNA Genes. Biochimie 1994, 76, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Mühlhausen, S.; Findeisen, P.; Plessmann, U.; Urlaub, H.; Kollmar, M. A Novel Nuclear Genetic Code Alteration in Yeasts and the Evolution of Codon Reassignment in Eukaryotes. Genome Res. 2016, 26, 945–955. [Google Scholar] [CrossRef]
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic Agriculture Wastes as Biomass Feedstocks for Second-Generation Bioethanol Production: Concepts and Recent Developments. 3 Biotech. 2015, 5, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Krassowski, T.; Coughlan, A.Y.; Shen, X.-X.; Zhou, X.; Kominek, J.; Opulente, D.A.; Riley, R.; Grigoriev, I.V.; Maheshwari, N.; Shields, D.C.; et al. Evolutionary Instability of CUG-Leu in the Genetic Code of Budding Yeasts. Nat. Commun. 2018, 9, 1887. [Google Scholar] [CrossRef]
- Suzuki, T. The ‘polysemous’ Codon_a Codon with Multiple Amino Acid Assignment Caused by Dual Specificity of tRNA Identity. EMBO J. 1997, 16, 1122–1134. [Google Scholar] [CrossRef]
- Knight, R.D.; Freeland, S.J.; Landweber, L.F. Rewiring the Keyboard: Evolvability of the Genetic Code. Nat. Rev. Genet. 2001, 2, 49–58. [Google Scholar] [CrossRef]
- Massey, S.E.; Moura, G.; Beltrão, P.; Almeida, R.; Garey, J.R.; Tuite, M.F.; Santos, M.A.S. Comparative Evolutionary Genomics Unveils the Molecular Mechanism of Reassignment of the CTG Codon in Candida spp. Genome Res. 2003, 13, 544–557. [Google Scholar] [CrossRef]
- Butler, G.; Rasmussen, M.D.; Lin, M.F.; Santos, M.A.S.; Sakthikumar, S.; Munro, C.A.; Rheinbay, E.; Grabherr, M.; Forche, A.; Reedy, J.L.; et al. Evolution of Pathogenicity and Sexual Reproduction in Eight Candida Genomes. Nature 2009, 459, 657–662. [Google Scholar] [CrossRef]
- Santos, M.A.; Perreau, V.M.; Tuite, M.F. Transfer RNA Structural Change Is a Key Element in the Reassignment of the CUG Codon in Candida albicans. EMBO J. 1996, 15, 5060–5068. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.S.; Cheesman, C.; Costa, V.; Moradas-Ferreira, P.; Tuite, M.F. Selective Advantages Created by Codon Ambiguity Allowed for the Evolution of an Alternative Genetic Code in Candida spp. Mol. Microbiol. 1999, 31, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, A.R.; Simões, J.; Lee, W.; Rung, J.; Weil, T.; Gut, I.G.; Gut, M.; Bayés, M.; Rizzetto, L.; Cavalieri, D.; et al. Reversion of a Fungal Genetic Code Alteration Links Proteome Instability with Genomic and Phenotypic Diversification. Proc. Natl. Acad. Sci. USA 2013, 110, 11079–11084. [Google Scholar] [CrossRef]
- Brown, A.J.P.; Budge, S.; Kaloriti, D.; Tillmann, A.; Jacobsen, M.D.; Yin, Z.; Ene, I.V.; Bohovych, I.; Sandai, D.; Kastora, S.; et al. Stress Adaptation in a Pathogenic Fungus. J. Exp. Biol. 2014, 217, 144–155. [Google Scholar] [CrossRef]
- Katsipoulaki, M.; Stappers, M.H.T.; Malavia-Jones, D.; Brunke, S.; Hube, B.; Gow, N.A.R. Candida albicans and Candida glabrata: Global Priority Pathogens. Microbiol. Mol. Biol. Rev. 2024, 88, e00021-23. [Google Scholar] [CrossRef]
- Rai, L.S.; Wijlick, L.V.; Bougnoux, M.; Bachellier-Bassi, S.; d’Enfert, C. Regulators of Commensal and Pathogenic Life-styles of an Opportunistic Fungus—Candida albicans. Yeast 2021, 38, 243–250. [Google Scholar] [CrossRef]
- Lass-Flörl, C.; Steixner, S. The Changing Epidemiology of Fungal Infections. Mol. Aspects Med. 2023, 94, 101215. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive Candidiasis. Nat. Rev. Dis. Primer 2018, 4, 18026. [Google Scholar] [CrossRef]
- Wall, G.; Lopez-Ribot, J.L. Current Antimycotics, New Prospects, and Future Approaches to Antifungal Therapy. Antibiotics 2020, 9, 445. [Google Scholar] [CrossRef]
- Segal, E.S.; Gritsenko, V.; Levitan, A.; Yadav, B.; Dror, N.; Steenwyk, J.L.; Silberberg, Y.; Mielich, K.; Rokas, A.; Gow, N.A.R.; et al. Gene Essentiality Analyzed by In Vivo Transposon Mutagenesis and Machine Learning in a Stable Haploid Isolate of Candida albicans. mBio 2018, 9, e02048-18. [Google Scholar] [CrossRef] [PubMed]
- Tautz, D.; Domazet-Lošo, T. The Evolutionary Origin of Orphan Genes. Nat. Rev. Genet. 2011, 12, 692–702. [Google Scholar] [CrossRef]
- Klausen, M.S.; Jespersen, M.C.; Nielsen, H.; Jensen, K.K.; Jurtz, V.I.; Sønderby, C.K.; Sommer, M.O.A.; Winther, O.; Nielsen, M.; Petersen, B.; et al. NetSurfP-2.0: Improved Prediction of Protein Structural Features by Integrated Deep Learning. Proteins Struct. Funct. Bioinforma. 2019, 87, 520–527. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly Accurate Protein Structure Prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Bisong, E. Google Colaboratory. In Building Machine Learning and Deep Learning Models on Google Cloud Platform; Apress: Berkeley, CA, USA, 2019; pp. 59–64. ISBN 978-1-4842-4469-2. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure Visualization for Researchers, Educators, and Developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]
- Chaillot, J.; Cook, M.A.; Corbeil, J.; Sellam, A. Genome-Wide Screen for Haploinsufficient Cell Size Genes in the Opportunistic Yeast Candida albicans. G3 Genes|Genomes|Genetics 2017, 7, 355–360. [Google Scholar] [CrossRef]
- Sellam, A.; Hogues, H.; Askew, C.; Tebbji, F.; Van Het Hoog, M.; Lavoie, H.; Kumamoto, C.A.; Whiteway, M.; Nantel, A. Experimental Annotation of the Human Pathogen Candida albicans Coding and Noncoding Transcribed Regions Using High-Resolution Tiling Arrays. Genome Biol. 2010, 11, R71. [Google Scholar] [CrossRef]
- Goodwin, T.J.D.; Ormandy, J.E.; Poulter, R.T.M. L1-like Non-LTR Retrotransposons in the Yeast Candida albicans. Curr. Genet. 2001, 39, 83–91. [Google Scholar] [CrossRef]
- Nobile, C.J.; Fox, E.P.; Nett, J.E.; Sorrells, T.R.; Mitrovich, Q.M.; Hernday, A.D.; Tuch, B.B.; Andes, D.R.; Johnson, A.D. A Recently Evolved Transcriptional Network Controls Biofilm Development in Candida albicans. Cell 2012, 148, 126–138. [Google Scholar] [CrossRef]
- Sárkány, Z.; Silva, A.; Pereira, P.J.B.; Macedo-Ribeiro, S. Ser or Leu: Structural Snapshots of Mistranslation in Candida albicans. Front. Mol. Biosci. 2014, 1, 27. [Google Scholar] [CrossRef]
- Feketová, Z.; Mašek, T.; Vopálenský, V.; Pospíšek, M. Ambiguous Decoding of the CUG Codon Alters the Functionality of the Candida albicans Translation Initiation Factor 4E: Candida albicans eIF4E. FEMS Yeast Res. 2010, 10, 558–569. [Google Scholar] [CrossRef][Green Version]
- Cutfield, J.F.; Sullivan, P.A.; Cutfield, S.M. Minor Structural Consequences of Alternative CUG Codon Usage (Ser for Leu) in Candida albicans Exoglucanase. Protein Eng. Des. Sel. 2000, 13, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Dostál, J.; Brynda, J.; Hrušková-Heidingsfeldová, O.; Sieglová, I.; Pichová, I.; Řezáčová, P. The Crystal Structure of the Secreted Aspartic Protease 1 from Candida parapsilosis in Complex with Pepstatin A. J. Struct. Biol. 2009, 167, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Botova, M.; Camacho-Zarco, A.R.; Tognetti, J.; Bessa, L.M.; Guseva, S.; Mikkola, E.; Salvi, N.; Maurin, D.; Herrmann, T.; Blackledge, M. A Specific Phosphorylation-Dependent Conformational Switch in SARS-CoV-2 Nucleocapsid Protein Inhibits RNA Binding. Sci. Adv. 2024, 10, eaax2323. [Google Scholar] [CrossRef] [PubMed]
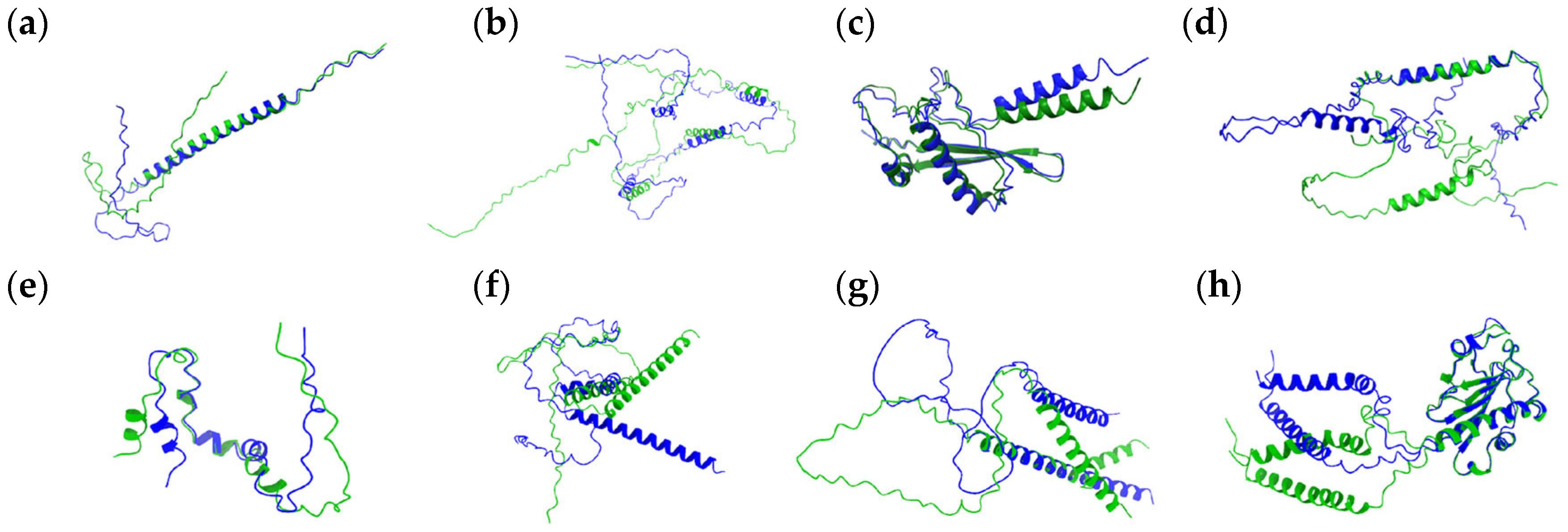

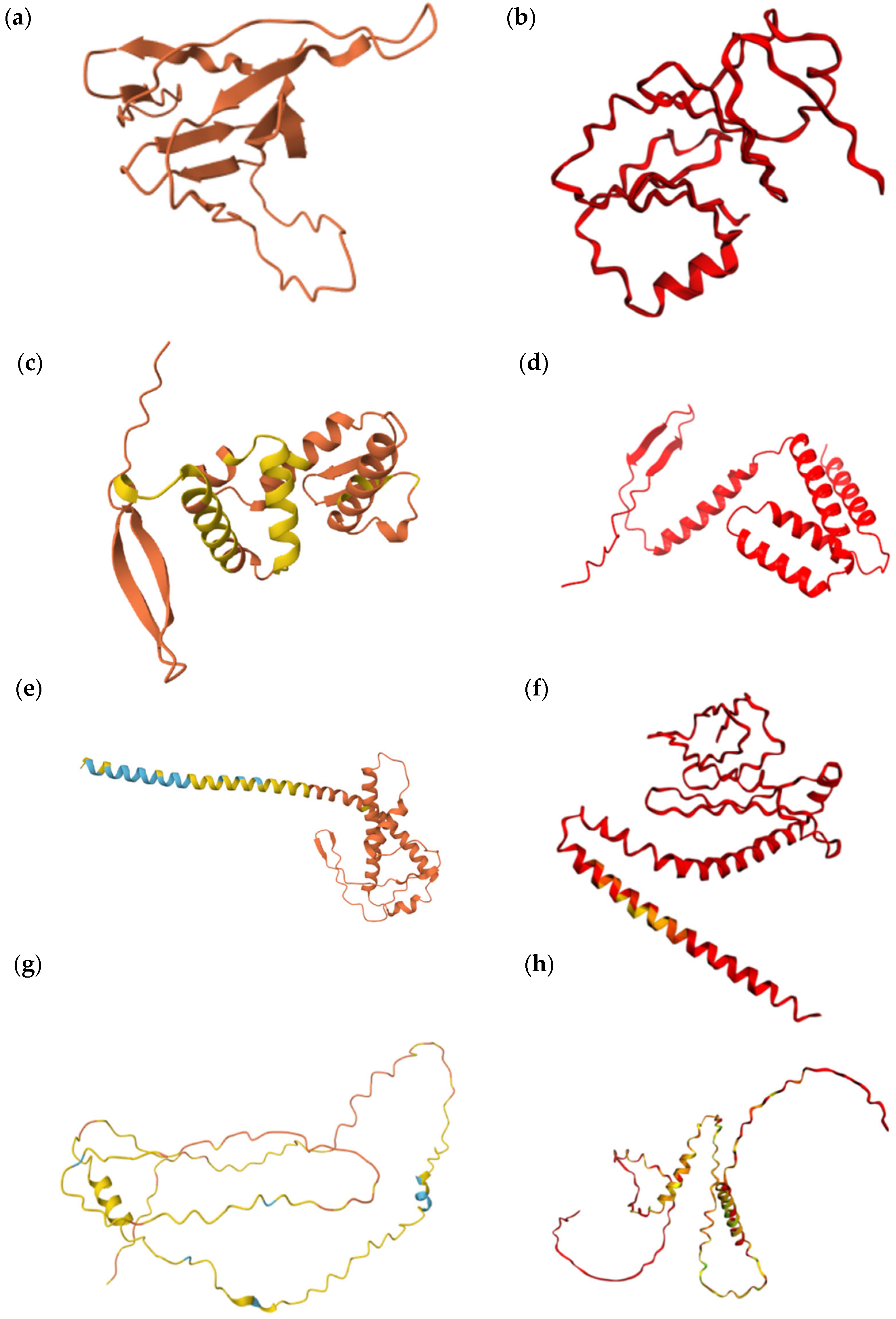
| Systematic Name | Uniprot Accession | Protein Length (AA) | Number of CUG Codons | Positions of the CUG-Encoded AA | Predicted Ser/Leu Exchange Effect |
|---|---|---|---|---|---|
| C1_00020C_A | Q5AB58 | 103 | 3 | 24, 92, 97 | P, insignificant |
| C1_04440W_A | Q59KZ4 | 122 | 1 | 13 | structure altered |
| C1_05250W_A | Q5A299 | 204 | 4 | 71, 100, 123, 180 | insignificant |
| C1_12040W_A | Q5A3K8 | 143 | 2 | 94, 101 | P, insignificant |
| C1_14580C_A | A0A1D8PG02 | 175 | 1 | 10 | P, insignificant |
| C1_14590C_A (TLO4) | A0A8H6F4J0 | 66 | 2 | 24, 35 | P, insignificant |
| C2_00310W_A | Q5ACW4 | 148 | 3 | 82, 94, 122 | structure altered |
| C2_01500W_A | Q5ALW9 | 144 | 1 | 102 | insignificant |
| C3_00120W_A | Q5A7K2 | 208 | 3 | 25, 33,151 | structure altered |
| C4_01710C_A | A0A1D8PLD5 | 142 | 7 | 18, 124, 125, 126, 127, 128, 132 | P, insignificant |
| C5_02130W_A | A0A1D8PNC2 | 192 | 3 | 157, 181, 185 | structure altered |
| C6_00270W_A | A0A1D8PPD0 | 230 | 2 | 100, 107 | insignificant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Čermáková, M.; Heidingsfeld, O. Predicting the Structural Effects of CUG Codon Translation on Uncharacterized Proteins in Candida albicans. J. Fungi 2025, 11, 638. https://doi.org/10.3390/jof11090638
Čermáková M, Heidingsfeld O. Predicting the Structural Effects of CUG Codon Translation on Uncharacterized Proteins in Candida albicans. Journal of Fungi. 2025; 11(9):638. https://doi.org/10.3390/jof11090638
Chicago/Turabian StyleČermáková, Michaela, and Olga Heidingsfeld. 2025. "Predicting the Structural Effects of CUG Codon Translation on Uncharacterized Proteins in Candida albicans" Journal of Fungi 11, no. 9: 638. https://doi.org/10.3390/jof11090638
APA StyleČermáková, M., & Heidingsfeld, O. (2025). Predicting the Structural Effects of CUG Codon Translation on Uncharacterized Proteins in Candida albicans. Journal of Fungi, 11(9), 638. https://doi.org/10.3390/jof11090638





