Sodium Cuminate Inhibits the Mycelial Growth of Penicillium digitatum by Inducing Oxidative Stress and Damaging the Cell Membrane
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Citrus Fruits
2.2. SC Preparation
2.3. The Antifungal Activity of SC Against P. digitatum
2.4. The Effects of SC on Disease Control and Quality of Citrus Fruit Inoculated with P. digitatum
2.5. Effects of SC on Naturally Occurring Diseases and Quality of Citrus Fruits
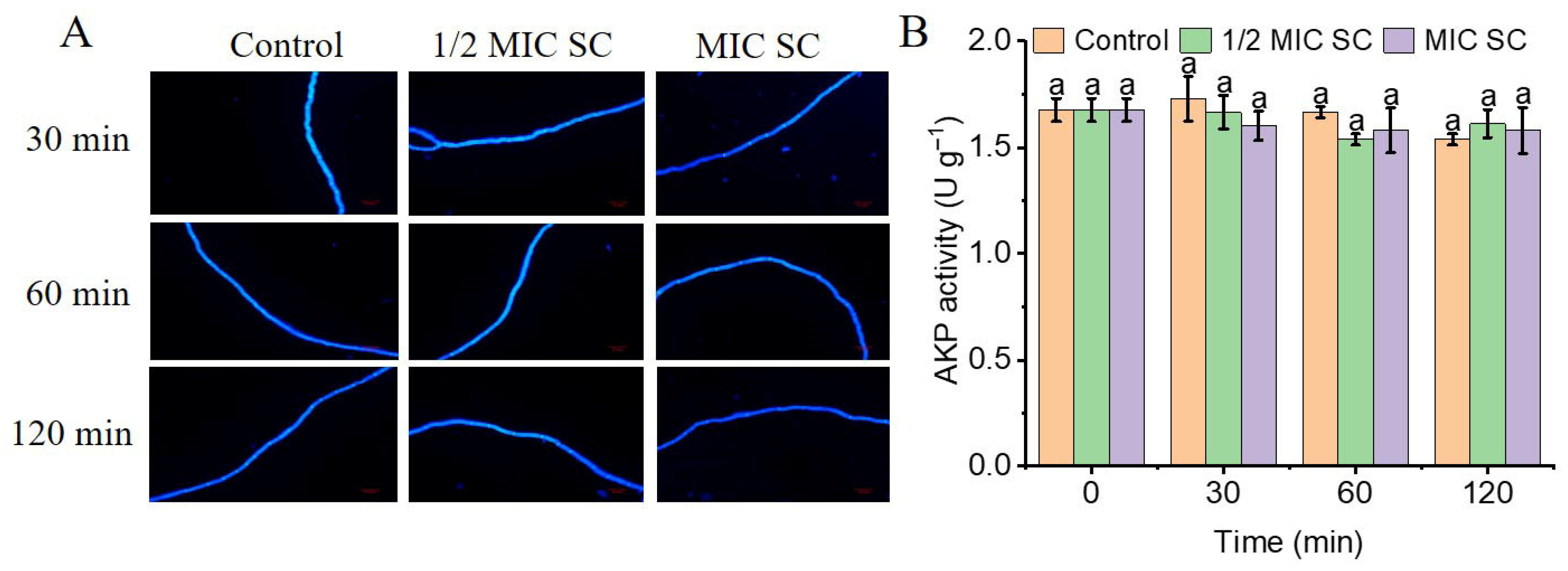
2.6. Effects of SC Treatment on the Cell Wall Integrity of P. digitatum
2.7. Effect of SC Treatment on the Cell Membrane Integrity of P. digitatum
2.8. Effects of SC Treatment on Oxidative Stress in P. digitatum
2.9. Effects of Exogenous Cysteine (Cys) or Diphenyleneiodonium Chloride (DPI) on ROS-Related Biochemical Parameters
2.10. Effects of Exogenous Cys and DPI on the Surface Morphology of SC-Treated P. digitatum
2.11. Effects of Exogenous Cys and DPI on the Cell Membrane Integrity of SC-Treated P. digitatum
2.12. Effects of Exogenous Cys and DPI on the Mitochondrial Membrane Potential (MMP) of SC-Treated P. digitatum
2.13. Statistical Analyses
3. Results
3.1. The Antifungal Activity of SC Against P. digitatum
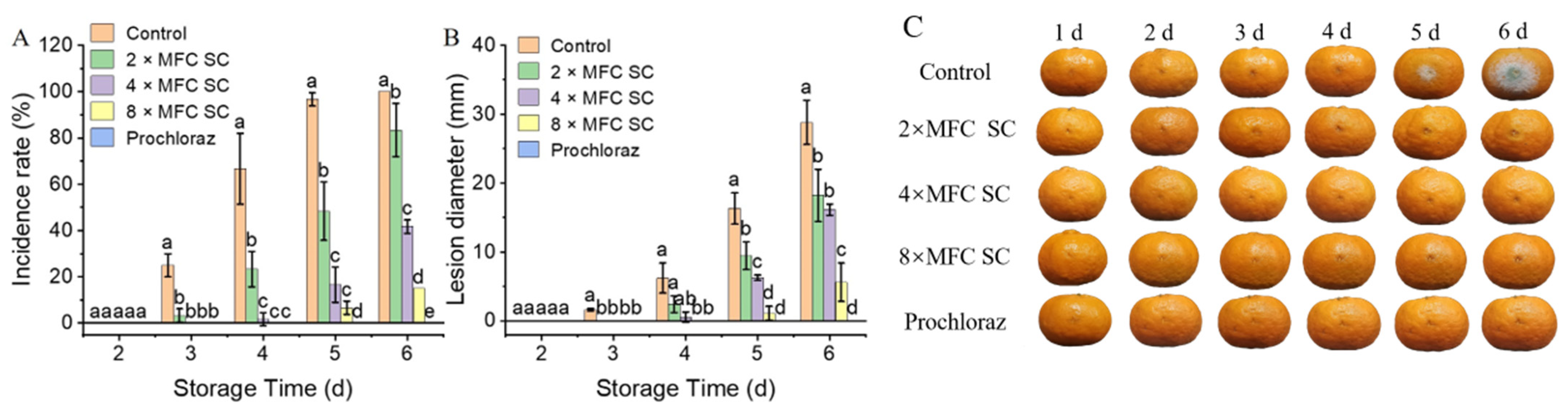
3.2. The Effect of SC on Green Mold in Citrus Fruit Incubated with P. digitatum
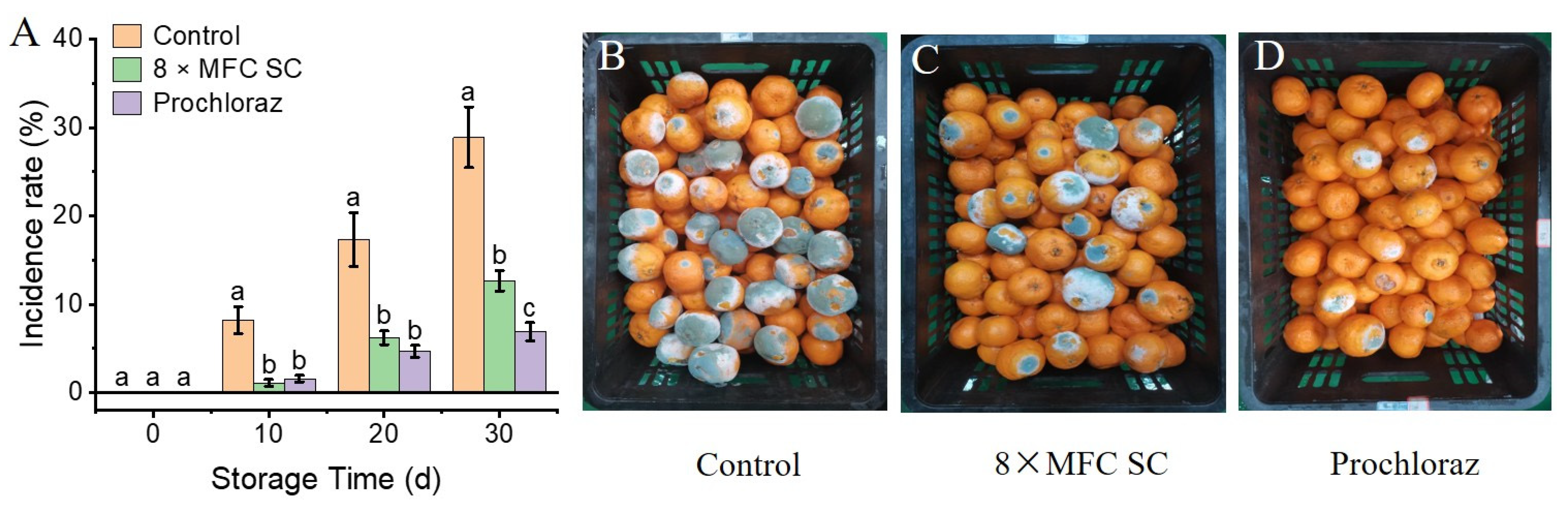
3.3. Effects of SC Treatment on Naturally Occurring Diseases in Citrus Fruits
3.4. SC Does Not Compromise the Cell Wall Integrity of P. digitatum
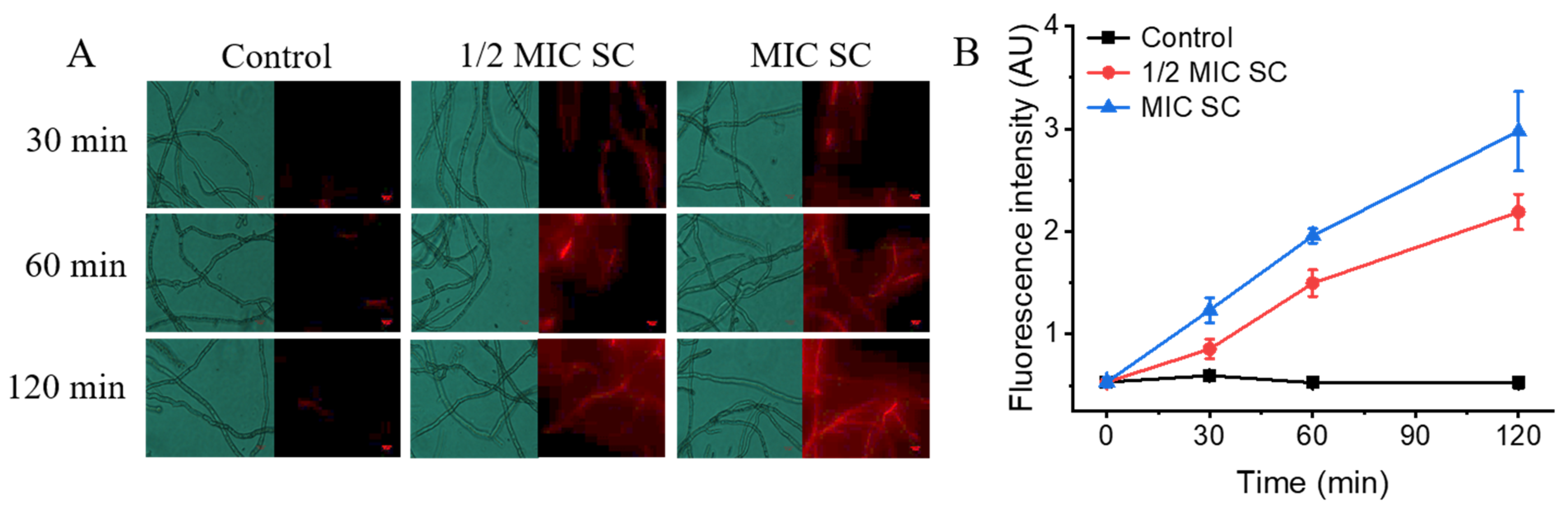
3.5. SC Treatment Impairs the Cell Membrane Integrity of P. digitatum
3.6. SC Induces a Massive Accumulation of ROS and Lipid Peroxidation in P. digitatum
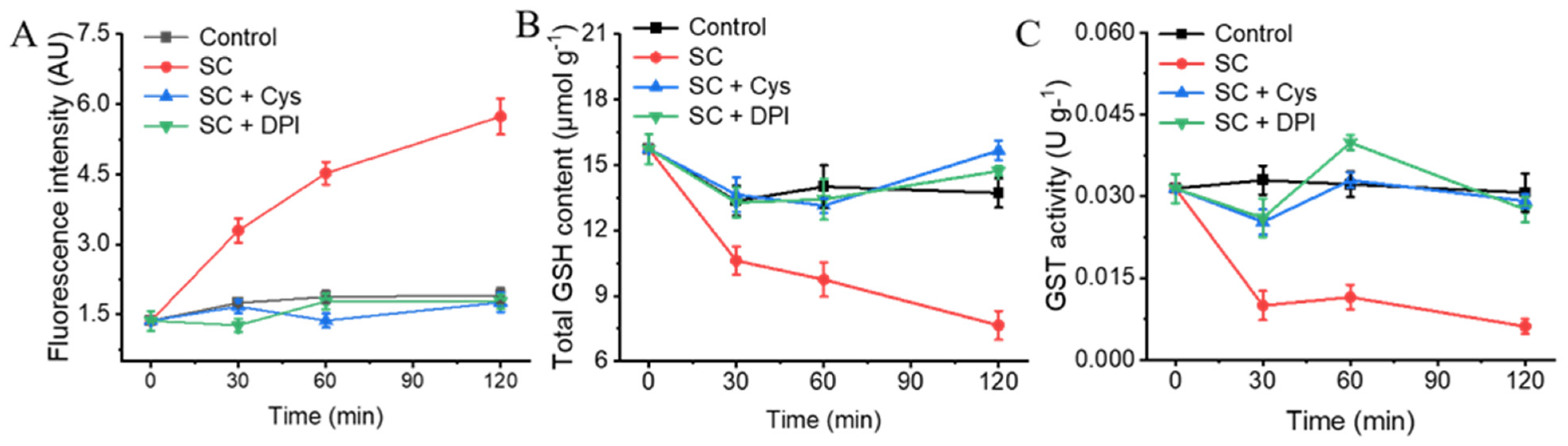
3.7. The Addition of Exogenous Cys and DPI Alleviates the Oxidative Stress Caused by SC
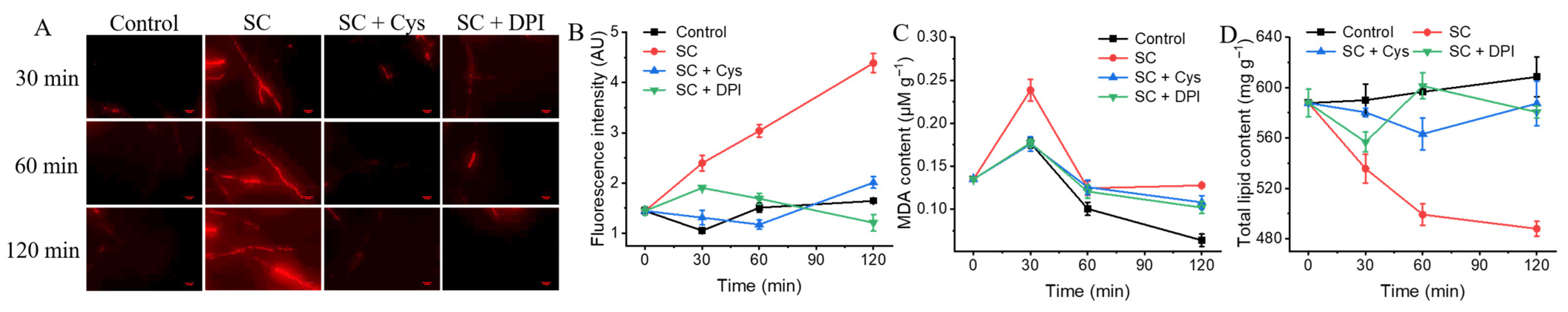
3.8. The Addition of Exogenous Cys and DPI Mitigates the Damage to Surface Morphology Caused by SC
3.9. The Addition of Exogenous Cys and DPI Mitigates the Damage to Cell Membrane Integrity and Lipid Peroxidation Caused by SC
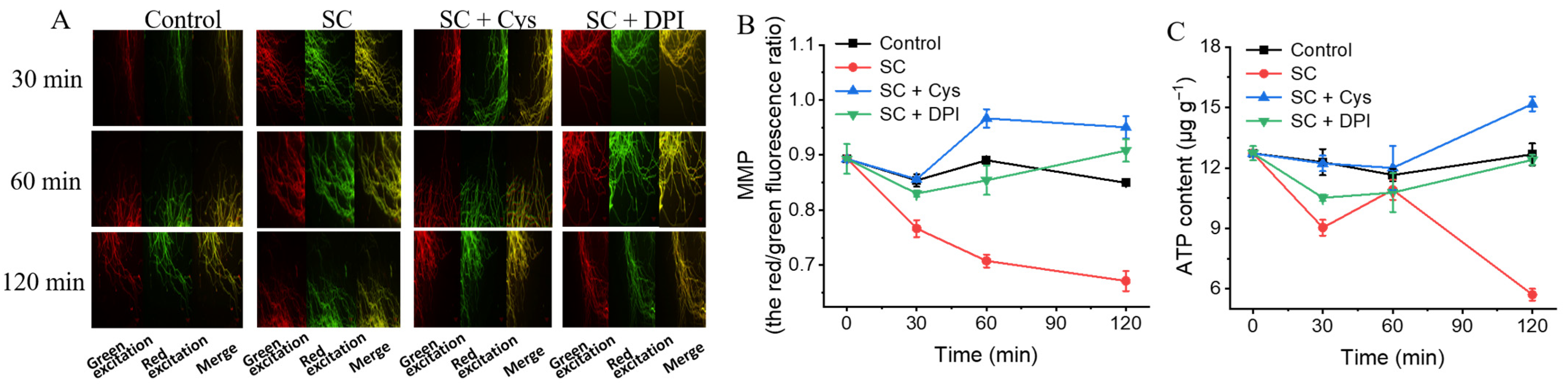
3.10. The Addition of Exogenous Cys and DPI Mitigates the Decrease in MMP Caused by SC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, F.; Zhong, X.; Wang, Y.; Zhao, J.; Lu, Y.; Long, C.A. Methylparaben suppresses the citrus postharvest pathogen Penicillium digitatum by undermining the cell wall and membrane and inducing the antioxidant system. Sci. Hortic. 2024, 333, 113253. [Google Scholar] [CrossRef]
- Chen, K.; Tian, Z.; He, H.; Long, C.A.; Jiang, F.T. Bacillus species as potential biocontrol agents against citrus diseases. Biol. Control 2020, 151, 104419. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Y.Q.; Liu, Q.Y.; Liu, S.Q.; Pan, H.; Cheng, Y.J.; Long, C.A. The GRAS salts of Na2SiO3 and EDTA-Na2 control citrus postharvest pathogens by disrupting the cell membrane. Foods 2023, 12, 2368. [Google Scholar] [CrossRef]
- Palou, L.; Ali, A.; Fallik, E.; Romanazzi, G. GRAS plant-and animal-derived compounds as alternatives to conventional fungicides for the control of postharvest diseases of fresh horticultural produce. Postharvest Biol. Technol. 2016, 122, 41–52. [Google Scholar] [CrossRef]
- Li, X.J.; Chen, O.; Wang, W.J.; Deng, L.L.; Yao, S.X.; Ming, J.; Zhang, H.Y.; Zeng, K.F. Advances and perspectives in biological control of postharvest fungal decay in citrus fruit utilizing yeast antagonists. Int. J. Food Microbiol. 2025, 432, 111093. [Google Scholar] [CrossRef]
- Liu, F.; Gao, R.Q.; Zhang, F.; Ren, Y.; Li, W.; He, B. Postharvest biocontrol of green mold (Penicillium digitatum) in citrus by Bacillus velezensis strain S161 and its mode of action. Biol. Control 2023, 187, 105392. [Google Scholar] [CrossRef]
- Riolo, M.; Villena, A.M.; Calpe, J.; Luz, C.; Meca, G.; Tuccitto, N.; Cacciola, S.O. A circular economy approach: A new formulation based on a lemon peel medium activated with lactobacilli for sustainable control of post-harvest fungal rots in fresh citrus fruit. Biol. Control 2024, 189, 105443. [Google Scholar] [CrossRef]
- Bhatta, U.K. Alternative management approaches of citrus diseases caused by Penicillium digitatum (green mold) and Penicillium italicum (blue mold). Front. Plant Sci. 2022, 12, 833328. [Google Scholar] [CrossRef]
- OuYang, Q.L.; Okwong, R.O.; Chen, Y.P.; Tao, N.G. Synergistic activity of cinnamaldehyde and citronellal against green mold in citrus fruits. Postharvest. Biol. Technol. 2020, 162, 111095. [Google Scholar] [CrossRef]
- Guimarães, J.E.R.; de la Fuente, B.; Pérez-Gago, M.B.; Andradas, C.; Carbó, R.; Mattiuz, B.H.; Palou, L. Antifungal activity of GRAS salts against Lasiodiplodia theobromae in vitro and as ingredients of hydroxypropyl methylcellulose-lipid composite edible coatings to control Diplodia stem-end rot and maintain postharvest quality of citrus fruits. Int. J. Food Microbiol. 2019, 301, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.Y.; Godana, E.A.; Wang, K.L.; Zhang, H.Y. A proteomic analysis of Wickerhamomyces anomalus incubated with chitosan reveals dynamic changes in protein expression and metabolic pathways. Postharvest Biol. Technol. 2024, 211, 112806. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Xie, Z.Y.; Guo, E.H.; Han, L.R.; Zhang, X.; Feng, J.T. Activity and biochemical characteristics of plant extract cuminic acid against Sclerotinia sclerotiorum. Crop. Prot. 2017, 101, 76–83. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, B. Pharmacodynamic analysis of cumin acid and its inhibition on Phytophthora capsici. Contemp. Chem. Ind. 2020, 49, 521–524, 528, (The Abstract is in English). [Google Scholar]
- Wang, Y.; Sun, Y.; Zhang, Y.; Zhang, X.; Feng, J.T. Antifungal activity and biochemical response of cuminic acid against Phytophthora capsici leonian. Molecules 2016, 21, 756. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Sun, Y.; Feng, J.T.; Zhang, X. Evaluating the potential value of natural product cuminic acid against plant pathogenic fungi in cucumber. Molecules 2017, 22, 1914. [Google Scholar] [CrossRef]
- Tamano, S.; Ikarashi, H.; Morinishi, Y.; Taga, K. Drag reduction and degradation of nonionic surfactant solutions with organic acid in turbulent pipe flow. J. Non-Newton. Fluid. 2015, 215, 1–7. [Google Scholar] [CrossRef]
- Matejczyk, M.; Świsłocka, R.; Golonko, A.; Lewandowski, W.; Hawrylik, E. Cytotoxic, genotoxic and antimicrobial activity of caffeic and rosmarinic acids and their lithium, sodium and potassium salts as potential anticancer compounds. Adv. Med. Sci. 2018, 63, 14–21. [Google Scholar] [CrossRef]
- Tao, N.G.; Wang, H.; Wang, C.F.; Ni, J.; Luo, Y.S. Research on the isolating of two citrus postharvestpathogens and their biological characteristics. Nat. Sci. J. Xiangtan Univ. 2013, 35, 75–78, (The abstract is in English). [Google Scholar]
- Yuan, X.X.; Meng, K.X.; Shi, S.W.; Wu, Y.Y.B.; Chen, X.M.; OuYang, Q.L.; Tao, N.G. Trans-2-hexenal inhibits the growth of imazalil-resistant Penicillium digitatum Pdw03 and delays green mold in postharvest citrus. Postharvest. Biol. Technol. 2023, 199, 112304. [Google Scholar] [CrossRef]
- OuYang, Q.L.; Duan, X.F.; Li, L.; Tao, N.G. Cinnamaldehyde exerts its antifungal activity by disrupting the cell wall integrity of Geotrichum citri-aurantii. Front. Microbiol. 2019, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- OuYang, Q.L.; Tao, N.G.; Zhang, M.L. A damaged oxidative phosphorylation mechanism is involved in the antifungal activity of citral against Penicillium digitatum. Front. Microbiol. 2018, 9, 239. [Google Scholar] [CrossRef]
- Hervieux, V.; Yaganza, E.S.; Arul, J.; Tweddell, R.J. Effect of organic and inorganic salts on the development of Helminthosporium solani, the causal agent of potato silver scurf. Plant Dise. 2002, 86, 1014–1018. [Google Scholar] [CrossRef]
- Lucera, A.; Costa, C.; Conte, A.; Nobile, M.A.D. Food applications of natural antimicrobial compounds. Front. Microbiol. 2012, 3, 287. [Google Scholar] [CrossRef]
- Cabezas-Pizarro, J.; Redondo-Solano, M.; Umaña-Gamboa, C.; Arias-Echandi, M.L. Antimicrobial activity of different sodium and potassium salts of carboxylic acid against some common foodborne pathogens and spoilage–associated bacteria. Rev. Argent. Microbiol. 2018, 50, 56–61. [Google Scholar] [CrossRef]
- Palou, L. Postharvest treatments with gras salts to control fresh fruit decay. Horticulturae 2018, 4, 46. [Google Scholar] [CrossRef]
- Parra, J.; Ripoll, G.; Orihuel-Iranzo, B. Potassium sorbate effects on citrus weight loss and decay control. Postharvest Biol. Technol. 2014, 96, 7–13. [Google Scholar] [CrossRef]
- Youssef, K.; Roberto, S.R. Applications of salt solutions before and after harvest affect the quality and incidence of postharvest gray mold of ‘Italia’ table grapes. Postharvest Biol. Technol. 2014, 87, 95–102. [Google Scholar] [CrossRef]
- Bu, J.; Yu, Y.; Aisikaer, G.; Aisikaer, G.; Ying, T.J. Postharvest UV-C irradiation inhibits the production of ethylene and the activity of cell wall-degrading enzymes during softening of tomato (Lycopersicon esculentum L.) fruits. Postharvest Biol. Technol. 2013, 86, 337–345. [Google Scholar] [CrossRef]
- Liu, K.; Zhou, X.; Fu, M. Inhibiting effects of epsilon-poly-lysine (ε-PL) on Pencillium digitatum and its involved mechanism. Postharvest Biol. Technol. 2017, 123, 94–101. [Google Scholar] [CrossRef]
- Ibe, C.; Oladele, R.O.; Alamir, O. Our pursuit for effective antifungal agents targeting fungal cell wall components: Where are we? Int. J. Antimicrob. Agents 2022, 59, 106477. [Google Scholar] [CrossRef]
- Dou, Y.; Dhanasekaran, S.; Ngea, G.L.N.; Yang, Q.Y.; Zhang, X.Y.; Zhao, L.N.; Wang, K.L.; Zhang, H.Y. Transcriptome analysis provides insights into potential mechanisms of epsilon-poly-L-lysine inhibiting Penicillium expansum invading apples. Postharvest Biol. Technol. 2024, 207, 112622. [Google Scholar] [CrossRef]
- Shu, C.; Cui, K.; Li, Q.; Cao, J.; Jiang, W. Epsilon-poly-l-lysine (ε-PL) exhibits multifaceted antifungal mechanisms of action that control postharvest Alternaria rot. Int. J. Food Microbiol. 2021, 348, 109224. [Google Scholar] [CrossRef]
- Liu, M.; Niu, Q.C.; Wang, Z.Y.; Qi, H.Y.; Liang, X.X.; Gai, Y.P.; Wang, B.S. Comparative physiological and transcriptome analysis provide insights into the inhibitory effect of 6-pentyl-2H-pyran-2-one on Clarireedia jacksoni. Pestic. Biochem. Physiol. 2023, 193, 105456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; Reymick, O.O.; Duan, B.; Ding, S.H.; Wang, R.R.; Tao, N.G. Combination of cinnamaldehyde/β-cyclodextrin inclusion complex and L-phenylalanine effectively reduces the postharvest green mold in citrus fruit. Pestic. Biochem. Physiol. 2024, 204, 106040. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.L.; Wong, R.S.M.; Lam, K.H.; Hung, L.K.; Chui, C.H. The role of reactive oxygen species in the biological activity of antimicrobial agents: An updated mini review. Chem-Biol. Interact. 2020, 320, 109023. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.L.; Zhang, B.Q.; Luo, X.F.; Li, A.P.; Zhang, S.Y.; An, J.X.; Zhang, Z.J.; Liu, Y.Q. Antifungal efficacy of sixty essential oils and mechanism of oregano essential oil against Rhizoctonia solani. Ind. Crops Prod. 2023, 191, 115975. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, J.; Lu, L.; Bold, T.; Li, X.; Wang, S.; Chang, Z.S.; Chen, J.; Kong, X.; Zheng, Y.X.; et al. Intracellular GSH/GST antioxidants system change as an earlier biomarker for toxicity evaluation of iron oxide nanoparticles. NanoImpact 2021, 23, 100338. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Shu, Q.; Xu, C.; Zheng, Q.; Guo, Z.; Wang, C.; Hao, Z.; Liu, X.; Wang, G.; et al. Copper clusters: An effective antibacterial for eradicating multidrug-resistant bacterial infection in vitro and in vivo. Adv. Funct. Mater. 2021, 31, 2008720. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Yin, J.; Lin, W. Organic fluorescent probes for detecting mitochondrial membrane potential. Coordin. Chem. Rev. 2020, 420, 213419. [Google Scholar] [CrossRef]
- Fan, L.; Wei, Y.Y.; Chen, Y.; Ouaziz, M.; Jiang, S.; Xu, F.; Wang, F.; Shao, X.F. Transcriptome analysis reveals the mechanism of antifungal peptide epinecidin-1 against Botrytis cinerea by mitochondrial dysfunction and oxidative stress. Postharvest Biol. Technol. 2024, 202, 105932. [Google Scholar] [CrossRef]
- Kwun, M.S.; Lee, D.G. Quercetin-induced yeast apoptosis through mitochondrial dysfunction under the accumulation of magnesium in Candida albicans. Fungal Biol. 2020, 124, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Plačková, P.; Šála, M.; Šmídková, M.; Dejmek, M.; Hrebabecky, H.; Nencka, R.; Thibaut, H.J.; Neyts, J.; Mertliková-Kaiserová, H. 9-Norbornyl-6-chloropurine (NCP) induces cell death through GSH depletion-associated ER stress and mitochondrial dysfunction. Free Radic. Biol. Med. 2016, 97, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Jin, C.; Zhong, Y.; Zhu, J.; Liu, Q.; Sun, D.; Feng, J.; Xia, X.; Peng, X. Involvement of NADPH oxidase in patulin-induced oxidative damage and cytotoxicity in HEK293 cells. Food Chem. Toxicol. 2021, 150, 112055. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, D.; Yang, K.; Xu, T.; Su, C.; Li, R.; Xiao, X.; Wang, Z. Sodium dehydroacetate confers broad antibiotic tolerance by remodeling bacterial metabolism. J. Hazard. Mate. 2022, 432, 128645. [Google Scholar] [CrossRef] [PubMed]


| SC (g L−1) | Inhibitory Rate (%) | |||
|---|---|---|---|---|
| 1 Day | 2 Day | 3 Day | 4 Day | |
| 0.2 | 100.00 ± 0.00 a | 81.25 ± 0.00 a | 78.23 ± 2.42 b | 70.29 ± 3.69 c |
| 0.4 | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 92.57 ± 2.62 b |
| 0.8 | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a |
| 1.6 | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a |
| Physiological Indicators | Treatments | 0 Day | 1 Day | 3 Day | 5 Day |
|---|---|---|---|---|---|
| Weight loss (%) | Control | 0.00 ± 0.00 a | 1.59 ± 0.27 a | 3.11 ± 0.43 a | 4.74 ± 0.57 a |
| Prochloraz | 0.00 ± 0.00 a | 1.18 ± 0.31 b | 2.49 ± 0.26 a | 3.77 ± 0.47 b | |
| 2× MFC SC | 0.00 ± 0.00 a | 1.21 ± 0.29 b | 2.58 ± 0.43 a | 3.85 ± 0.52 b | |
| 4× MFC SC | 0.00 ± 0.00 a | 1.10 ± 0.16 b | 2.55 ± 0.40 a | 3.45 ± 0.55 b | |
| 8× MFC SC | 0.00 ± 0.00 a | 1.08 ± 0.24 b | 2.01 ± 0.66 a | 3.29 ± 0.83 b | |
| Coloration index | Control | 5.29 ± 0.40 a | 5.52 ± 0.41 a | 5.77 ± 0.44 a | 6.08 ± 0.43 a |
| Prochloraz | 5.15 ± 0.31 a | 5.31 ± 0.24 a | 5.40 ± 0.21 a | 5.69 ± 0.27 a | |
| 2× MFC SC | 5.17 ± 0.41 a | 5.35 ± 0.43 a | 5.58 ± 0.43 a | 5.82 ± 0.40 a | |
| 4× MFC SC | 5.34 ± 0.45 a | 5.64 ± 0.44 a | 5.85 ± 0.47 a | 6.06 ± 0.39 a | |
| 8× MFC SC | 5.28 ± 0.69 a | 5.64 ± 0.64 a | 5.81 ± 0.63 a | 5.91 ± 0.69 a | |
| pH | Control | 2.63 ± 0.03 a | 2.93 ± 0.00 a | 2.72 ± 0.01 a | 2.78 ± 0.01 a |
| Prochloraz | 2.63 ± 0.03 a | 2.75 ± 0.01 a | 2.72 ± 0.02 a | 2.76 ± 0.02 a | |
| 2× MFC SC | 2.63 ± 0.03 a | 2.79 ± 0.01 a | 2.75 ± 0.01 a | 2.83 ± 0.01 a | |
| 4× MFC SC | 2.63 ± 0.03 a | 2.71 ± 0.03 a | 2.78 ± 0.01 a | 2.77 ± 0.02 a | |
| 8× MFC SC | 2.63 ± 0.03 a | 2.71 ± 0.01 a | 2.76 ± 0.01 a | 2.77 ± 0.01 a | |
| TSS (%) | Control | 15.94 ± 0.28 a | 14.84 ± 0.40 b | 15.70 ± 0.35 a | 16.38 ± 0.47 a |
| Prochloraz | 15.94 ± 0.28 a | 17.24 ± 0.30 a | 16.90 ± 0.54 a | 16.79 ± 0.09 a | |
| 2× MFC SC | 15.94 ± 0.28 a | 15.02 ± 0.37 b | 15.96 ± 0.99 a | 16.40 ± 0.22 a | |
| 4× MFC SC | 15.94 ± 0.28 a | 16.60 ± 0.61 a | 16.02 ± 0.28 a | 16.23 ± 0.38 a | |
| 8× MFC SC | 15.94 ± 0.28 a | 16.48 ± 0.31 a | 16.77 ± 1.00 a | 16.72 ± 0.50 a | |
| TA (%) | Control | 1.44 ± 0.18 a | 1.03 ± 0.08 b | 1.66 ± 0.23 a | 1.45 ± 0.10 a |
| Prochloraz | 1.44 ± 0.18 a | 1.47 ± 0.08 a | 1.48 ± 0.13 a | 1.63 ± 0.12 a | |
| 2× MFC SC | 1.44 ± 0.18 a | 1.33 ± 0.06 a | 1.71 ± 0.28 a | 1.47 ± 0.17 a | |
| 4× MFC SC | 1.44 ± 0.18 a | 1.56 ± 0.29 a | 1.33 ± 0.18 a | 1.44 ± 0.10 a | |
| 8× MFC SC | 1.44 ± 0.18 a | 1.48 ± 0.05 a | 1.56 ± 0.12 a | 1.61 ± 0.13 a | |
| Vc content (mg 100 g−1) | Control | 27.32 ± 1.52 a | 39.28 ± 1.29 a | 28.79 ± 2.82 c | 31.18 ± 2.53 b |
| Prochloraz | 27.32 ± 1.52 a | 33.44 ± 0.99 b c | 37.30 ± 1.02 a b | 35.72 ± 1.02 a b | |
| 2× MFC SC | 27.32 ± 1.52 a | 37.77 ± 3.69 a b | 35.42 ± 1.18 a b | 36.36 ± 2.90 a | |
| 4× MFC SC | 27.32 ± 1.52 a | 35.33 ± 1.23 b c | 33.63 ± 1.23 b | 38.53 ± 3.63 a | |
| 8× MFC SC | 27.32 ± 1.52 a | 31.28 ± 2.67 c | 39.00 ± 2.31 a | 37.59 ± 0.85 a | |
| Hardness (N) | Control | 5.21 ± 0.27 a | 6.07 ± 0.07 a | 5.88 ± 0.14 a | 5.05 ± 0.19 b |
| Prochloraz | 5.21 ± 0.27 a | 5.92 ± 0.12 a | 5.97 ± 0.11 a | 5.97 ± 0.09 a | |
| 2× MFC SC | 5.21 ± 0.27 a | 6.00 ± 0.15 a | 5.97 ± 0.08 a | 5.91 ± 0.12 a | |
| 4× MFC SC | 5.21 ± 0.27 a | 6.14 ± 0.09 a | 6.01 ± 0.07 a | 5.93 ± 0.10 a | |
| 8× MFC SC | 5.21 ± 0.27 a | 5.92 ± 0.04 a | 6.06 ± 0.10 a | 6.00 ± 0.09 a |
| Physiological Indicators | Treatments | 0 Day | 10 Day | 20 Day | 30 Day |
|---|---|---|---|---|---|
| Weight loss rate (%) | Control | 0.00 ± 0.00 a | 2.78 ± 0.63 c | 3.76 ± 0.61 a | 5.10 ± 0.81 a |
| Prochloraz | 0.00 ± 0.00 a | 1.08 ± 0.69 a | 2.38 ± 1.19 b | 3.23 ± 1.01 b | |
| 8× MFC SC | 0.00 ± 0.00 a | 1.76 ± 0.71 a b | 2.53 ± 0.96 b | 3.41 ± 0.83 b | |
| Coloration index | Control | 5.10 ± 0.57 a | 5.85 ± 0.81 a | 6.39 ± 0.53 a | 6.67 ± 0.47 a |
| Prochloraz | 4.46 ± 0.57 a | 5.31 ± 0.41 a | 6.09 ± 0.32 a | 6.44 ± 0.38 a | |
| 8× MFC SC | 4.88 ± 0.81 a | 5.60 ± 0.67 a | 6.19 ± 0.48 a | 6.56 ± 0.30 a | |
| pH | Control | 2.63 ± 0.03 a | 2.73 ± 0.01 a | 2.81 ± 0.01 a | 2.89 ± 0.01 a |
| Prochloraz | 2.63 ± 0.03 a | 2.83 ± 0.01 a | 2.83 ± 0.07 a | 2.95 ± 0.01 a | |
| 8× MFC SC | 2.63 ± 0.03 a | 2.64 ± 0.01 a | 2.86 ± 0.02 a | 2.99 ± 0.01 a | |
| TSS (%) | Control | 15.94 ± 0.28 a | 16.68 ± 0.49 a | 15.61 ± 0.13 a | 16.46 ± 0.48 a |
| Prochloraz | 15.94 ± 0.28 a | 15.48 ± 0.29 a | 15.72 ± 0.13 a | 16.67 ± 0.17 a | |
| 8× MFC SC | 15.94 ± 0.28 a | 16.43 ± 0.30 a | 15.93 ± 0.15 a | 16.84 ± 0.18 a | |
| TA (%) | Control | 1.14 ± 0.23 a | 1.41 ± 0.08 a | 1.26 ± 0.05 a | 1.40 ± 0.08 a |
| Prochloraz | 1.14 ± 0.23 a | 1.47 ± 0.08 a | 1.28 ± 0.06 a | 1.37 ± 0.05 a | |
| 8× MFC SC | 1.14 ± 0.23 a | 1.66 ± 0.12 a | 1.41 ± 0.08 a | 1.44 ± 0.11 a | |
| Vc content (mg 100 g−1) | Control | 27.32 ± 1.52 a | 33.91 ± 2.83 a | 27.60 ± 0.86 c | 29.58 ± 0.16 b |
| Prochloraz | 27.32 ± 1.52 a | 31.18 ± 2.08 a | 30.62 ± 0.59 b | 31.46 ± 0.16 a b | |
| 8× MFC SC | 27.32 ± 1.52 a | 33.53 ± 1.33 a | 32.35 ± 0.71 a | 33.63 ± 2.47 a | |
| Hardness (N) | Control | 5.21 ± 0.27 a | 5.46 ± 0.37 a | 5.11 ± 0.07 b | 5.10 ± 0.06 b |
| Prochloraz | 5.21 ± 0.27 a | 5.91 ± 0.06 a | 5.66 ± 0.19 a | 5.63 ± 0.05 a | |
| 8× MFC SC | 5.21 ± 0.27 a | 5.70 ± 0.05 a | 5.54 ± 0.15 a | 5.58 ± 0.07 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Zhang, Y.; Tan, X.; Li, L.; OuYang, Q.; Tao, N. Sodium Cuminate Inhibits the Mycelial Growth of Penicillium digitatum by Inducing Oxidative Stress and Damaging the Cell Membrane. J. Fungi 2025, 11, 612. https://doi.org/10.3390/jof11090612
Yang M, Zhang Y, Tan X, Li L, OuYang Q, Tao N. Sodium Cuminate Inhibits the Mycelial Growth of Penicillium digitatum by Inducing Oxidative Stress and Damaging the Cell Membrane. Journal of Fungi. 2025; 11(9):612. https://doi.org/10.3390/jof11090612
Chicago/Turabian StyleYang, Mingchen, Yonghua Zhang, Xiaoli Tan, Lu Li, Qiuli OuYang, and Nengguo Tao. 2025. "Sodium Cuminate Inhibits the Mycelial Growth of Penicillium digitatum by Inducing Oxidative Stress and Damaging the Cell Membrane" Journal of Fungi 11, no. 9: 612. https://doi.org/10.3390/jof11090612
APA StyleYang, M., Zhang, Y., Tan, X., Li, L., OuYang, Q., & Tao, N. (2025). Sodium Cuminate Inhibits the Mycelial Growth of Penicillium digitatum by Inducing Oxidative Stress and Damaging the Cell Membrane. Journal of Fungi, 11(9), 612. https://doi.org/10.3390/jof11090612







