Potential Antioxidant and Neuroprotective Effect of Polysaccharide Isolated from Digüeñe Cyttaria espinosae
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Collection and Identification of C. espinosae
2.2.1. Collection
2.2.2. Identification
2.3. Isolation and Purification of Fruiting Body Polysaccharides
2.4. Elemental Analysis of CePs
2.5. GC-MS Analysis of CePs
2.6. FT-IR Characterisation of CePs
2.7. Determination of Total Sugar Content of CePS
2.8. Determination of Total Phenolic Content of CePs
2.9. Determination of Protein Content of CePs
2.10. Antioxidant Activity of CePs
2.10.1. DPPH Radical Scavenging Activity of CePs
2.10.2. ABTS Radical Scavenging Activity of CePs
2.10.3. Total Antioxidant Capacity (TEAC)
2.11. Biological Assays
2.11.1. PC12 Cells
2.11.2. Cytotoxicity Assay (MTT)
2.11.3. Aggregation of Soluble Oligomers of the Aβ1–40 Peptide
2.11.4. Neuroprotection Assay Against Aβ-Generated Toxicity (MTT)
2.12. Zebrafish Embryo Toxicity Assay
2.13. Statistical Analysis
3. Results
3.1. Collection and Authentication of Ascocarps
3.2. Polysaccharides Characterization
3.2.1. Yield of Polysaccharides
3.2.2. Elemental Composition Analysis
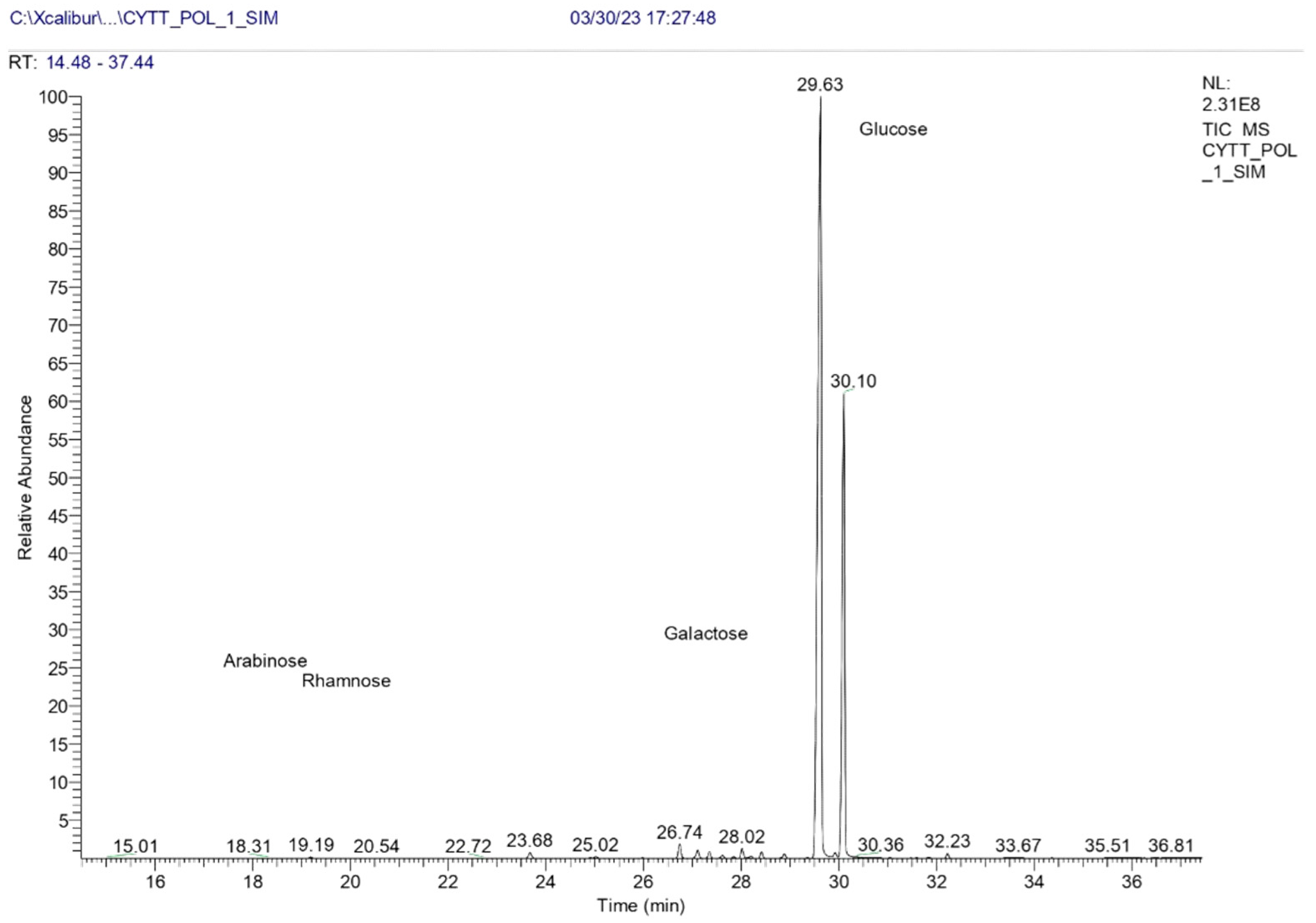
3.2.3. Monomeric Composition
3.2.4. FT−IR Analysis
3.2.5. Total Sugar Content
3.2.6. Total Protein Content
3.3. Antioxidant Assays
3.3.1. Radical Neutralization Assay (DPPH)
3.3.2. Radical Neutralization Test (ABTS•+)
3.3.3. Total Phenolic Content
3.4. Neuroprotection Assays
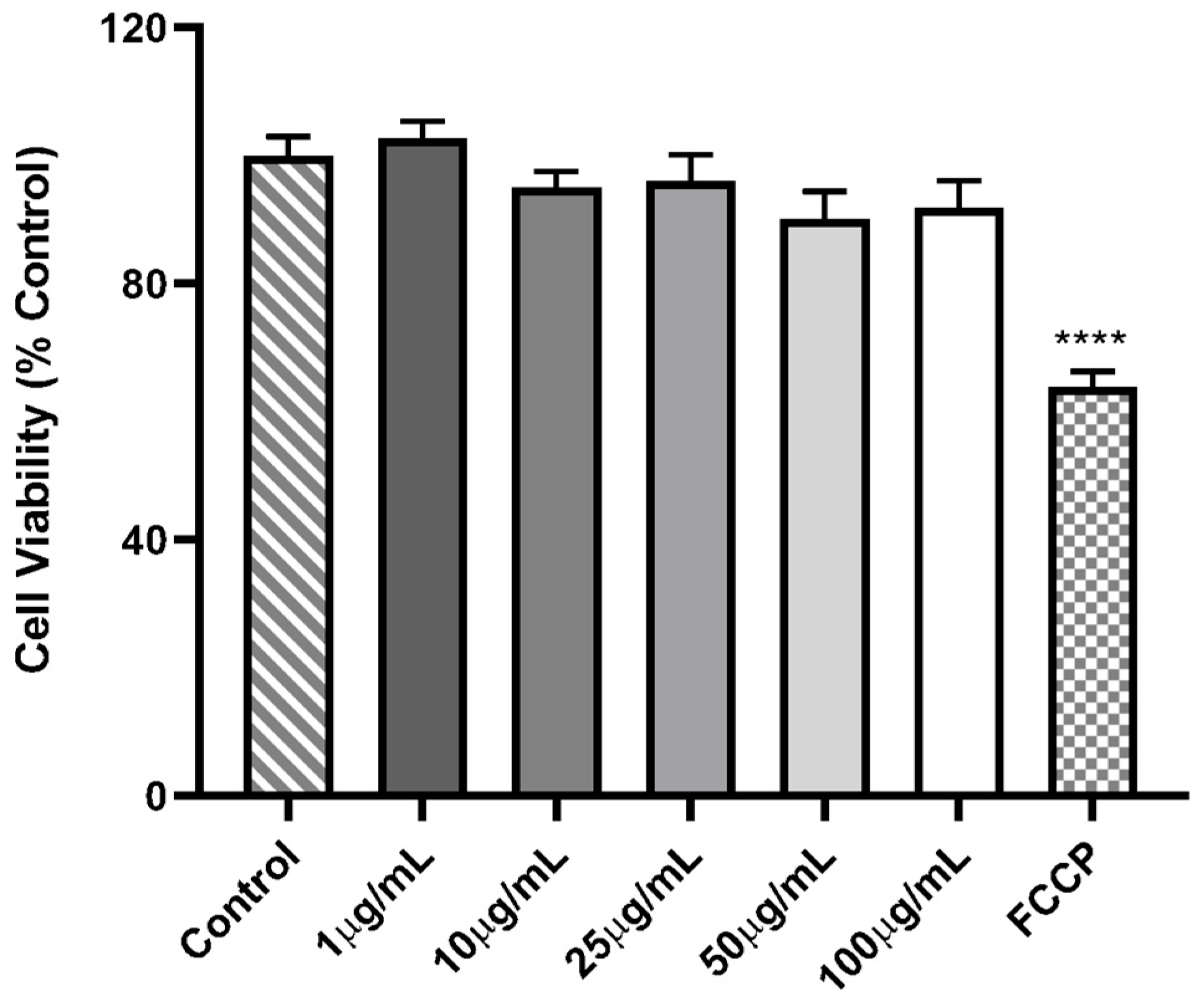
3.4.1. Cell Viability Assay
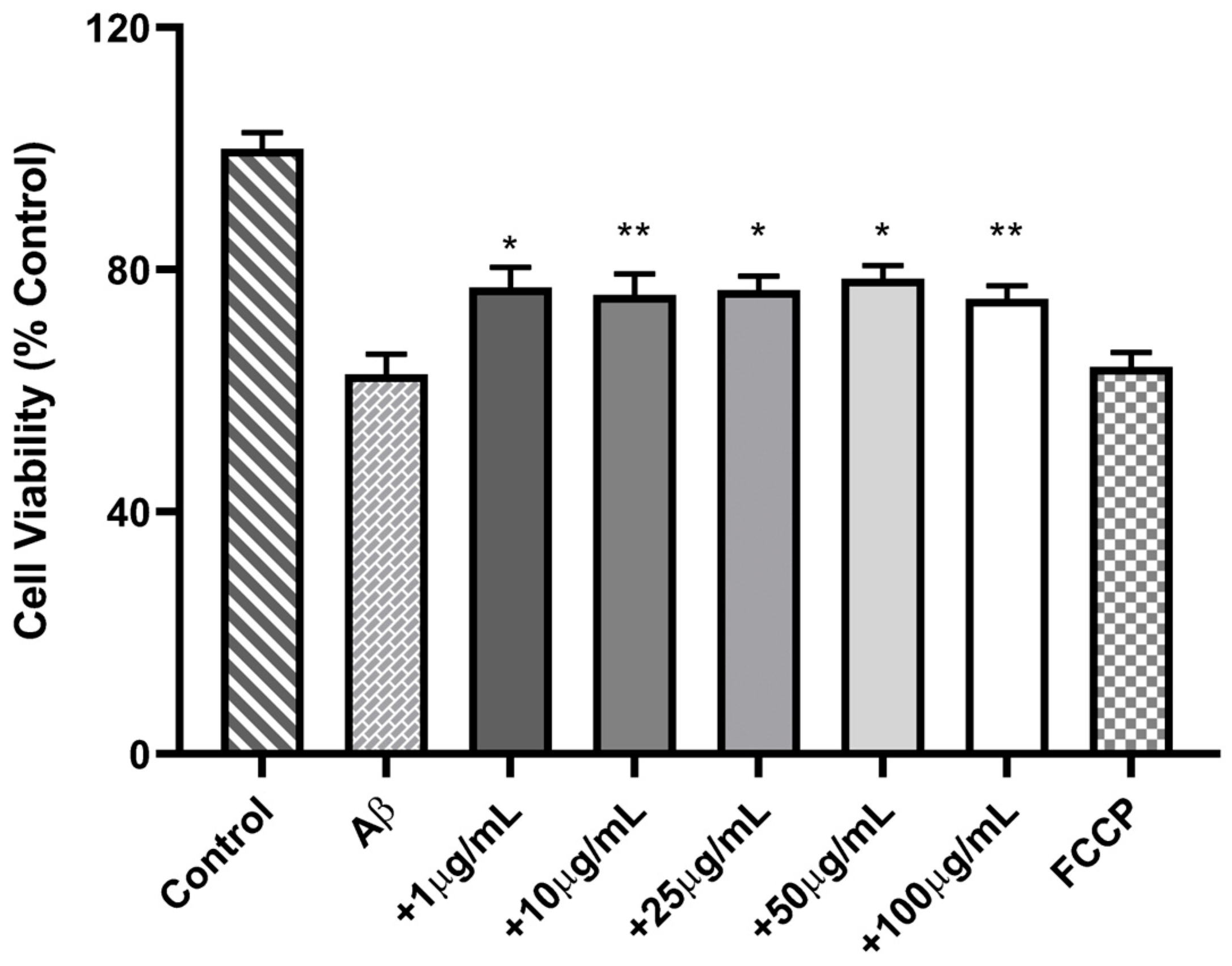
3.4.2. Cell Neuroprotective Assay
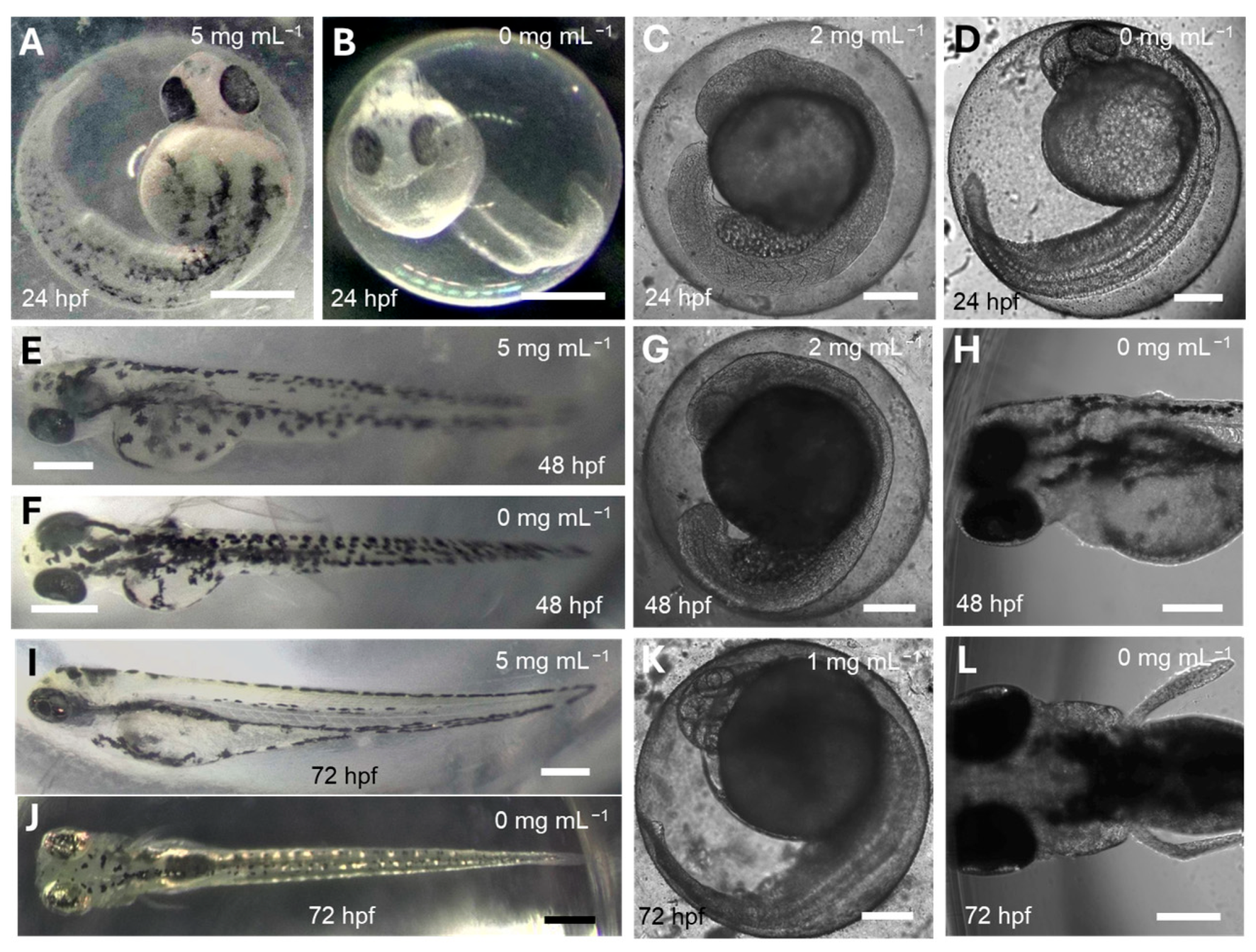
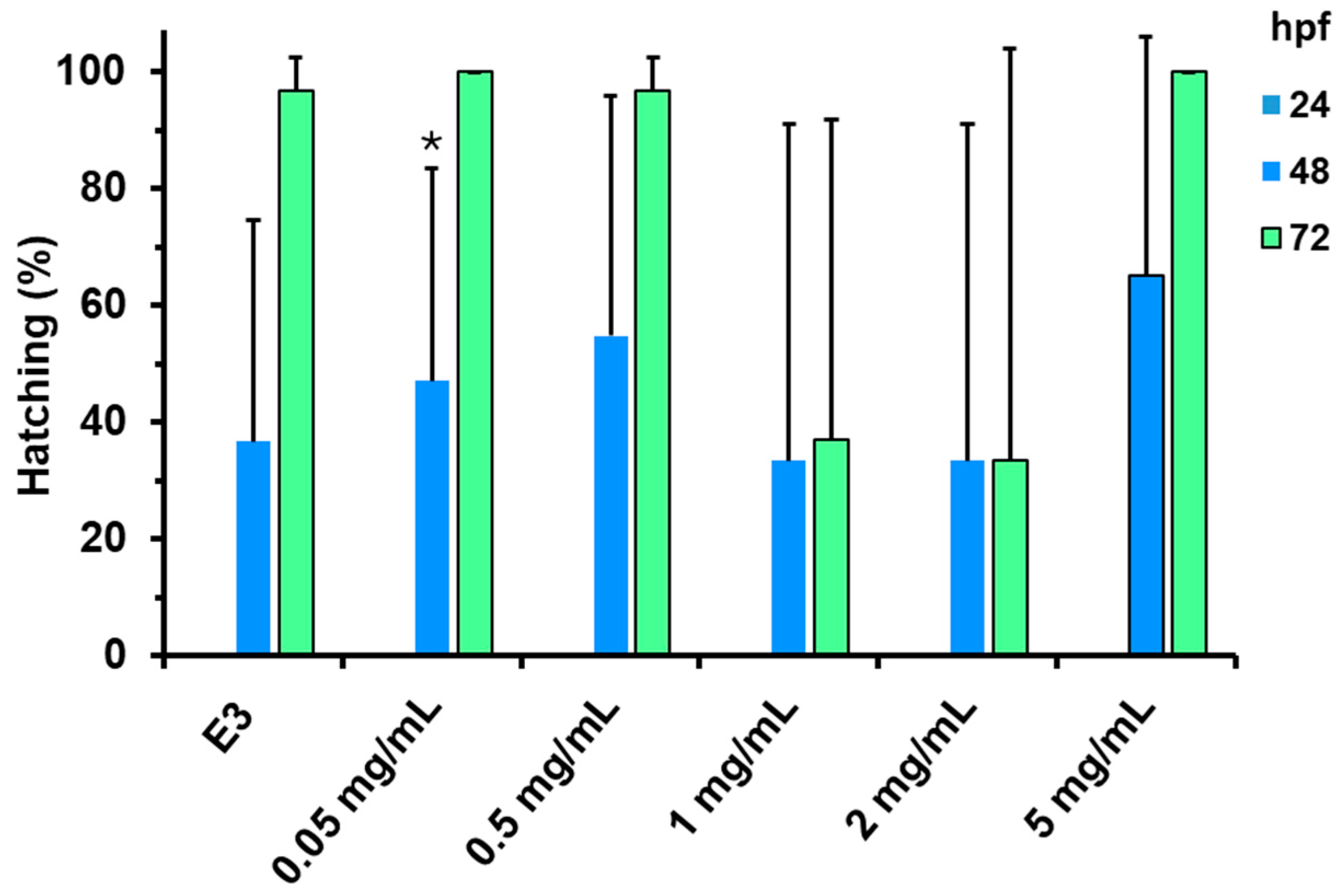
3.5. In Vivo Zebrafish Toxicity Assay
4. Discussion
4.1. Collection and Authentication of Ascocarps
4.2. Polysaccharides Characterization/Chemical Assessment
4.2.1. Yield of Isolation of CePs
4.2.2. Total Carbon (C), Hydrogen (H), Nitrogen (N) and Sulphur (S)
4.2.3. Analysis of Monosaccharide Composition
4.2.4. FT-IR Spectra Analysis
4.2.5. Analysis of Total Sugar Content
4.2.6. Analysis of Total Protein Content
4.3. Antioxidant Activity
4.3.1. In Vitro DPPH Assay
4.3.2. In Vitro ABTS Assay
4.4. Analysis of TEAC Assay
4.5. Analysis of Total Phenolic Content
4.6. Neuroprotective Activity
4.7. Toxicity of the Extract
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis-(3-ethylbenzothiazolin-6-sulfonic acid) |
| AD | Alzheimer’s disease |
| APP | Amyloid precursor protein |
| Ara | Arabinose |
| Aβ | β-Amyloid Peptide |
| DMEM | Dulbecco’s Modified Half Eagle |
| DMSO | Dimethyl sulfoxide |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| FCCP | Carbonylcyanide-p-(trifluoromethoxy) phenylhydrazone |
| FTIR | Fourier transform infrared spectroscopy |
| GAE | Gallic Acid Equivalents |
| Gal | Galactose |
| Glc | Glucose |
| IC50 | Half the maximum inhibitory concentration |
| LPS | Lipopolysaccharides or endotoxins |
| Man | Mannose |
| MPP+ | 1-methyl-4-phenylpyridinium |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NEP | Neprilysin |
| PBS | Phosphate-buffered saline |
| PC-12 | Cell line derived from rat pheochromocytoma |
| DW | Dry weight |
| CePs | Polysaccharides from Cyttaria espinosae |
| ROS | Reactive oxygen species |
| TEAC | Trolox Equivalent Antioxidant Capacity |
References
- Lu, H.; Lou, H.; Hu, J.; Liu, Z.; Chen, Q. Macrofungi: A Review of Cultivation Strategies, Bioactivity, and Application of Mushrooms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2333–2356. [Google Scholar] [CrossRef]
- Niego, A.G.; Rapior, S.; Thongklang, N.; Raspé, O.; Jaidee, W.; Lumyong, S.; Hyde, K.D. Macrofungi as a Nutraceutical Source: Promising Bioactive Compounds and Market Value. J. Fungi 2021, 7, 397. [Google Scholar] [CrossRef]
- Wang, X.-M.; Zhang, J.; Wu, L.-H.; Zhao, Y.-L.; Li, T.; Li, J.-Q.; Wang, Y.-Z.; Liu, H.-G. A Mini-Review of Chemical Composition and Nutritional Value of Edible Wild-Grown Mushrooms from China. Food Chem. 2014, 151, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, E.A.; El Enshasy, H.; Wadaan, M.A.M.; Aziz, R. Mushrooms: A Potential Natural Source of Anti-Inflammatory Compounds for Medical Applications. Mediat. Inflamm. 2014, 2014, 805841. [Google Scholar] [CrossRef] [PubMed]
- Weis, A.L.; Wasser, S.P. Therapeutic Effects of Substances Occurring in Higher Basidiomycetes Mushrooms: A Modern Perspective. Crit. Rev. Immunol. 1999, 19, 32. [Google Scholar] [CrossRef]
- Bills, G.F.; Gloer, J.B. Biologically Active Secondary Metabolites from the Fungi. Microbiol. Spectr. 2016, 4, 1087–1119. [Google Scholar] [CrossRef]
- Ma, G.; Yang, W.; Zhao, L.; Pei, F.; Fang, D.; Hu, Q. A Critical Review on the Health Promoting Effects of Mushrooms Nutraceuticals. Food Sci. Hum. Wellness 2018, 7, 125–133. [Google Scholar] [CrossRef]
- Phan, C.-W.; David, P.; Naidu, M.; Wong, K.-H.; Sabaratnam, V. Therapeutic Potential of Culinary-Medicinal Mushrooms for the Management of Neurodegenerative Diseases: Diversity, Metabolite, and Mechanism. Crit. Rev. Biotechnol. 2015, 35, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Ir, R.; Jeewon, R.; Doble, M.; Hyde, K.D.; Kaliappan, I.; Jeyaraman, R.; Reddi, R.N.; Krishnan, J.; Li, M.; et al. A Mechanistic Review on Medicinal Mushrooms-Derived Bioactive Compounds: Potential Mycotherapy Candidates for Alleviating Neurological Disorders. Planta Medica 2020, 86, 1161–1175. [Google Scholar] [CrossRef]
- Li, I.-C.; Lin, S.; Tsai, Y.-T.; Hsu, J.-H.; Chen, Y.-L.; Lin, W.-H.; Chen, C.-C. Cordyceps cicadae Mycelia and Its Active Compound HEA Exert Beneficial Effects on Blood Glucose in Type 2 Diabetic Db/Db Mice: Effect of Cordyceps Cicadae Mycelia in Db/Db Mice. J. Sci. Food Agric. 2019, 99, 606–612. [Google Scholar] [CrossRef]
- Wang, D.; Wang, J.; Wang, D.; Yu, X.; Olatunji, O.J.; Ouyang, Z.; Wei, Y. Neuroprotective Effects of Butanol Fraction of Cordyceps Cicadae on Glutamate-Induced Damage in PC12 Cells Involving Oxidative Toxicity. Chem. Biodivers. 2018, 15, e1700385. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Huang, W.-M.; Tang, P.-C.; Zhang, X.; Zhang, X.-Y.; Yu, B.-C.; Fan, Y.-Y.; Ge, X.-Q.; Zhang, X.-L. Neuroprotective Effects of Natural Cordycepin on LPS-Induced Parkinson’s Disease through Suppressing TLR4/NF-κB/NLRP3-Mediated Pyroptosis. J. Funct. Foods 2020, 75, 104274. [Google Scholar] [CrossRef]
- Lew, S.Y.; Lim, S.H.; Lim, L.W.; Wong, K.H. Neuroprotective Effects of Hericium Erinaceus (Bull.: Fr.) Pers. against High-Dose Corticosterone-Induced Oxidative Stress in PC-12 Cells. BMC Complement. Med. Ther. 2020, 20, 340. [Google Scholar] [CrossRef] [PubMed]
- Schmeda-Hirschmann, G.; Loyola, J.; Razmilic, I.; Reyes, S. Los “digüeñes” o “quireñes” (Cyttaria spp., Discomycetes): Un recurso alimenticio nativo del centro y sur de Chile. Universum 1995, 10, 129–142. Available online: https://hdl.handle.net/20.500.14001/32140 (accessed on 20 August 2025).
- Salazar Vidal, V.E. Comparación de Parámetros Químico-Nutricionales de las Especies del Género Cyttaria Más Consumidas en Chile. Master’s Thesis, Universidad de Concepción, Facultad de Ciencias Forestales, Concepción, Chile, 2019. Available online: https://repositorio.udec.cl/bitstreams/19cd8ec1-589f-4adf-8b15-23b37fa0060c/download (accessed on 20 August 2025).
- Gamundi, I.J. Las Cyttariales sudamericanas (Fungi-Ascomycetes). Darwiniana 1971, 16, 461–510. [Google Scholar]
- Villalobos-Pezos, J.; González, P.; Córdova, E.; Duran, R.; Antezana, M. Effects of Drying Treatments on the Physicochemical Characteristics and Antioxidant Properties of the Edible Wild Mushroom Cyttaria espinosae Lloyd. J. Fungi 2024, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Sorokulova, I.; Lantukh, D.; Kruchinina, M.; Sabitov, A. Biopolymers for sample collection, protection, and preservation. Appl. Microbiol. Biotechnol. 2015, 99, 5995–6003. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, F.A.; Abdala-Díaz, R.; Hernández, V.; Pedreros, P.; Aranda, M.; Cabrera-Pardo, J.R.; Pérez, C.; Becerra, J.; Urrutia, R. Invasive Diatom Didymosphenia geminata as a Source of Polysaccharides with Antioxidant and Immunomodulatory Effects on Macrophage Cell Lines. J. Appl. Phycol. 2020, 32, 93–102. [Google Scholar] [CrossRef]
- Abdala Díaz, R.T.; Casas Arrojo, V.; Arrojo Agudo, M.A.; Cárdenas, C.; Dobretsov, S.; Figueroa, F.L. Immunomodulatory and Antioxidant Activities of Sulfated Polysaccharides from Laminaria ochroleuca, Porphyra umbilicalis, and Gelidium corneum. Mar. Biotechnol. 2019, 21, 577–587. [Google Scholar] [CrossRef]
- Giuliano, S.; Mistek, E.; Lednev, I.K. Forensic Phenotype Profiling Based on the Attenuated Total Reflection Fourier Transform-Infrared Spectroscopy of Blood: Chronological Age of the Donor. ACS Omega 2020, 5, 27026–27031. [Google Scholar] [CrossRef]
- Wróbel, T.P.; Marzec, K.M.; Majzner, K.; Kochan, K.; Bartuś, M.; Chłopicki, S.; Barańska, M. Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR) Spectroscopy of a Single Endothelial Cell. Analyst 2012, 137, 4135. [Google Scholar] [CrossRef] [PubMed]
- Kurzyna-Szklarek, M.; Cybulska, J.; Zdunek, A. Analysis of the Chemical Composition of Natural Carbohydrates—An Overview of Methods. Food Chem. 2022, 394, 133466. [Google Scholar] [CrossRef]
- Folin, O.; Ciocalteu, V. On Tyrosine and Tryptophane Determinations in Proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Crisóstomo-Ayala, K.; Hernández de La Torre, M.; Pedreño, M.A.; Hernández, J.A.; Pérez, C.; Bustos, E.; Sánchez-Olate, M.; Ríos, D. Seasonal Changes in Photosynthesis, Phenolic Content, Antioxidant Activity, and Anatomy of Apical and Basal Leaves of Aristotelia chilensis. Biol. Plant. 2021, 65, 342–350. [Google Scholar] [CrossRef]
- Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenolic-compound-extraction systems for fruit and vegetable samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef]
- Usuldin, S.R.A.; Wan-Mohtar, W.A.A.Q.I.; Ilham, Z.; Jamaludin, A.A.; Abdullah, N.R.; Rowan, N. In Vivo Toxicity of Bioreactor-Grown Biomass and Exopolysaccharides from Malaysian Tiger Milk Mushroom Mycelium for Potential Future Health Applications. Sci. Rep. 2021, 11, 23079. [Google Scholar] [CrossRef]
- Busquet, F.; Strecker, R.; Rawlings, J.M.; Belanger, S.E.; Braunbeck, T.; Carr, G.J.; Cenijn, P.; Fochtman, P.; Gourmelon, A.; Hübler, N.; et al. OECD Validation Study to Assess Intra- and Inter-Laboratory Reproducibility of the Zebrafish Embryo Toxicity Test for Acute Aquatic Toxicity Testing. Regul. Toxicol. Pharmacol. 2014, 69, 496–511. [Google Scholar] [CrossRef]
- Song, Y.-S.; Dai, M.-Z.; Zhu, C.-X.; Huang, Y.-F.; Liu, J.; Zhang, C.-D.; Xie, F.; Peng, Y.; Zhang, Y.; Li, C.-Q.; et al. Validation, Optimization, and Application of the Zebrafish Developmental Toxicity Assay for Pharmaceuticals under the ICH S5(R3) Guideline. Front. Cell Dev. Biol. 2021, 9, 721130. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio); University of Oregon Press: Eugene, OR, USA, 1995. [Google Scholar]
- García-Márquez, J.; Moreira, B.R.; Valverde-Guillén, P.; Latorre-Redoli, S.; Caneda-Santiago, C.T.; Acién, G.; Martínez-Manzanares, E.; Marí-Beffa, M.; Abdala-Díaz, R.T. In Vitro and In Vivo Effects of Ulvan Polysaccharides from Ulva rigida. Pharmaceutics 2023, 16, 660. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Ocaña, M.C.; Martínez-Poveda, B.; Marí-Beffa, M.; Quesada, A.R.; Medina, M.Á. Fasentin Diminishes Endothelial Cell Proliferation, Differentiation and Invasion in a Glucose Metabolism-Independent Manner. Sci. Rep. 2020, 10, 6132. [Google Scholar] [CrossRef] [PubMed]
- Salazar Vidal, V. Actualización del Conocimiento del Género Cyttaria Berk. (Cyttariales, Ascomycota) en Chile. Bol. Micol. 2020, 35. [Google Scholar] [CrossRef]
- Salazar Vidal, V. Hongos Silvestres Comestibles Nativos de Chile: Reconocimiento, Recolección Sostenible y Recetas; Diseño Sostenible SpA—Ediciones Libro Verde: Santiago, Chile, 2022; ISBN 978-956-09553-4-0. [Google Scholar]
- Guo, Q.; Ai, L.; Cui, S.W. Fourier Transform Infrared Spectroscopy (FTIR) for Carbohydrate Analysis. In SpringerBriefs in Molecular Science; Springer International Publishing: Cham, Switzerland, 2018; pp. 69–71. ISBN 9783319963693. [Google Scholar]
- Smith, B.C. Infrared Spectral Interpretation: A Systematic Approach; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Baker, J.T. Infrared Spectra of Sugars and Their Derivatives. In Infrared Spectroscopy: Principles and Spectral Interpretation; Wiley-Blackwell: Hoboken, NJ, USA, 2009; pp. 92–107. [Google Scholar]
- Bunzel, M.; Schendel, R.R. Determination of (Total) Phenolics and Antioxidant Capacity in Food and Ingredients. In Food Analysis; Nollet, L.M.L., Toldrá, F., Eds.; Springer: Cham, Switzerland, 2017; pp. 55–468. [Google Scholar] [CrossRef]
- Pizarro, C.S.; Ronco, M.A.M.; Gotteland, R.M. ß-glucanos: ¿qué tipos existen y cuáles son sus beneficios en la salud? ß-glucans: What types exist and what are their health benefits? Rev. Chil. Nutr. 2014, 41, 439–446. [Google Scholar] [CrossRef]
- Pontón, J. La pared celular de los hongos y el mecanismo de acción de la anidulafungina. Rev. Iberoam. Micol. 2008, 25, 78–82. [Google Scholar] [CrossRef]
- Grün, C.H.; Hochstenbach, F.; Humbel, B.M.; Verkleij, A.J.; Sietsma, J.H.; Klis, F.M.; Kamerling, J.P.; Vliegenthart, J.F.G. The Structure of Cell Wall Alpha-Glucan from Fission Yeast. Glycobiology 2005, 15, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Hochstenbach, F.; Klis, F.M.; van den Ende, H.; van Donselaar, E.; Peters, P.J.; Klausner, R.D. Identification of a Putative Alpha-Glucan Synthase Essential for Cell Wall Construction and Morphogenesis in Fission Yeast. Proc. Natl. Acad. Sci. USA 1998, 95, 9161–9166. [Google Scholar] [CrossRef]
- Ruiz-Herrera, J.; Ortiz-Castellanos, L. Cell Wall Glucans of Fungi. A Review. Cell Surf. 2019, 5, 100022. [Google Scholar] [CrossRef]
- Fujikawa, T.; Sakaguchi, A.; Nishizawa, Y.; Kouzai, Y.; Minami, E.; Yano, S.; Koga, H.; Meshi, T.; Nishimura, M. Surface α-1,3-Glucan Facilitates Fungal Stealth Infection by Interfering with Innate Immunity in Plants. PLoS Pathog. 2012, 8, e1002882. [Google Scholar] [CrossRef]
- Rappleye, C.A.; Engle, J.T.; Goldman, W.E. RNA Interference in Histoplasma Capsulatum Demonstrates a Role for Alpha-(1,3)-Glucan in Virulence: RNAi and Fungal Virulence. Mol. Microbiol. 2004, 53, 153–165. [Google Scholar] [CrossRef]
- Zhao, T.; Yang, M.; Ma, L.; Liu, X.; Ding, Q.; Chai, G.; Lu, Y.; Wei, H.; Zhang, S.; Ding, C. Structural Modification and Biological Activity of Polysaccharides. Molecules 2023, 28, 5416. [Google Scholar] [CrossRef]
- Qi, J.; Kim, S.M. Effects of the Molecular Weight and Protein and Sulfate Content of Chlorella ellipsoidea Polysaccharides on Their Immunomodulatory Activity. Int. J. Biol. Macromol. 2018, 107 Pt A, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, Z.; Ye, L.; Kang, Z.; Zhang, X.; Huang, Y.; Zhang, B.; Zou, Y. Comparison of the Partial Structure and Antioxidant Activity of Polysaccharides from Two Species of Chinese Truffles. Molecules 2020, 25, 4345. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Li, P.; Luo, Q.; Yan, J.; Zhang, J.; Jin, X.; Huang, W. Effect of γ-Irradiation on the Structure and Antioxidant Activity of Polysaccharide Isolated from the Fruiting Bodies of Morchella sextelata. Biosci. Rep. 2020, 40, BSR20194522. [Google Scholar] [CrossRef] [PubMed]
- Erbiai, E.H.; da Silva, L.P.; Saidi, R.; Lamrani, Z.; Esteves da Silva, J.C.G.; Maouni, A. Chemical Composition, Bioactive Compounds, and Antioxidant Activity of Two Wild Edible Mushrooms Armillaria mellea and Macrolepiota procera from Two Countries (Morocco and Portugal). Biomolecules 2021, 11, 575. [Google Scholar] [CrossRef]
- Peterson, K.R.; Pfister, D.H. Phylogeny of Cyttaria Inferred from Nuclear and Mitochondrial Sequence and Morphological Data. Mycologia 2010, 102, 1398–1416. [Google Scholar] [CrossRef]
- Leiva-Portilla, D.J.; Rodríguez-Núñez, K.E.; Rodríguez-Ramos, F.J.; Delgadillo Acevedo, Á.; Uribe, E. Impact on Physicochemical Composition and Antioxidant Activity of the Wild Edible Mushroom Cyttaria espinosae Subjected to Drying. Chem. Biodivers. 2020, 17, e2000642. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Tsai, C.-L.; Lien, Y.-Y.; Lee, M.-S.; Sheu, S.-C. High Molecular Weight Polysaccharides from Hericium erinaceus Against Amyloid Beta-Induced Neurotoxicity. BMC Complement. Altern. Med. 2016, 16, 170. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, R.; Yang, Z.; Wen, Q.; Cao, X.; Zhao, N.; Yan, J. Protective Effects of Polysaccharides in Neurodegenerative Diseases. Front. Aging Neurosci. 2022, 14, 917629. [Google Scholar] [CrossRef]
- Barcan, A.S.; Barcan, R.A.; Vamanu, E. Therapeutic Potential of Fungal Polysaccharides in Gut Microbiota Regulation: Implications for Diabetes, Neurodegeneration, and Oncology. J. Fungi 2024, 10, 394. [Google Scholar] [CrossRef]
- Zhang, X.; Duan, Y.; Xue, J.; Chen, S.; Wang, H. Edible Mushroom Polysaccharides: Structural Characteristics, Chemical Modification Strategies, and Structure-Activity Relationship: A Review. Int. J. Biol. Macromol. 2025, 320, 145888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xu, Y.; Xie, L.; Shu, X.; Zhang, S.; Wang, Y.; Wang, H.; Dong, Q.; Peng, W. The Function and Application of Edible Fungal Polysaccharides. Adv. Appl. Microbiol. 2024, 127, 45–142. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-Q.; Song, A.-X.; Wong, W.-T.; Wu, J.-Y. Isolation and Assessment of a Highly-Active Anti-Inflammatory Exopolysaccharide from Mycelial Fermentation of a Medicinal Fungus Cs-HK1. Int. J. Mol. Sci. 2021, 22, 2450. [Google Scholar] [CrossRef]
- Sheng, L.; Chen, J.; Li, J.; Zhang, W. An Exopolysaccharide from Cultivated Cordyceps Sinensis and Its Effects on Cytokine Expressions of Immunocytes. Appl. Biochem. Biotechnol. 2011, 163, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Roda, E.; Priori, E.C.; Ratto, D.; De Luca, F.; Di Iorio, C.; Angelone, P.; Locatelli, C.A.; Desiderio, A.; Goppa, L.; Savino, E.; et al. Neuroprotective Metabolites of Hericium Erinaceus Promote Neuro-Healthy Aging. Int. J. Mol. Sci. 2021, 22, 6379. [Google Scholar] [CrossRef]
- Fontaine, T.; Delangle, A.; Simenel, C.; Coddeville, B.; van Vliet, S.J.; van Kooyk, Y.; Bozza, S.; Moretti, S.; Schwarz, F.; Trichot, C.; et al. Galactosaminogalactan, a New Immunosuppressive Polysaccharide of Aspergillus fumigatus. PLoS Pathog. 2011, 7, e1002372. [Google Scholar] [CrossRef]
- Ge, F.; Chen, Y.; Wang, B.; Zhou, W.; Du, B.; Hou, L. Bioactive Polysaccharides from Hericium erinaceus: Extraction, Structure, Bioactivities, and Applications. Molecules 2025, 30, 1850. [Google Scholar] [CrossRef]
- Lowenthal, M.S.; Kilpatrick, E.L.; Phinney, K.W. Separation of Monosaccharides Hydrolyzed from Glycoproteins without the Need for Derivatization. Anal. Bioanal. Chem. 2015, 407, 5453–5462. [Google Scholar] [CrossRef]
- Baeva, E.; Bleha, R.; Lavrova, E.; Sushytskyi, L.; Čopíková, J.; Jablonsky, I.; Klouček, P.; Synytsya, A. Polysaccharides from Basidiocarps of Cultivating Mushroom Pleurotus ostreatus: Isolation and Structural Characterization. Molecules 2019, 24, 2740. [Google Scholar] [CrossRef]
- Patel, D.K.; Seo, Y.-R.; Dutta, S.D.; Lee, O.H.; Lim, K.-T. Influence of Maitake (Grifola frondosa) Particle Sizes on Human Mesenchymal Stem Cells and in Vivo Evaluation of Their Therapeutic Potential. Biomed Res. Int. 2020, 2020. [Google Scholar] [CrossRef]
- Khaskheli, S.G.; Liu, Y.; Khaskheli, A.A.; Dong, X. Unveiling the Medicinal Potential of Edible Mushroom Polysaccharides: Structure, Function, and Therapeutic Insights. Food Biomacromol. 2024, 1, 45–57. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Niksic, M.; van Griensven, L.; Vrvic, M.; Jakovljevic, D. Polysaccharides of Higher Fungi: Biological Role, Structure, and Antioxidative Activity. Hem. Ind. 2014, 68, 305–320. [Google Scholar] [CrossRef]
- Li, H.; Ding, F.; Xiao, L.; Shi, R.; Wang, H.; Han, W.; Huang, Z. Food-Derived Antioxidant Polysaccharides and Their Pharmacological Potential in Neurodegenerative Diseases. Nutrients 2017, 9, 778. [Google Scholar] [CrossRef]
- Vamanu, E. Antioxidant Properties of Polysaccharides Obtained by Batch Cultivation of Pleurotus Ostreatus Mycelium. Nat. Prod. Res. 2013, 27, 1115–1118. [Google Scholar] [CrossRef]
- Albornoz, V.; Casas-Arrojo, V.; Figueroa, F.; Hernández, V.; Pérez, C.; Rajchenberg, M.; Smith, C.T.; Becerra, J.; Cabrera-Pardo, J.R.; Campos, V.L. Evaluation of Cytotoxic Effect Against Tumour Cells of the Acidic Polysaccharides of the Fungus Nothophellinus andinopatagonicus. J. Chil. Chem. Soc. 2022, 67, 5418–5424. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, M.; Zhao, L.; Chen, L.; Ding, Z. Novel Insights into the Mechanism Underlying High Polysaccharide Yield in Submerged Culture of Ganoderma lucidum Revealed by Transcriptome and Proteome Analyses. Microorganisms 2023, 11, 772. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Hu, X.; Zhang, Y.; Liu, T. Studies on the Purification of Polysaccharides Separated from Tremella fuciformis and Their Neuroprotective Effect. Mol. Med. Rep. 2016, 13, 3985–3992. [Google Scholar] [CrossRef] [PubMed]
- Abdelatif, S. Antioxidant and antimicrobial activities of some mushroom extracts against food-related microbes. J. Biol. Chem. 2013, 8, 13–148. [Google Scholar]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Methodological Aspects about In Vitro Evaluation of Antioxidant Properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef]
- Zhong, Q.; Wei, B.; Wang, S.; Ke, S.; Chen, J.; Zhang, H.; Wang, H. The Antioxidant Activity of Polysaccharides Derived from Marine Organisms: An Overview. Mar. Drugs 2019, 17, 674. [Google Scholar] [CrossRef]
- Guo, S.; Duan, W.; Wang, Y.; Chen, L.; Yang, C.; Gu, X.; Xue, Q.; Li, R.; Zhang, Z. Component Analysis and Anti-Colorectal Cancer Mechanism via AKT/mTOR Signalling Pathway of Sanghuangporus vaninii Extracts. Molecules 2022, 27, 1153. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Xu, X.L.; Li, S.; Zhang, R.; Le, W.D. Neuroprotective effects of naturally sourced bioactive polysaccharides: An update. Neural Regen Res. 2022, 17, 1907–1912. [Google Scholar] [CrossRef]
- Gao, Q.-H.; Fu, X.; Zhang, R.; Wang, Z.; Guo, M. Neuroprotective effects of plant polysaccharides: A review of the mechanisms. Int. J. Biol. Macromol. 2018, 106, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.W.; Lee, J.-E.; Lee, C.; Kim, Y.T. Natural Products and Their Neuroprotective Effects in Degenerative Brain Diseases: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 11223. [Google Scholar] [CrossRef] [PubMed]
- Kamei, H.; Duan, C. Hypoxic Treatment of Zebrafish Embryos and Larvae. En Methods in Molecular Biology; Springer: New York, NY, USA, 2018; pp. 195–203. ISBN 9781493976645. [Google Scholar]
- Graham, A.M.; Presnell, J.S. Hypoxia Inducible Factor (HIF) Transcription Factor Family Expansion, Diversification, Divergence and Selection in Eukaryotes. PLoS ONE 2017, 12, e0179545. [Google Scholar] [CrossRef]
- Rusdi, N.; Kue, C.S.; Yu, K.-X.; Lau, B.F.; Chung, L.Y.; Kiew, L.V. Assessment of Potential Anticancer Activity of Brown Seaweed Compounds Using Zebrafish Phenotypic Assay. Nat. Prod. Commun. 2019, 14, 1934578X1985790. [Google Scholar] [CrossRef]
- Wan-Mohtar, W.A.A.Q.I.; Taufek, N.M.; Thiran, J.P.; Rahman, J.F.P.; Yerima, G.; Subramaniam, K.; Rowan, N. Investigations on the Use of Exopolysaccharide Derived from Mycelial Extract of Ganoderma lucidum as Functional Feed Ingredient for Aquaculture-Farmed Red Hybrid Tilapia (Oreochromis sp.). Future Foods 2021, 3, 100018. [Google Scholar] [CrossRef]




| Yield a (%) | Total Phenolics (mg GAE g−1 DW) b | Total Sugar Content (g/100 g) b | Total Protein (g/100 g) b |
|---|---|---|---|
| 8.71 ± 0.77 | 0.801 ± 0.34 | 77.36 ± 6129 | 1.57 ± 0.76 |
| Elemental Composition of CePs (%) | |||
|---|---|---|---|
| TC | TH | TN | TS |
| 37.85 ± 0.5 | 6.593 ± 0.2 | 0.679 ± 0.09 | 0.000 ± 0.002 |
| Header | Monosaccharide | Retention Time (min) | Peak Area | % Mass |
|---|---|---|---|---|
| 1 | D (+) Gal isomer 1 | 28.01 | 10,354,902 | 0.60 |
| 2 | D (+) Gal isomer 2 | 28.88 | 5,186,177 | 0.30 |
| 3 | D (+) Glc isomer 1 | 29.63 | 1,187,411,268 | 69.14 |
| 4 | D (+) Glc isomer 2 | 30.10 | 512,578,595 | 29.85 |
| 5 | D (+) Glc isomer 3 | 32.23 | 4,288,197 | 0.25 |
| 6 | D (+) Rha isomer 1 | 19.18 | 1,159,481 | 0.07 |
| 7 | D (+) Rha isomer 2 | 19.50 | 238,482 | 0.01 |
| 8 | D (+) Rha isomer 3 | 21.83 | 114,412 | 0.01 |
| 9 | D (+) Ara isomer 1 | 18.30 | 157,850 | 0.01 |
| 10 | D (+) Ara isomer2 | 18.64 | 143,961 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, C.; Figueroa, F.A.; Tello, I.; Abdala-Díaz, R.T.; Marí-Beffa, M.; Salazar-Vidal, V.; Becerra, J.; Gavilán, J.; Fuentealba, J. Potential Antioxidant and Neuroprotective Effect of Polysaccharide Isolated from Digüeñe Cyttaria espinosae. J. Fungi 2025, 11, 637. https://doi.org/10.3390/jof11090637
Pérez C, Figueroa FA, Tello I, Abdala-Díaz RT, Marí-Beffa M, Salazar-Vidal V, Becerra J, Gavilán J, Fuentealba J. Potential Antioxidant and Neuroprotective Effect of Polysaccharide Isolated from Digüeñe Cyttaria espinosae. Journal of Fungi. 2025; 11(9):637. https://doi.org/10.3390/jof11090637
Chicago/Turabian StylePérez, Claudia, Fabián A. Figueroa, Ignacio Tello, Roberto T. Abdala-Díaz, Manuel Marí-Beffa, Viviana Salazar-Vidal, José Becerra, Javiera Gavilán, and Jorge Fuentealba. 2025. "Potential Antioxidant and Neuroprotective Effect of Polysaccharide Isolated from Digüeñe Cyttaria espinosae" Journal of Fungi 11, no. 9: 637. https://doi.org/10.3390/jof11090637
APA StylePérez, C., Figueroa, F. A., Tello, I., Abdala-Díaz, R. T., Marí-Beffa, M., Salazar-Vidal, V., Becerra, J., Gavilán, J., & Fuentealba, J. (2025). Potential Antioxidant and Neuroprotective Effect of Polysaccharide Isolated from Digüeñe Cyttaria espinosae. Journal of Fungi, 11(9), 637. https://doi.org/10.3390/jof11090637







