Pathogenic Roles of Polyketide Synthase CLPKS18 and (R)-(-)-Mellein from Curvularia lunata in Maize Leaf Spot
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Maize Cultivars
2.2. Transcriptome Sequencing and Analysis
2.3. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) Verification
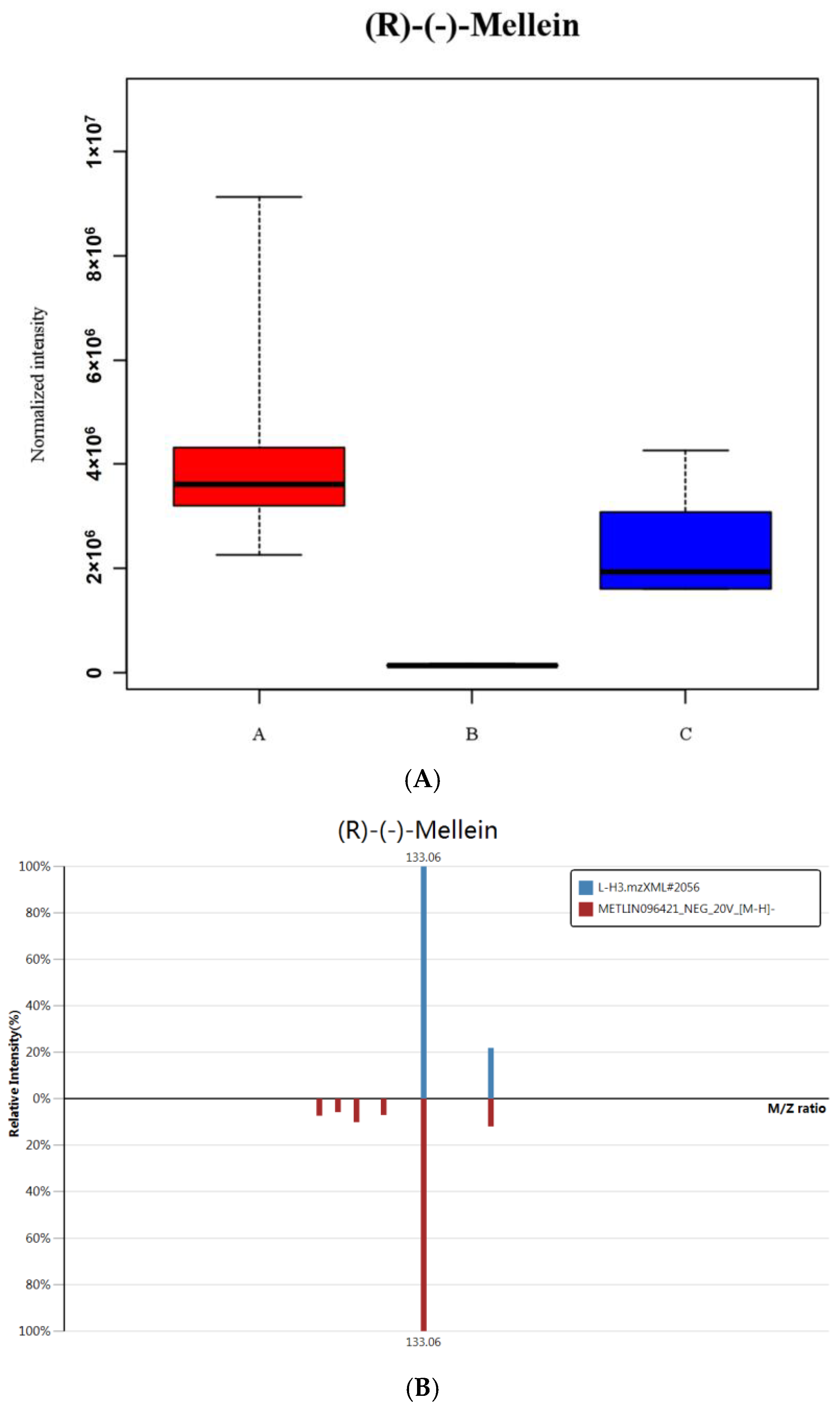
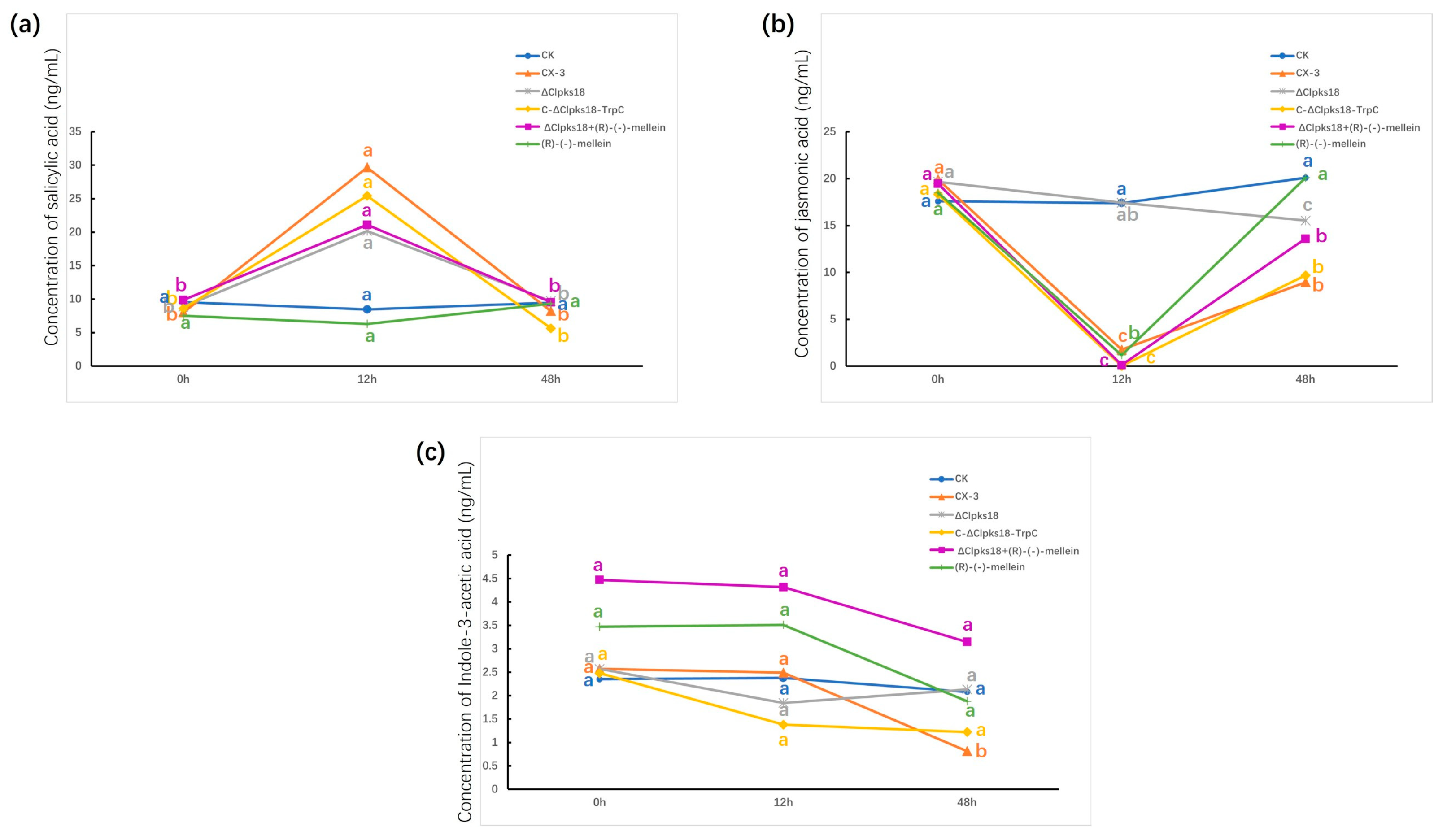
2.4. Extraction and Detection of JA, SA and IAA in Maize Leaves
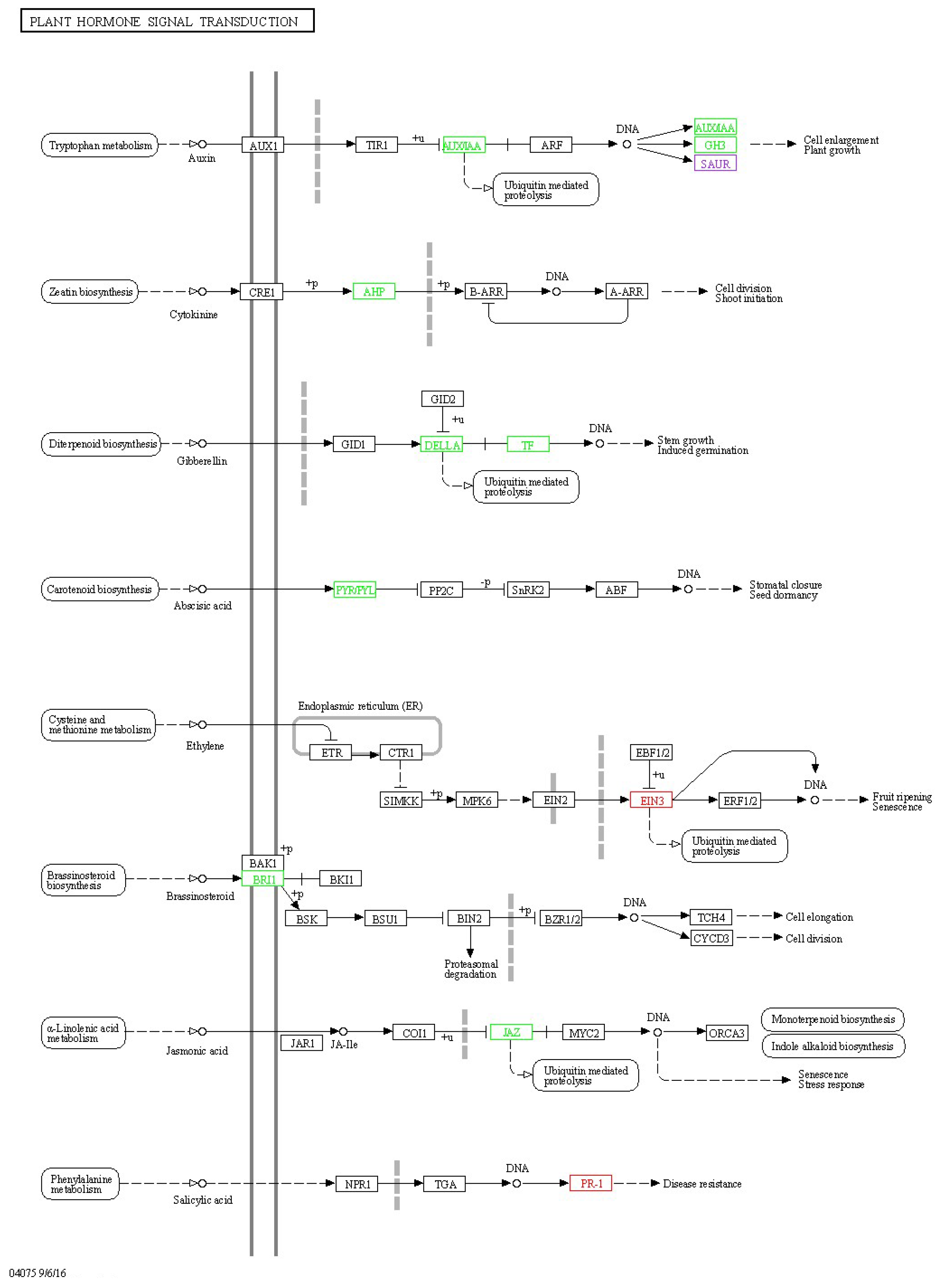
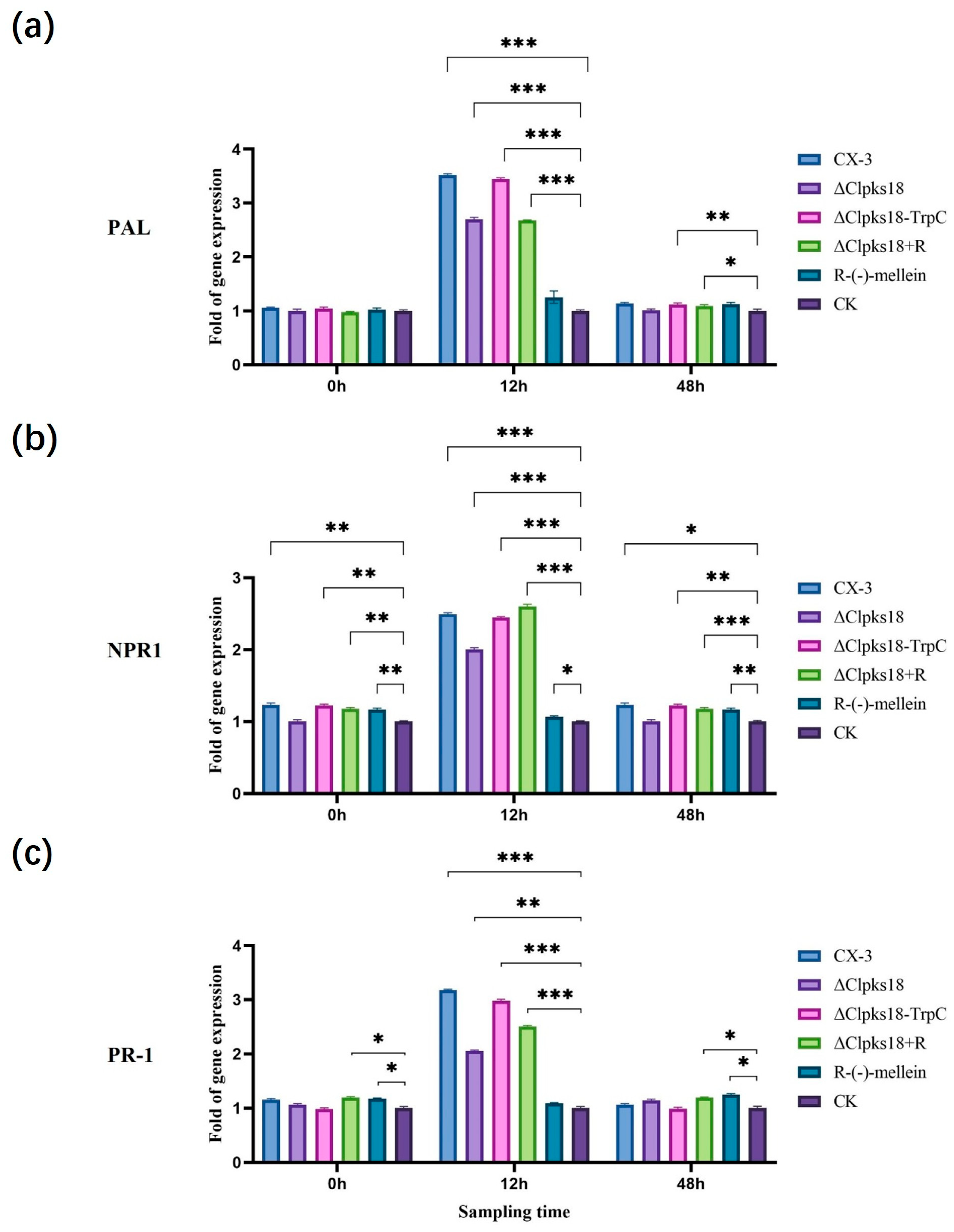
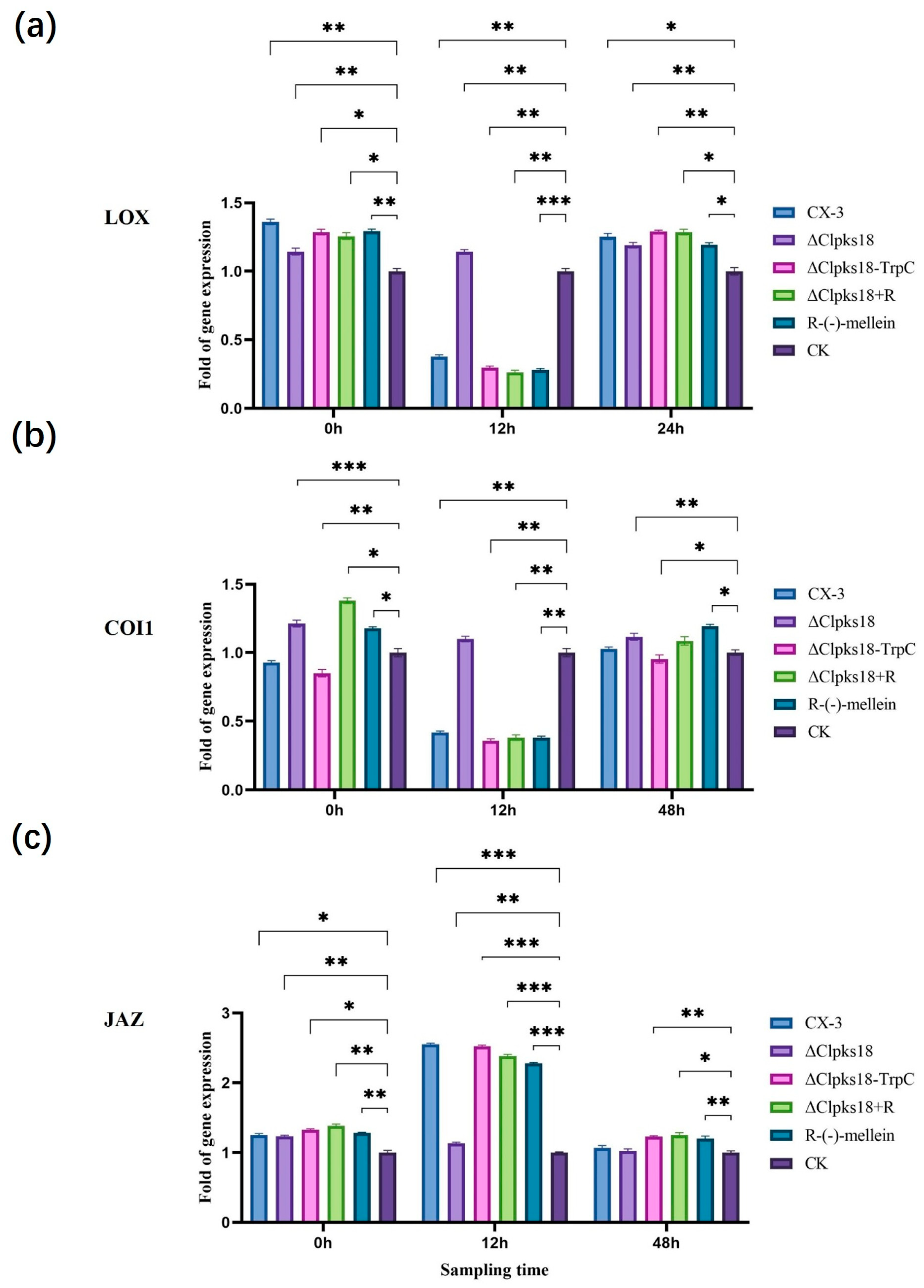
2.5. Signaling Pathway-Related Gene Analysis
3. Results
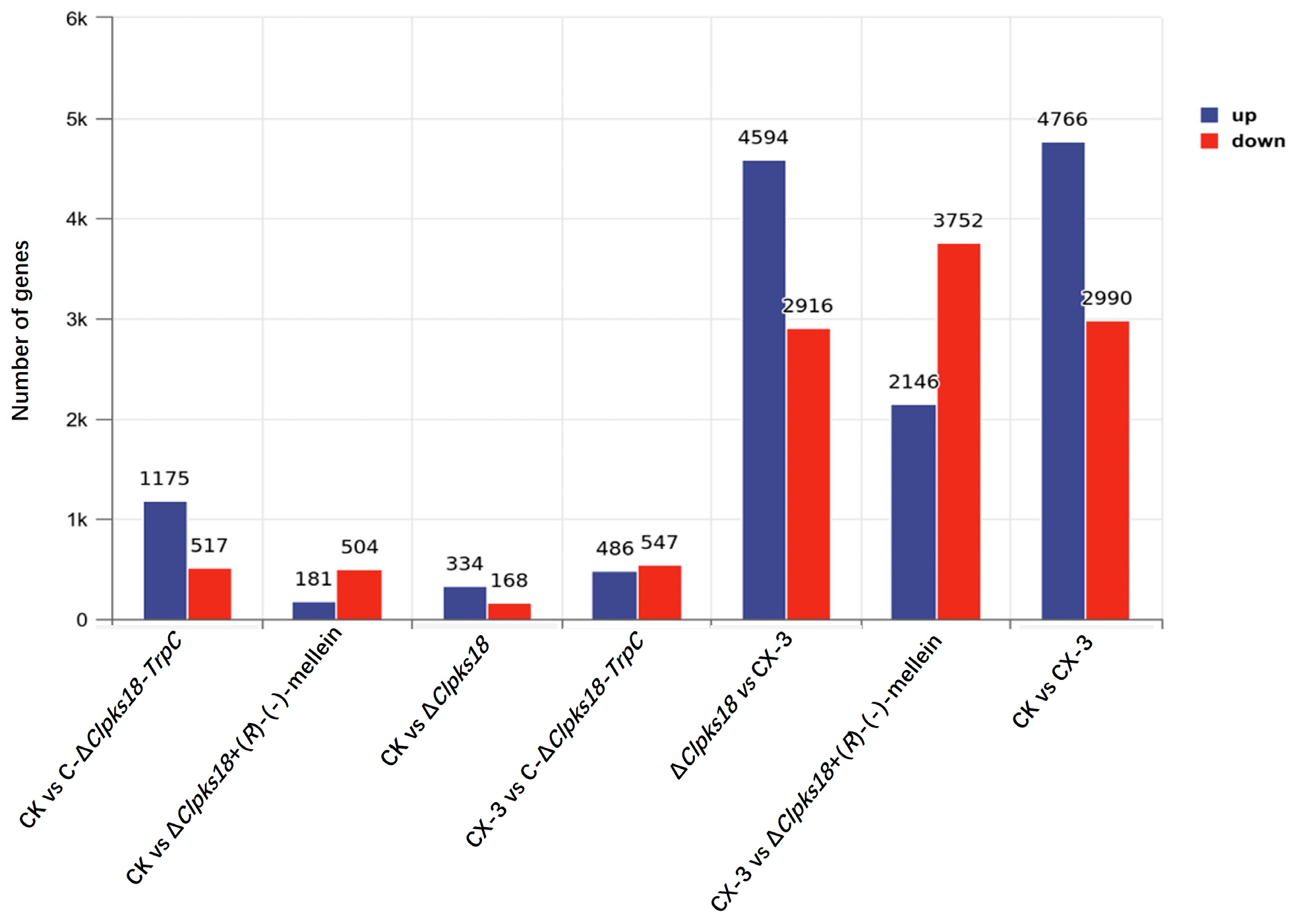
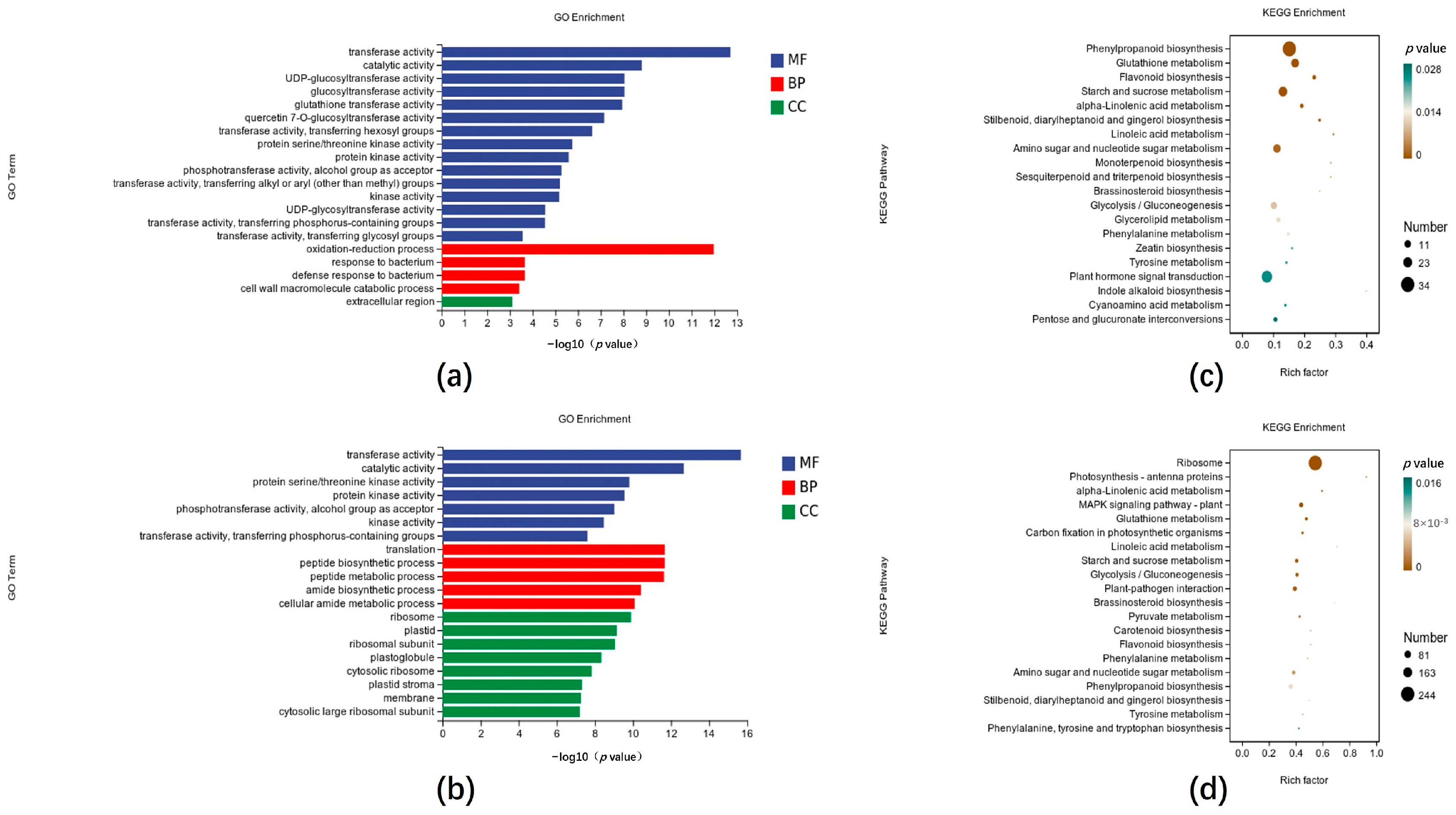
3.1. Different Expression Patterns Between the CX-3 and ΔClpks18 Treatments
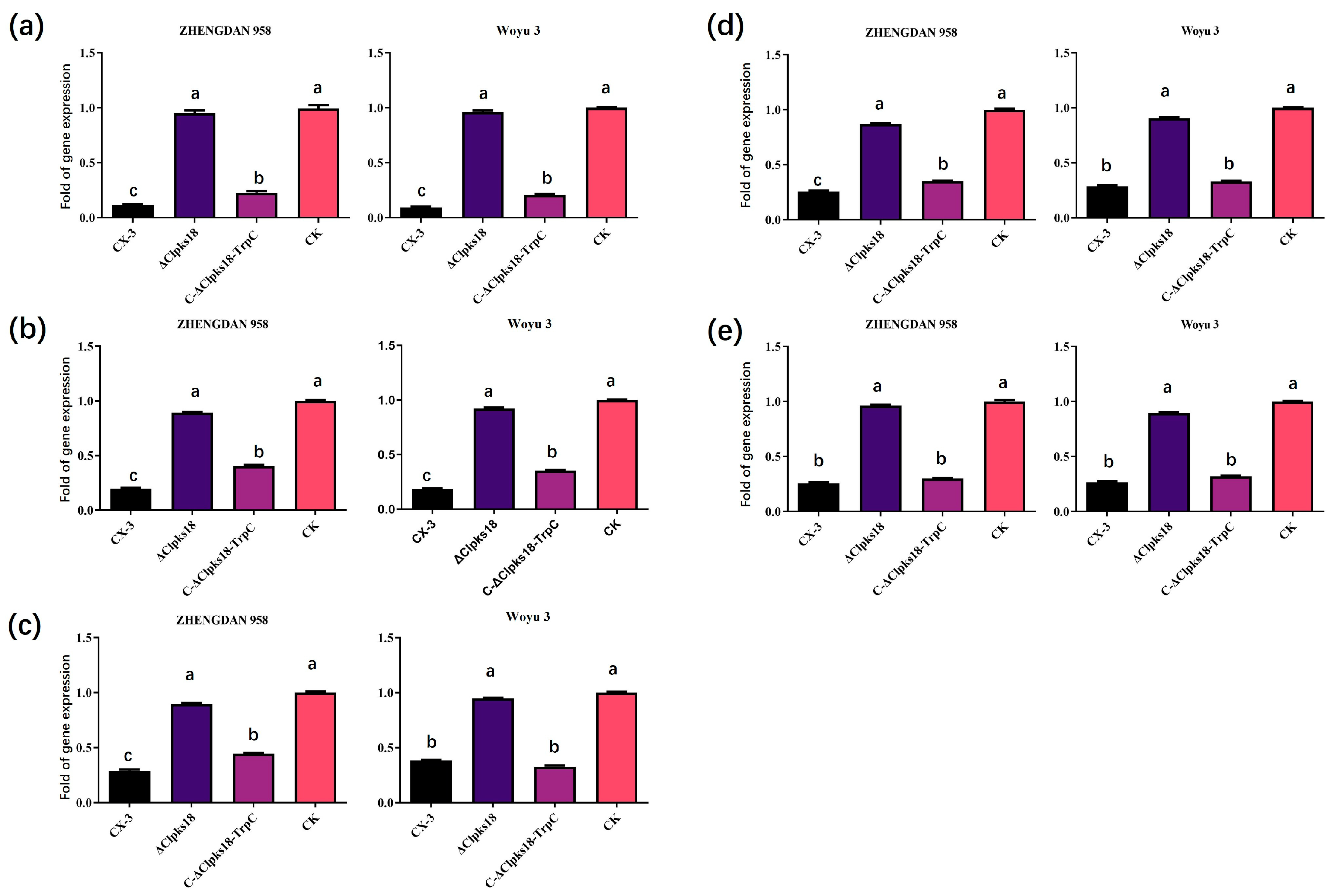
3.2. Quantitative PCR Validation Is Consistent with Transcriptome
3.3. Effects of Clpks18 on Hormone Production and Signaling Systems in Maize
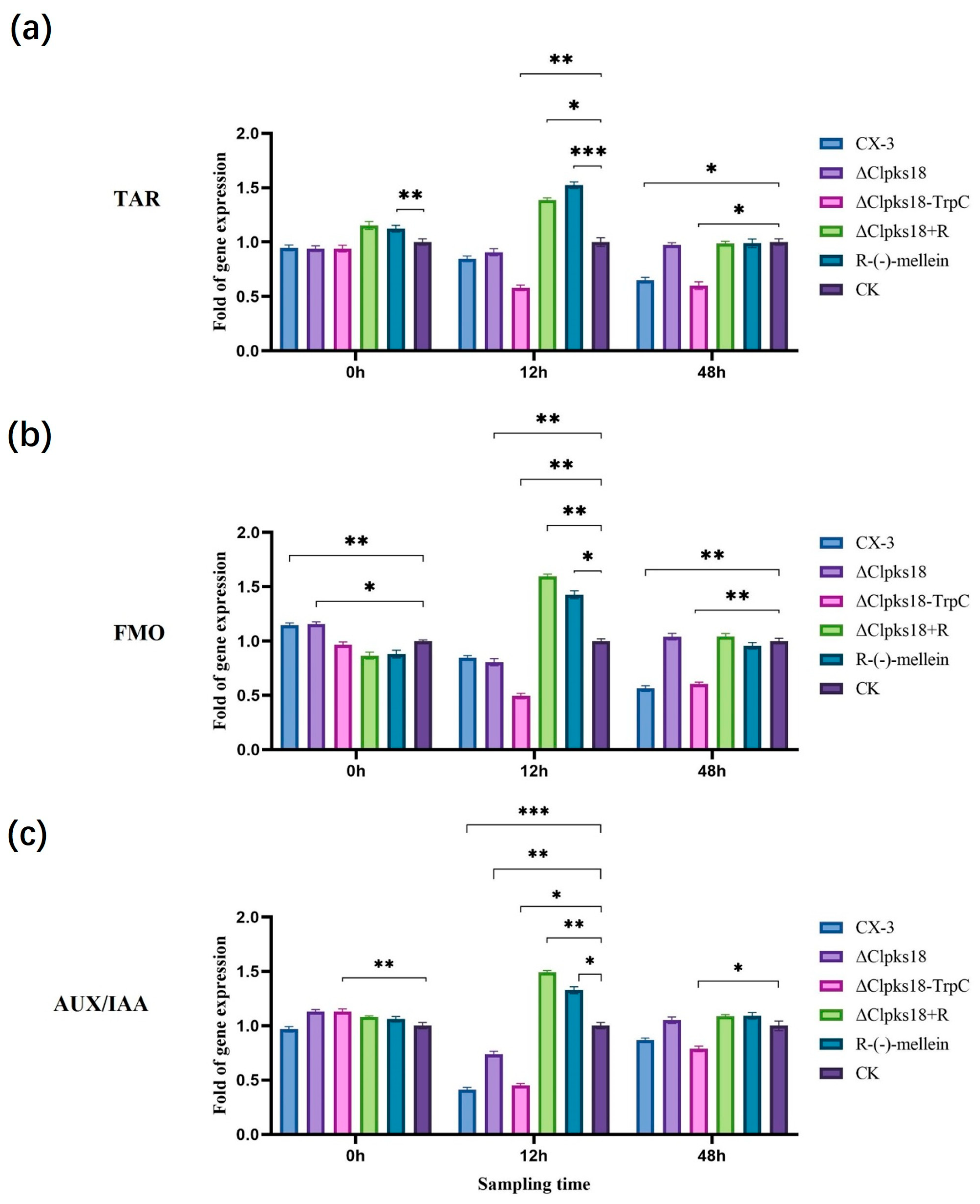
3.4. Comparison and Analysis of Effects of CLPKS18 and (R)-(-)-Mellein on SA, JA and IAA Production and Signaling Pathways in Maize
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Primer Name | Sequence (5′ to 3′) |
|---|---|
| LOC100274569F | TGAGTACCGCAAGGATGGTG |
| LOC100274569R | GCGCGACGATACACTAGACA |
| LOC103641361F | CCCGAACATTCCCGTTTGGT |
| LOC103641361R | AAGGACTCTGTGCCGTTTGG |
| LOC100384229F | TGACGAAAATCCAGCCAGCA |
| LOC100384229R | AGCAGGAAATGGTGTTAGCGA |
| LOC100193303F | TCCAGATCCTCTCCGCCAAC |
| LOC100193303R | TGCAAACAGGCACAAATGCG |
| LOC103653849F | TACTTCAACCACCCGCTGT |
| LOC103653849R | CACATGCTGCTACCACTACCA |
| LOC103649700F | GTCACGGATTTCCTCCGGTC |
| LOC103649700R | TGATGGCTGTGTACTGCGG |
| LOC100037821F | CCTTGGTTATCCGTAGCCCG |
| LOC100037821R | CTTGGCCTTGAGAAGAAGCG |
| lox1f | GACCCTCGCCTCAAGAACC |
| lox1r | GAACGGGGAAACGCAAACAA |
| LOC100273108F | CCCAGTTCTCCTCCTTCGAC |
| LOC100273108R | AGCTCTGGACCCTGAACATC |
| LOC103638892F | CCGTTCTTCAAGCCCGAGTC |
| LOC103638892R | GTGGTGGTTGGTGTTGCTCG |
| LOC100280173F | CCGGCTTGTTCTGCTCGATA |
| LOC100280173R | CATCATCGGTTTCCCCAACG |
| LOC100384215F | GCGAGAAGCTCAAGTCCCC |
| LOC100384215R | TGTCATCTCCTCCTCTTGCG |
| LOC100281196F | TCGCCTTTGTTGGCAGATGA |
| LOC100281196R | CTGGCGTCTCATTAAGGTCCA |
| LOC100286221F | AGATCATGTGGCGGAGCAC |
| LOC100286221R | CAGCAACGATTTGGGACAGC |
| ZmUBI-F | GGAAAAACCATAACCCTGGA |
| ZmUBI-R | ATATGGAGAGAGGGCACCAG |
Appendix B


References
- Liu, T.; Wang, Y.; Ma, B.; Hou, J.; Jin, Y.; Zhang, Y.; Ke, X.; Tai, L.; Zuo, Y.; Dey, K. Clg2p interacts with Clf and ClUrase to regulate appressorium formation, pathogenicity and conidial morphology in Curvularia lunata. Sci. Rep. 2016, 6, 24047. [Google Scholar] [CrossRef]
- Gao, J.; Gao, S.; Li, Y.; Chen, J. Genome-wide prediction and analysis of the classical secreted proteins of Curvularia lunata. J. Plant Prot. 2015, 42, 869–876. [Google Scholar] [CrossRef]
- Jing, F. The Pathogenesis of the Cell-degrading Enzymes Produced by Curvularia lunata. Rain Fed. Crops 2002, 22, 164–167. [Google Scholar]
- Liu, T.; Liu, L.; Jiang, X.; Huang, X.; Chen, J. A new furanoid toxin produced by Curvularia lunata, the causal agent of maize Curvularia leaf spot. Can. J. Plant Pathol. 2009, 31, 22–27. [Google Scholar] [CrossRef]
- Xu, S.; Chen, J.; Liu, L.; Wang, X.; Huang, X.; Zhai, Y. Proteomics Associated with Virulence Differentiation of Curvularia lunata in Maize in China. J. Integr. Plant Biol. 2007, 49, 487–496. [Google Scholar] [CrossRef]
- Ayer, W.A.; Shewchuk, L.M. Metabolites of Nectria fuckeliana. J. Nat. Prod. 1986, 49, 947–948. [Google Scholar] [CrossRef]
- Venkatasubbaiah, P.; Baudoin, A.B.A.M.; Chilton, W.S. Leaf Spot of Hemp Dogbane Caused by Stagonospora apocyni, and its Phytotoxins. J. Phytopathol. 1992, 135, 309–316. [Google Scholar] [CrossRef]
- Li, Y.; Gai, Z.; Wang, C.; Li, P.; Li, B. Identification of Mellein as a Pathogenic Substance of Botryosphaeria dothidea by UPLC-MS/MS Analysis and Phytotoxic Bioassay. J. Agric. Food Chem. 2021, 69, 8471–8481. [Google Scholar] [CrossRef]
- Matei, P.M.; Sánchez-Báscones, M.; Bravo-Sánchez, C.T.; Martín-Ramos, P.; Martín-Villullas, M.T.; García-González, M.C.; Hernández-Navarro, S.; Navas-Gracia, L.M.; Martín-Gil, J. Hygienization and control of Diplodia seriata fungus in vine pruning waste composting and its seasonal variability in open and closed systems. Waste Manag. 2016, 58, 126–134. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, S.; Dou, K.; Ren, J.; Chen, J. The Interpretation of the Role of a Polyketide Synthase ClPKS18 in the Pathogenicity of Curvularia lunata. Front. Microbiol. 2022, 13, 853140. [Google Scholar] [CrossRef]
- Chooi, Y.-H.; Krill, C.; Barrow, R.A.; Chen, S.; Trengove, R.; Oliver, R.P.; Solomon, P.S. An in planta-expressed polyketide synthase produces (R)-mellein in the wheat pathogen Parastagonospora nodorum. Appl. Environ. Microbiol. 2015, 81, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Condon, B.J.; Leng, Y.; Wu, D.; Bushley, K.E.; Ohm, R.A.; Otillar, R.; Martin, J.; Schackwitz, W.; Grimwood, J.; MohdZainudin, N.; et al. Comparative genome structure, secondary metabolite, and effector coding capacity across Cochliobolus pathogens. PLoS Genet. 2013, 9, e1003233. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J.D. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Adie, B.A.; Pérez-Pérez, J.; Pérez-Pérez, M.M.; Godoy, M.; Sánchez-Serrano, J.J.; Schmelz, E.A.; Solano, R. ABA is an essential signal for plant resistance to pathogens affecting JA biosynthesis and the activation of defenses in Arabidopsis. Plant Cell 2007, 19, 1665–1681. [Google Scholar] [CrossRef]
- NY/T 1248.10-2016; Technical Specification for Identification of Pests and Diseases of Maize—Part 10: Curvularia Leaf Spot. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2016.
- Bao, D.; Chang, S.; Li, X.; Qi, Y. Advances in the study of auxin early response genes: Aux/IAA, GH3, and SAUR. Crop J. 2024, 12, 964–978. [Google Scholar] [CrossRef]
- Liu, S.; Wang, C.; Liu, X.; Navas-Castillo, J.; Zang, L.; Fan, Z.; Zhu, X.; Zhou, T. Tomato chlorosis virus-encoded p22 suppresses auxin signalling to promote infection via interference with SKP1-Cullin-F-box(TIR1) complex assembly. Plant Cell Environ. 2021, 44, 3155–3172. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Pajerowska-Mukhtar, K.; Culler, A.H.; Dong, X. Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Curr. Biol. 2007, 17, 1784–1790. [Google Scholar] [CrossRef]
- Ye, J.; Zhong, T.; Zhang, D.; Ma, C.; Wang, L.; Yao, L.; Zhang, Q.; Zhu, M.; Xu, M. The Auxin-Regulated Protein ZmAuxRP1 Coordinates the Balance between Root Growth and Stalk Rot Disease Resistance in Maize. Mol. Plant 2019, 12, 360–373. [Google Scholar] [CrossRef]
- Li, K.; Yu, R.; Fan, L.-M.; Wei, N.; Chen, H.; Deng, X.W. DELLA-mediated PIF degradation contributes to coordination of light and gibberellin signalling in Arabidopsis. Nat. Commun. 2016, 7, 11868. [Google Scholar] [CrossRef]
- Zhao, H.; Bao, Y. PIF4: Integrator of light and temperature cues in plant growth. Plant Sci. 2021, 313, 111086. [Google Scholar] [CrossRef]
- Du, H.; Chang, Y.; Huang, F.; Xiong, L. GID1 modulates stomatal response and submergence tolerance involving abscisic acid and gibberellic acid signaling in rice. J. Integr. Plant Biol. 2015, 57, 954–968. [Google Scholar] [CrossRef]
- Ci, J.; Wang, X.; Wang, Q.; Zhao, F.; Yang, W.; Cui, X.; Jiang, L.; Ren, X.; Yang, W. Genome-wide analysis of gibberellin-dioxygenases gene family and their responses to GA applications in maize. PLoS ONE 2021, 16, e0250349. [Google Scholar] [CrossRef]
- Yang, D.-L.; Li, Q.; Deng, Y.-W.; Lou, Y.-G.; Wang, M.-Y.; Zhou, G.-X.; Zhang, Y.-Y.; He, Z.-H. Altered Disease Development in the eui Mutants and Eui Overexpressors Indicates that Gibberellins Negatively Regulate Rice Basal Disease Resistance. Mol. Plant 2008, 1, 528–537. [Google Scholar] [CrossRef]
- Katsir, L.; Schilmiller, A.L.; Staswick, P.E.; He, S.Y.; Howe, G.A. COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc. Natl. Acad. Sci. USA 2008, 105, 7100–7105. [Google Scholar] [CrossRef]
- Ruan, J.; Zhou, Y.; Zhou, M.; Yan, J.; Khurshid, M.; Weng, W.; Cheng, J.; Zhang, K. Jasmonic Acid Signaling Pathway in Plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef]
- Han, Y.; Luthe, D. Key Genes in the JAZ Signaling Pathway Are Up-Regulated Faster and More Abundantly in Caterpillar-Resistant Maize. J. Chem. Ecol. 2022, 48, 179–195. [Google Scholar] [CrossRef]
- Yao, J.; Withers, J.; He, S.Y. Pseudomonas syringae infection assays in Arabidopsis. Methods Mol. Biol. 2013, 1011, 63–81. [Google Scholar] [CrossRef]
- Mutka, A.M.; Fawley, S.; Tsao, T.; Kunkel, B.N. Auxin promotes susceptibility to Pseudomonas syringae via a mechanism independent of suppression of salicylic acid-mediated defenses. Plant J. 2013, 74, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.Y.; Huang, Y.H.; Manawasinghe, I.S.; Wanasinghe, D.N.; Liu, J.W.; Shu, Y.X.; Zhao, M.P.; Xiang, M.M.; Luo, M. Stagonosporopsis pogostemonis: A Novel Ascomycete Fungus Causing Leaf Spot and Stem Blight on Pogostemon cablin (Lamiaceae) in South China. Pathogens 2021, 10, 1093. [Google Scholar] [CrossRef] [PubMed]
- Reveglia, P.; Billones-Baaijens, R.; Niem, J.; Masi, M.; Cimmino, A.; Evidente, A.; Savocchia, S. Production of Phytotoxic Metabolites by Botryosphaeriaceae in Naturally Infected and Artificially Inoculated Grapevines. Plants 2021, 10, 802. [Google Scholar] [CrossRef]
- Solis-Ortiz, C.S.; Gonzalez-Bernal, J.; Kido-Díaz, H.A.; Peña-Uribe, C.A.; López-Bucio, J.S.; López-Bucio, J.; Guevara-García, Á.A.; García-Pineda, E.; Villegas, J.; Campos-García, J.; et al. Bacterial cyclodipeptides elicit Arabidopsis thaliana immune responses reducing the pathogenic effects of Pseudomonas aeruginosa PAO1 strains on plant development. J. Plant Physiol. 2022, 275, 153738. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Shang, L.; Wang, S.; Wang, X.; Li, Y.; Zhang, S.; Wang, J.; Chen, J. Pathogenic Roles of Polyketide Synthase CLPKS18 and (R)-(-)-Mellein from Curvularia lunata in Maize Leaf Spot. J. Fungi 2025, 11, 627. https://doi.org/10.3390/jof11090627
Lu Z, Shang L, Wang S, Wang X, Li Y, Zhang S, Wang J, Chen J. Pathogenic Roles of Polyketide Synthase CLPKS18 and (R)-(-)-Mellein from Curvularia lunata in Maize Leaf Spot. Journal of Fungi. 2025; 11(9):627. https://doi.org/10.3390/jof11090627
Chicago/Turabian StyleLu, Zhixiang, Lin Shang, Shaoqing Wang, Xinhua Wang, Yaqian Li, Shunping Zhang, Jing Wang, and Jie Chen. 2025. "Pathogenic Roles of Polyketide Synthase CLPKS18 and (R)-(-)-Mellein from Curvularia lunata in Maize Leaf Spot" Journal of Fungi 11, no. 9: 627. https://doi.org/10.3390/jof11090627
APA StyleLu, Z., Shang, L., Wang, S., Wang, X., Li, Y., Zhang, S., Wang, J., & Chen, J. (2025). Pathogenic Roles of Polyketide Synthase CLPKS18 and (R)-(-)-Mellein from Curvularia lunata in Maize Leaf Spot. Journal of Fungi, 11(9), 627. https://doi.org/10.3390/jof11090627









