Trichoderma harzianum Cellobiohydrolase Thph2 Induces Reactive Oxygen Species-Mediated Resistance Against Southern Corn Leaf Blight in Maize
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions
2.2. Biological Materials and Culture Conditions
2.3. RNA-Seq Data Analysis and Transcript Quantification
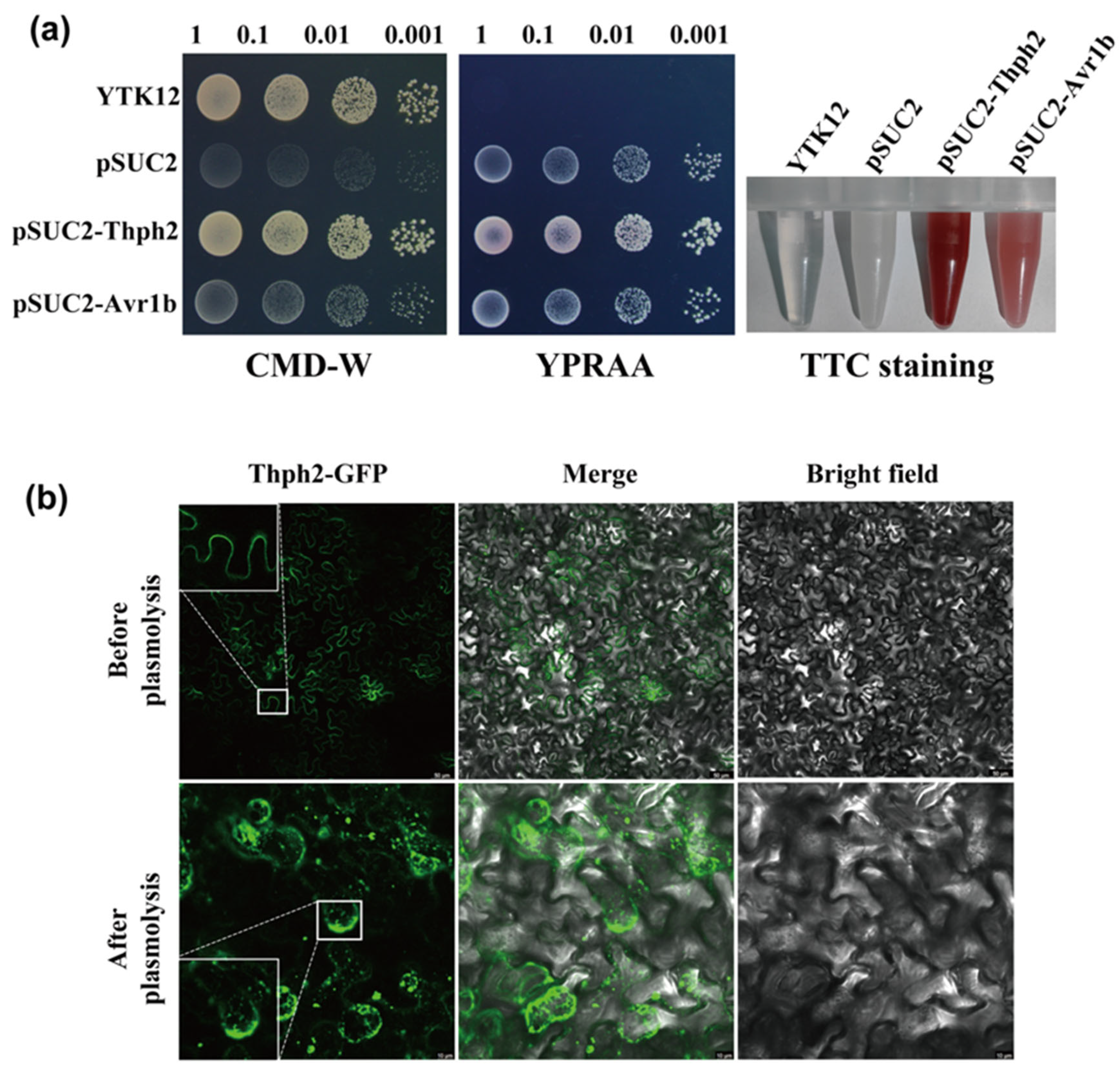
2.4. Validation of Signal Peptide Function
2.5. Thph2 Function Analysis in N. benthamiana
2.6. Detection of Reactive Oxygen Species (ROS) and Cell Death in Plant Systems
2.7. Western Blotting Assay
2.8. Expression and Purification of Proteins in Yeast
2.9. Quantitative Gene Expression Profiling by Reverse Transcription qPCR
2.10. Statistical Analysis
3. Results
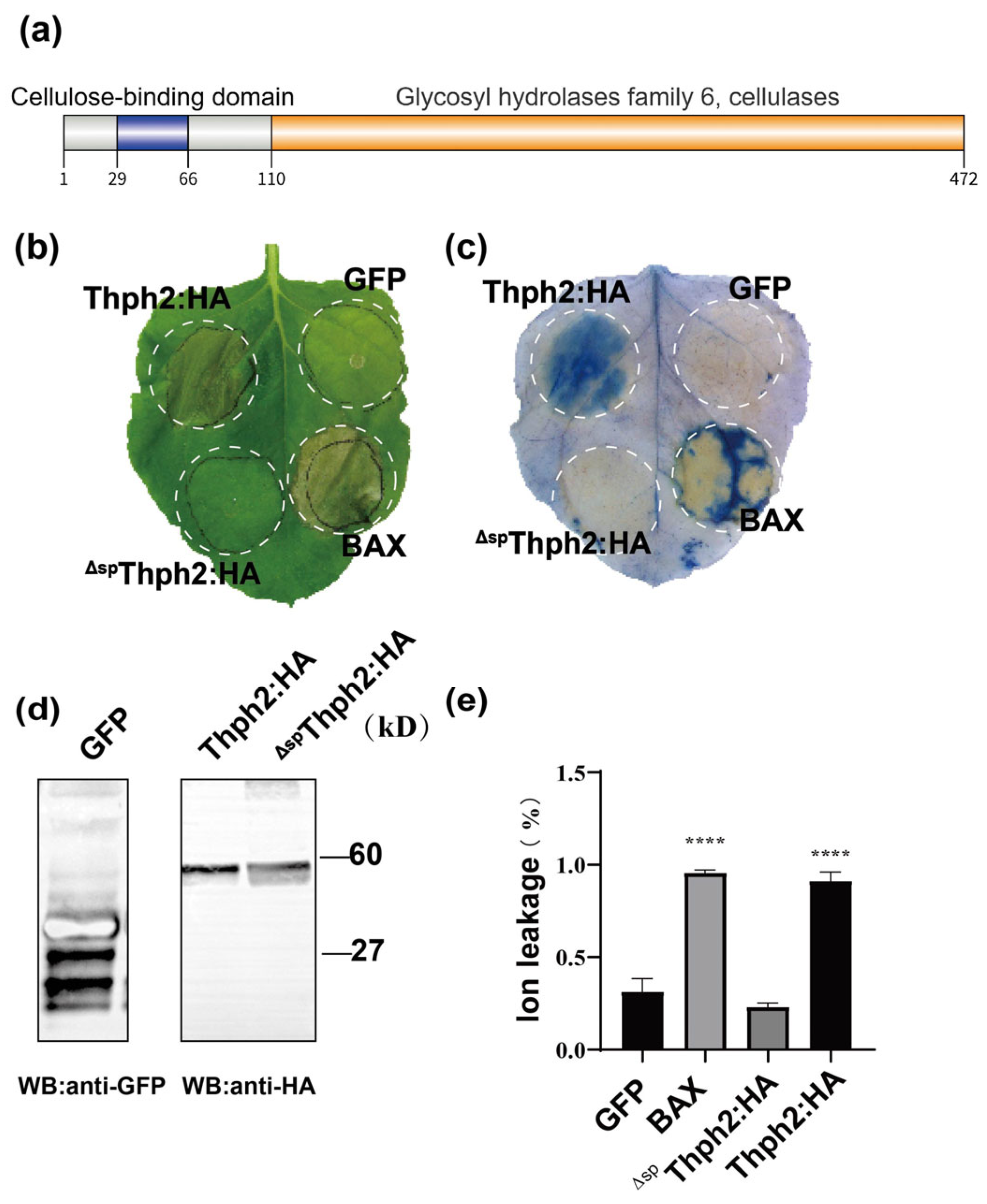
3.1. Thph2 Can Induce N. benthamiana Cell PCD
3.2. Signal Peptide-Dependent Programmed Cell Death Induction by Thph2
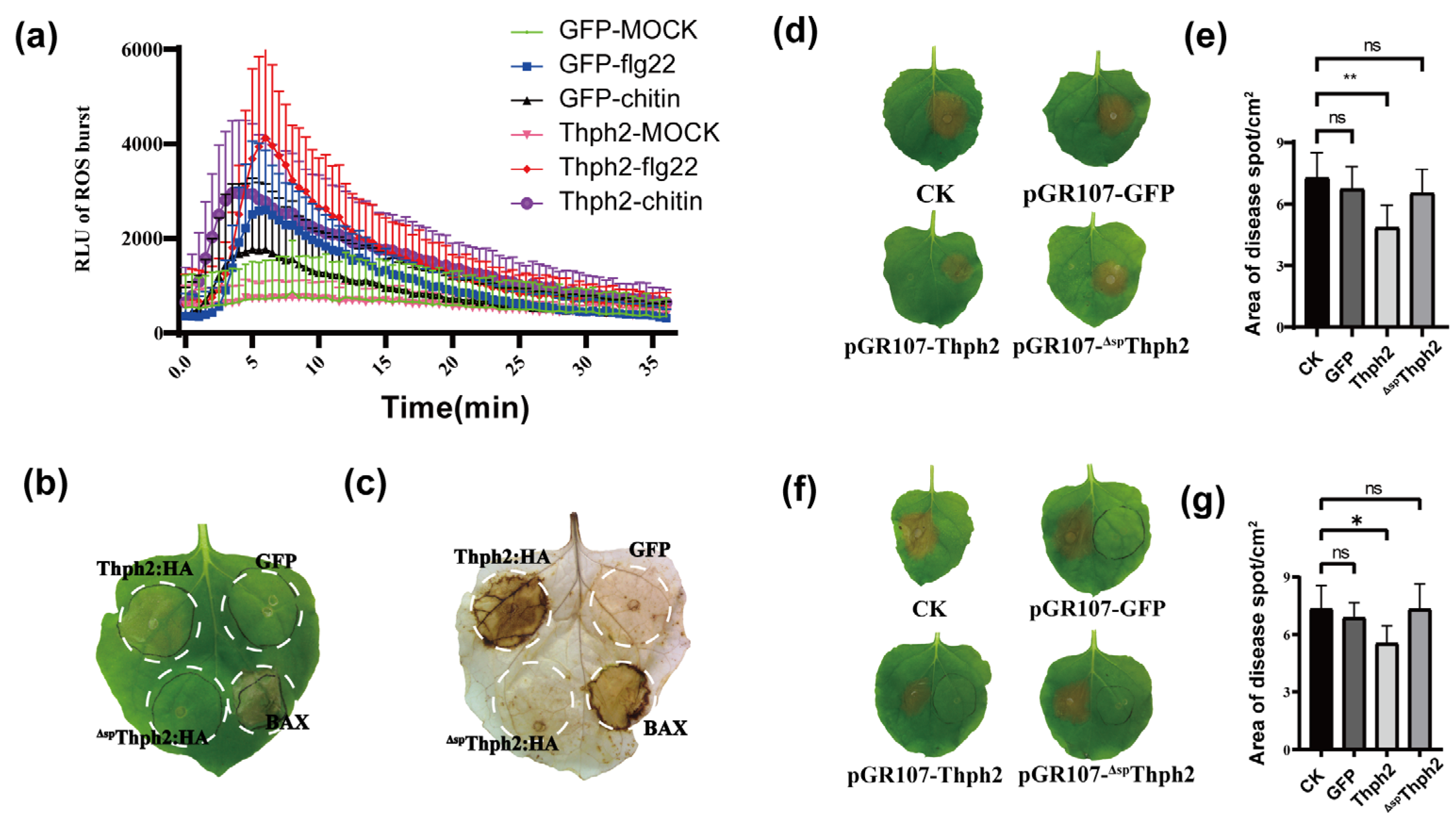
3.3. Thph2 Activates Immune Responses in N. Benthamiana
3.4. Thph2 Enhances Disease Resistance in N. benthamiana Leaves
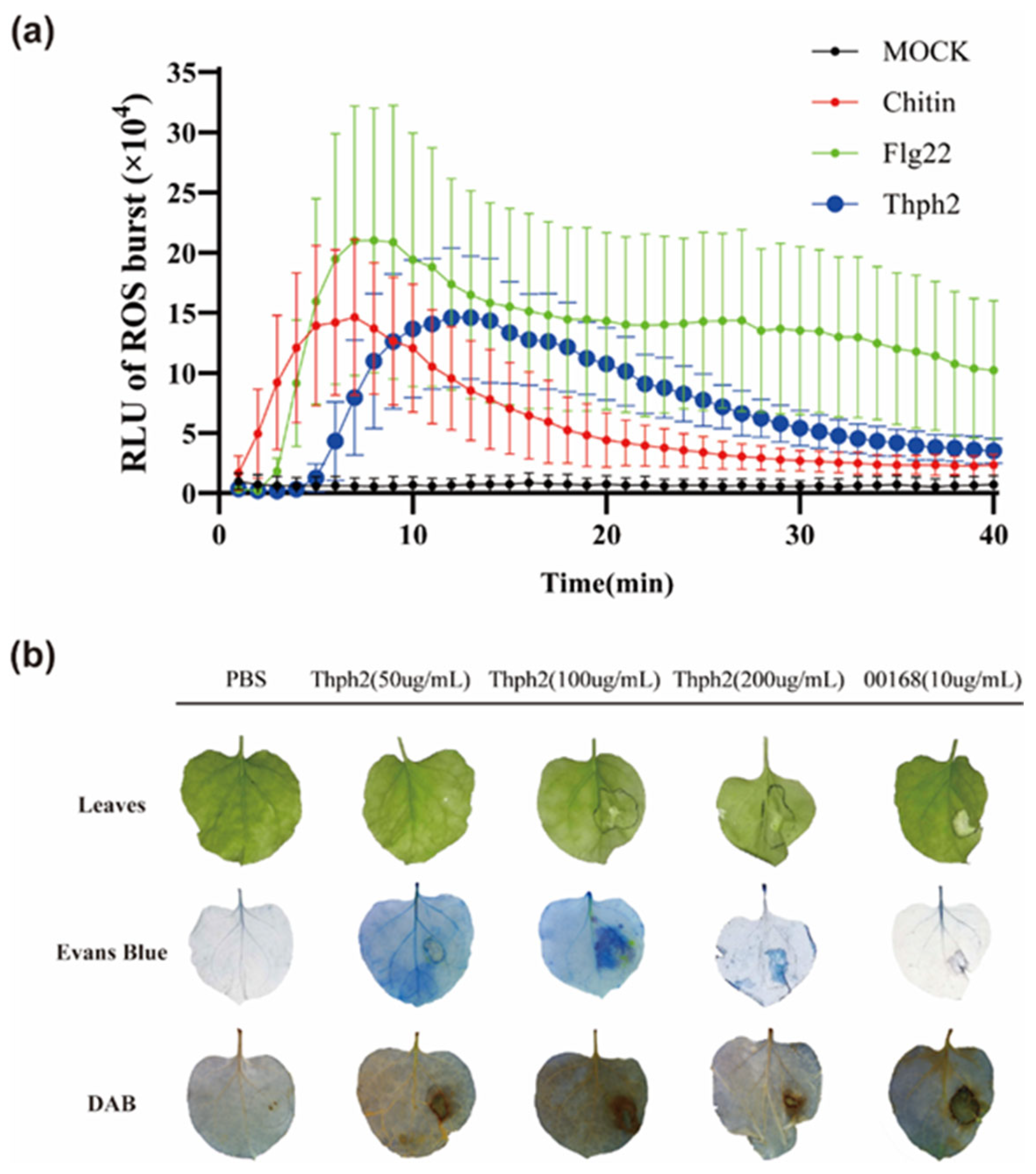
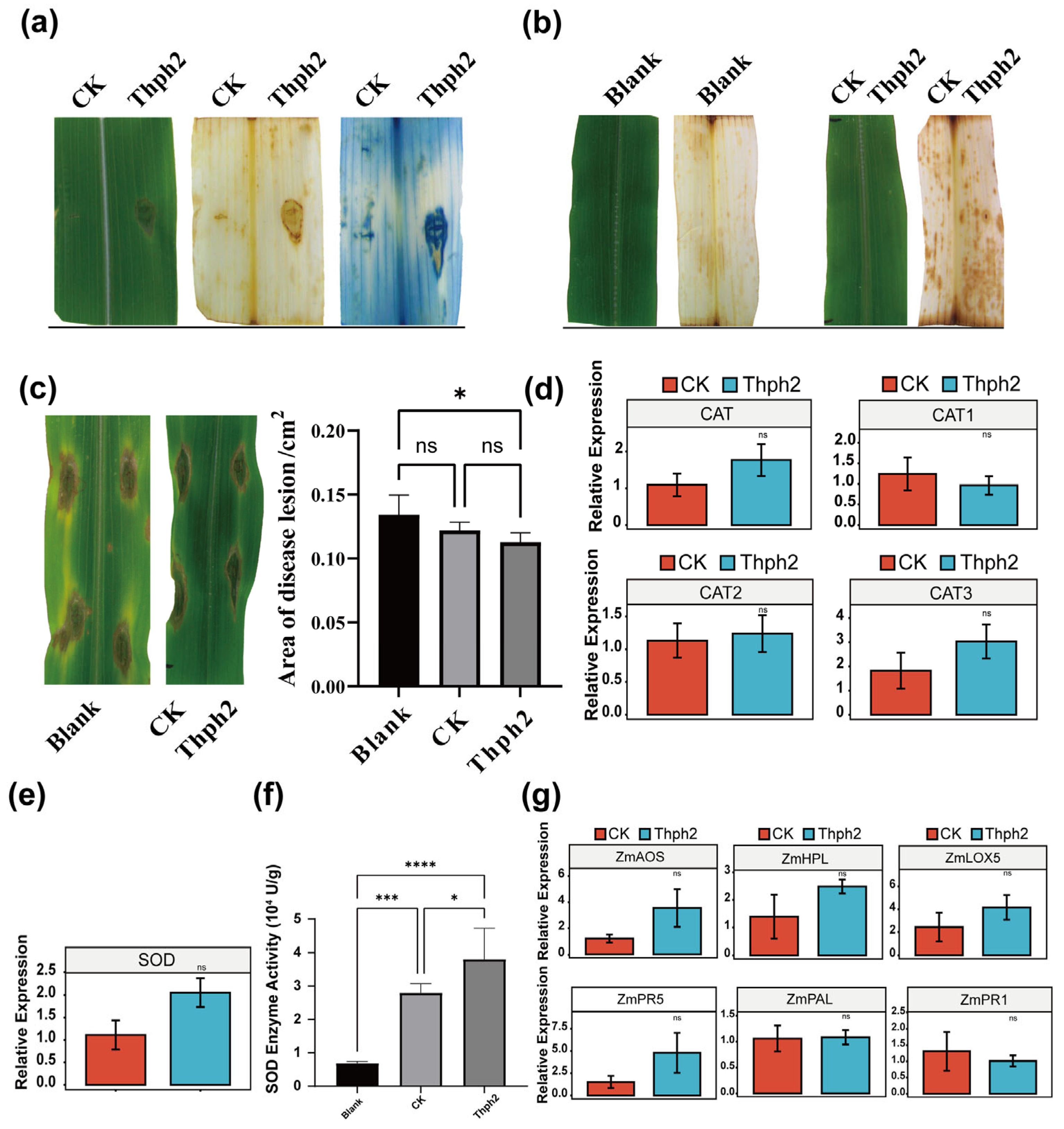
3.5. Thph2 Can Induce ROS Accumulation in Maize Leaves, Enhance Resistance to SCLB
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Katiyar-Agarwal, S.; Jin, H. Role of Small RNAs in Host-Microbe Interactions. Annu. Rev. Phytopathol. 2010, 48, 225–246. [Google Scholar] [CrossRef]
- Chisholm, S.T.; Coaker, G.; Day, B.; Staskawicz, B.J. Host-Microbe Interactions: Shaping the Evolution of the Plant Immune Response. Cell 2006, 124, 803–814. [Google Scholar] [CrossRef]
- Lu, Y.; Tsuda, K. Intimate Association of PRR- and NLR-Mediated Signaling in Plant Immunity. MPMI 2020, 34, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Kanyuka, K.; Rudd, J.J. Cell Surface Immune Receptors: The Guardians of the Plant’s Extracellular Spaces. Curr. Opin. Plant Biol. 2019, 50, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Boller, T.; Felix, G. A Renaissance of Elicitors: Perception of Microbe-Associated Molecular Patterns and Danger Signals by Pattern-Recognition Receptors. Annu. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Dangl, J.L. The Plant Immune System. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Wu, S.; Shan, L.; He, P. Microbial Signature-Triggered Plant Defense Responses and Early Signaling Mechanisms. Plant Sci. 2014, 228, 118–126. [Google Scholar] [CrossRef]
- Lorito, M.; Woo, S.L.; Harman, G.E.; Monte, E. Translational Research on Trichoderma: From ’Omics to the Field. Annu. Rev. Phytopathol. 2010, 48, 395–417. [Google Scholar] [CrossRef]
- Rubartelli, A.; Lotze, M.T. Inside, Outside, Upside down: Damage-Associated Molecular-Pattern Molecules (DAMPs) and Redox. Trends Immunol. 2007, 28, 429–436. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Fan, L.; Fu, K.; Yu, C.; Wang, M.; Xia, H.; Sun, J.; Li, Y.; Chen, J. Cellulase from Trichoderma Harzianum Interacts with Roots and Triggers Induced Systemic Resistance to Foliar Disease in Maize. Sci. Rep. 2016, 6, 35543. [Google Scholar] [CrossRef]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-Beneficial Effects of Trichoderma and of Its Genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma Species-Opportunistic, Avirulent Plant Symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Ton, J.; Van Pelt, J.A.; Van Loon, L.C.; Pieterse, C.M.J. Differential Effectiveness of Salicylate-Dependent and Jasmonate/Ethylene-Dependent Induced Resistance in Arabidopsis. MPMI 2002, 15, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-D.; Borrego, E.J.; Kenerley, C.M.; Kolomiets, M.V. Oxylipins Other Than Jasmonic Acid Are Xylem-Resident Signals Regulating Systemic Resistance Induced by Trichoderma Virens in Maize. Plant Cell 2019, 32, 166–185. [Google Scholar] [CrossRef] [PubMed]
- Zaid, R.; Koren, R.; Kligun, E.; Gupta, R.; Leibman-Markus, M.; Mukherjee, P.K.; Kenerley, C.M.; Bar, M.; Horwitz, B.A. Gliotoxin, an Immunosuppressive Fungal Metabolite, Primes Plant Immunity: Evidence from Trichoderma Virens -Tomato Interaction. MBio 2022, 13, e00389-22. [Google Scholar] [CrossRef]
- Coppola, M.; Cascone, P.; Di Lelio, I.; Woo, S.L.; Lorito, M.; Rao, R.; Pennacchio, F.; Guerrieri, E.; Digilio, M.C. Trichoderma Atroviride P1 Colonization of Tomato Plants Enhances Both Direct and Indirect Defense Barriers Against Insects. Front. Physiol. 2019, 10, 813. [Google Scholar] [CrossRef]
- Panda, S.; Kazachkova, Y.; Aharoni, A. Catch-22 in Specialized Metabolism: Balancing Defense and Growth. J. Exp. Bot. 2021, 72, 6027–6041. [Google Scholar] [CrossRef]
- Buscaill, P.; van der Hoorn, R.A.L. Defeated by the Nines: Nine Extracellular Strategies to Avoid Microbe-Associated Molecular Patterns Recognition in Plants. Plant Cell 2021, 33, 2116–2130. [Google Scholar] [CrossRef]
- Lang, B.; Chen, J. Trichoderma Harzianum Cellulase Gene Thph2 Affects Trichoderma Root Colonization and Induces Resistance to Southern Leaf Blight in Maize. J. Fungi 2023, 9, 1168. [Google Scholar] [CrossRef]
- Sachdev, S.; Singh, R.P. Isolation Characterisation and Screening of Native Microbial Isolates for Biocontrol of Fungal Pathogens of Tomato. Clim. Change Environ. Sustain. 2018, 6, 46–58. [Google Scholar] [CrossRef]
- Martinez, C.; Blanc, F.; Le Claire, E.; Besnard, O.; Nicole, M.; Baccou, J.-C. Salicylic Acid and Ethylene Pathways Are Differentially Activated in Melon Cotyledons by Active or Heat-Denatured Cellulase fromTrichoderma Longibrachiatum. Plant Physiol. 2001, 127, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dou, K.; Gao, Y.; Li, Y. Mechanism and application of Trichoderma spp. in biological control of corn diseases. MYCOSYSTEMA 2014, 33, 1154–1167. [Google Scholar] [CrossRef]
- Nawrocka, J.; Małolepsza, U. Diversity in Plant Systemic Resistance Induced by Trichoderma. Biol. Control 2013, 67, 149–156. [Google Scholar] [CrossRef]
- Fernández, E.; Segarra, G.; Trillas, M.I. Physiological Effects of the Induction of Resistance by Compost or Trichoderma Asperellum Strain T34 against Botrytis Cinerea in Tomato. Biol. Control 2014, 78, 77–85. [Google Scholar] [CrossRef]
- Chen, J. Advances on Trichoderma-induce Plant Resistance against Diseases. Chin. J. Biol. Control 2015, 31, 733–741. [Google Scholar] [CrossRef]
- Bhat, M.K. Cellulases and Related Enzymes in Biotechnology. Biotechnol. Adv. 2000, 18, 355–383. [Google Scholar] [CrossRef]
- Boisset, C.; Fraschini, C.; Schülein, M.; Henrissat, B.; Chanzy, H. Imaging the Enzymatic Digestion of Bacterial Cellulose Ribbons Reveals the Endo Character of the Cellobiohydrolase Cel6A fromHumicola Insolensand Its Mode of Synergy with Cellobiohydrolase Cel7A. Appl. Environ. Microbiol. 2000, 66, 1444–1452. [Google Scholar] [CrossRef]
- Wang, B.; Xia, L. High Efficient Expression of Cellobiase Gene from Aspergillus Niger in the Cells of Trichoderma Reesei. Bioresour. Technol. 2011, 102, 4568–4572. [Google Scholar] [CrossRef]
- Singh, R.; Srivastava, R.P.; Ram, L. Southern Corn Leaf Blight—An Important Disease of Maize: An Extension Fact Sheet. Indian Res. J. Ext. Educ. 2012, 2, 239–241. [Google Scholar]
- Jacobs, T. Why Do Plant Cells Divide? Plant Cell 1997, 9, 1021–1029. [Google Scholar] [CrossRef]
- Morris, S.W.; Vernooij, B.; Titatarn, S.; Starrett, M.; Thomas, S.; Wiltse, C.C.; Frederiksen, R.A.; Bhandhufalck, A.; Hulbert, S.; Uknes, S. Induced Resistance Responses in Maize. MPMI 1998, 11, 643–658. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.M.; Flors, V.; García-Agustín, P.; Jakab, G.; Mauch, F.; Newman, M.-A.; Pieterse, C.M.J.; Poinssot, B.; Pozo, M.J.; et al. Priming: Getting Ready for Battle. MPMI 2006, 19, 1062–1071. [Google Scholar] [CrossRef]
- Feussner, I.; Wasternack, C. The LIPOXYGENASE PATHWAY. Annu. Rev. Plant Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef]
- Farag, M.A.; Fokar, M.; Abd, H.; Zhang, H.; Allen, R.D.; Paré, P.W. (Z)-3-Hexenol Induces Defense Genes and Downstream Metabolites in Maize. Planta 2004, 220, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.M.; Weeden, N.F.; Martín, A. Linkage among Isozyme, RFLP and RAPD Markers in Vicia Faba. Theor. Appl. Genet. 1993, 85, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Akshaya, R.L.; Rohini, M.; He, Z.; Partridge, N.C.; Selvamurugan, N. MiR-4638-3p Regulates Transforming Growth Factor-Β1-Induced Activating Transcription Factor-3 and Cell Proliferation, Invasion, and Apoptosis in Human Breast Cancer Cells. Int. J. Biol. Macromol. 2022, 222, 1974–1982. [Google Scholar] [CrossRef]
- Hermosa, R.; Rubio, M.B.; Cardoza, R.E.; Nicolás, C.; Monte, E.; Gutiérrez, S. The Contribution of Trichoderma to Balancing the Costs of Plant Growth and Defense. Int. Microbiol. 2013, 16, 69–80. [Google Scholar] [CrossRef]
- Anand, G.; Leibman-Markus, M.; Elkabetz, D.; Bar, M. Method for the Production and Purification of Plant Immuno-Active Xylanase from Trichoderma. IJMS 2021, 22, 4214. [Google Scholar] [CrossRef]
- Inglis, G.D.; Kawchuk, L.M. Comparative Degradation of Oomycete, Ascomycete, and Basidiomycete Cell Walls by Mycoparasitic and Biocontrol Fungi. Can. J. Microbiol. 2002, 48, 60–70. [Google Scholar] [CrossRef]
- He, J.; Kong, M.; Qian, Y.; Gong, M.; Lv, G.; Song, J. Cellobiose Elicits Immunity in Lettuce Conferring Resistance toBotrytis Cinerea. J. Exp. Bot. 2022, 74, 1022–1038. [Google Scholar] [CrossRef]
- Fan, L.; Fu, K.; Yu, C.; Li, Y.; Li, Y.; Chen, J. Thc6 Protein, Isolated from Trichoderma Harzianum, Can Induce Maize Defense Response against Curvularia Lunata. J. Basic Microbiol. 2014, 55, 591–600. [Google Scholar] [CrossRef]
- Ma, Y.; Han, C.; Chen, J.; Li, H.; He, K.; Liu, A.; Li, D. Fungal Cellulase Is an Elicitor but Its Enzymatic Activity Is Not Required for Its Elicitor Activity. Mol. Plant Pathol. 2014, 16, 14–26. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Messner, R.; Gruber, F.; Mach, R.L.; Kubicek-Pranz, E.M. The Trichoderma Cellulase Regulatory Puzzle: From the Interior Life of a Secretory Fungus. Enzyme Microb. Technol. 1993, 15, 90–99. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Herrera-Estrella, A.; Seidl-Seiboth, V.; Martinez, D.A.; Druzhinina, I.S.; Thon, M.; Zeilinger, S.; Casas-Flores, S.; Horwitz, B.A.; Mukherjee, P.K.; et al. Comparative Genome Sequence Analysis Underscores Mycoparasitism as the Ancestral Life Style of Trichoderma. Genome Biol. 2011, 12, R40. [Google Scholar] [CrossRef]
- Kubicek, C.P. Systems Biological Approaches towards Understanding Cellulase Production by Trichoderma Reesei. J. Biotechnol. 2012, 163, 133–142. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lang, B.; Liu, H.; Si, G.; Zhang, X.; Zhang, C.; Wang, J.; Chen, J. Trichoderma harzianum Cellobiohydrolase Thph2 Induces Reactive Oxygen Species-Mediated Resistance Against Southern Corn Leaf Blight in Maize. J. Fungi 2025, 11, 629. https://doi.org/10.3390/jof11090629
Lang B, Liu H, Si G, Zhang X, Zhang C, Wang J, Chen J. Trichoderma harzianum Cellobiohydrolase Thph2 Induces Reactive Oxygen Species-Mediated Resistance Against Southern Corn Leaf Blight in Maize. Journal of Fungi. 2025; 11(9):629. https://doi.org/10.3390/jof11090629
Chicago/Turabian StyleLang, Bo, Hongyi Liu, Gaoyue Si, Xifen Zhang, Cheng Zhang, Jing Wang, and Jie Chen. 2025. "Trichoderma harzianum Cellobiohydrolase Thph2 Induces Reactive Oxygen Species-Mediated Resistance Against Southern Corn Leaf Blight in Maize" Journal of Fungi 11, no. 9: 629. https://doi.org/10.3390/jof11090629
APA StyleLang, B., Liu, H., Si, G., Zhang, X., Zhang, C., Wang, J., & Chen, J. (2025). Trichoderma harzianum Cellobiohydrolase Thph2 Induces Reactive Oxygen Species-Mediated Resistance Against Southern Corn Leaf Blight in Maize. Journal of Fungi, 11(9), 629. https://doi.org/10.3390/jof11090629





