Acidification and Nutrient Imbalances Drive Fusarium Wilt Severity in Banana (Musa spp.) Grown on Tropical Latosols
Abstract
1. Introduction
2. Materials and Methods
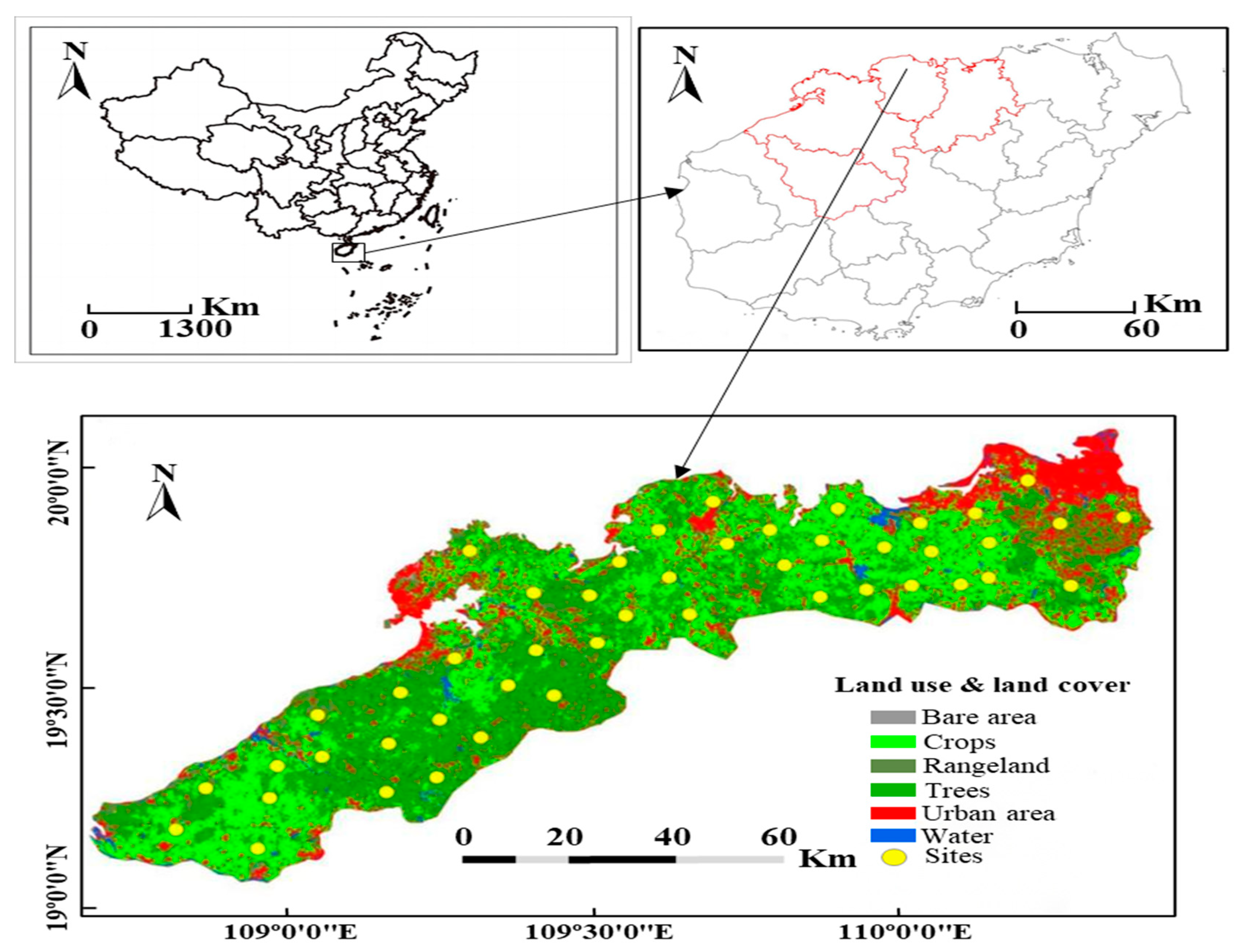
2.1. Study Sites and Soil Sampling
2.2. Analysis of Soil Samples
2.3. Pathogen Quantification
2.4. Data Analysis
3. Results
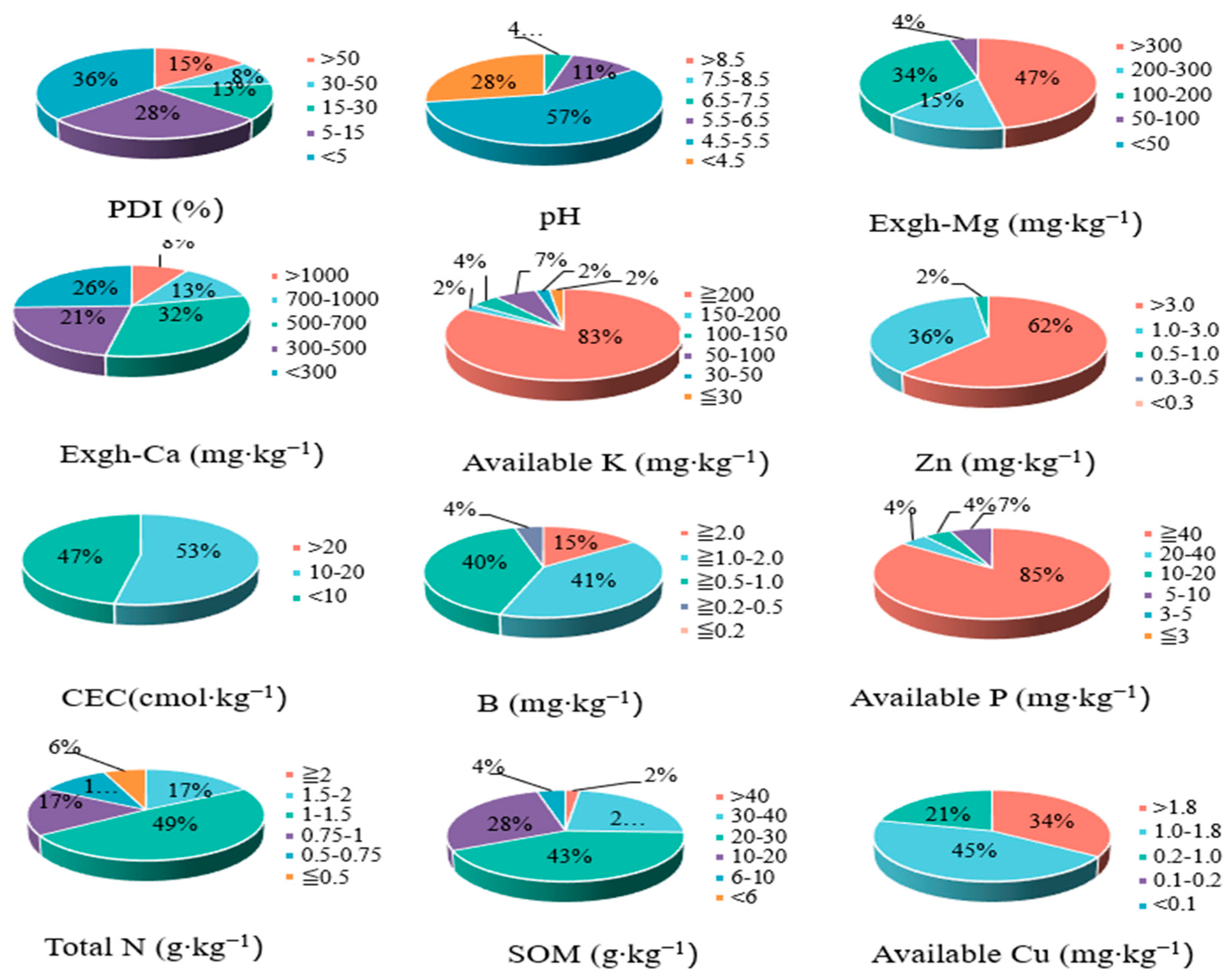
3.1. Properties of Tropical Banana Soil on Hainan Island
3.1.1. Soil pH, Cation Exchange Capacity (CEC), and Organic C
3.1.2. Macronutrient Status
3.1.3. Micronutrient Status
3.2. Effect of Cultivation Time on Fusarium Wilt in Banana
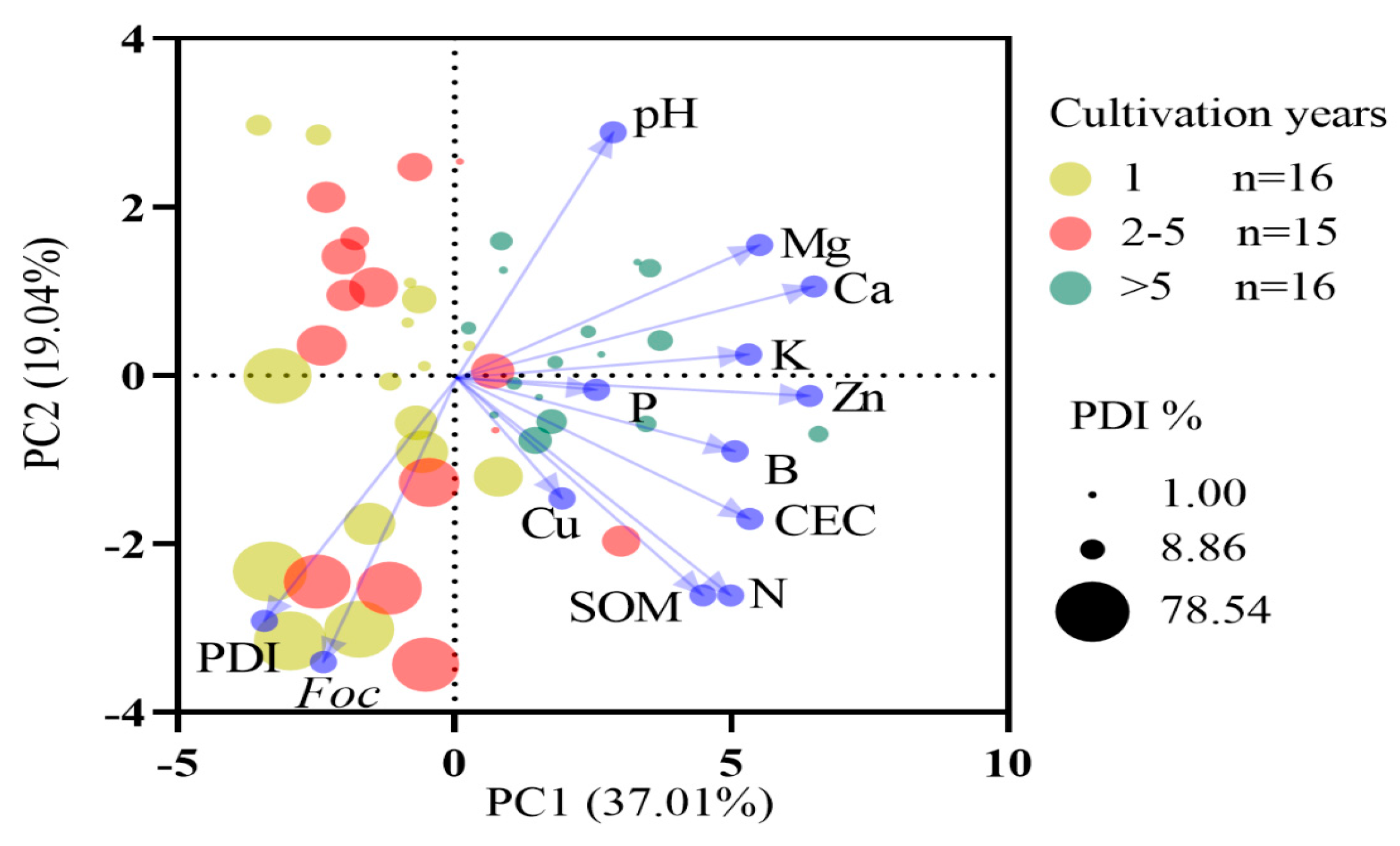
3.3. Relationships Between Soil Properties and Fusarium Wilt in Banana
4. Discussion
4.1. Relationships Between Soil Chemical Properties and Fusarium Wilt in Banana
4.2. Properties of Banana Tropical Soil on Hainan Island
4.3. Effect of Cultivation Time on Fusarium Wilt in Banana
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ding, Z.; Zhao, F.; Zhu, Z.; Ali, E.F.; Shaheen, S.M.; Rinklebe, J.; Eissa, M.A. Green nanosilica enhanced the salt-tolerance defenses and yield of Williams banana: A field trial for using saline water in low fertile arid soil. Environ. Exp. Bot. 2022, 197, 104843. [Google Scholar] [CrossRef]
- World Banana Production. Available online: https://www.atlasbig.com/en-us/countries-banana-production (accessed on 1 September 2023).
- Liu, Z.; Wang, B.; Tao, C.; Ou, Y.; Lu, N.; Shen, Z.; Li, R.; Shen, Q. Characteristics and correlation of Fusarium oxysporum and soil nutrients around banana roots. Acta Pedol. Sin. 2023, 60, 1134–1145. [Google Scholar]
- Xie, J.H. Fruit scientific research in New China in the past 70 years: Banana. J. Fruit Sci. 2019, 36, 1429–1440. [Google Scholar]
- Jie, J.; Wang, Z.F.; Cao, L.X.; Tan, H.M.; Inderbitzin, P.; Jiang, Z.D.; Zhou, S. Artificial inoculation of banana tissue culture plantlets with indigenous endophytes originally derived from native banana plants. Biol. Control 2009, 51, 427–434. [Google Scholar] [CrossRef]
- Huang, Y.H.; Wang, R.C.; Li, C.H.; Zuo, C.W.; Wei, Y.R.; Zhang, L.; Yi, G.J. Control of Fusarium wilt in banana with Chinese leek. Eur. J. Plant Pathol. 2012, 134, 87–95. [Google Scholar] [CrossRef] [PubMed]
- NY/T 4235-2022; Technical Specification for Prevention and Control of Fusarium Wilt of Banana. China Agricultural Press: Beijing, China, 2023.
- Orr, R.; Nelson, P.N. Impacts of soil abiotic attributes on Fusarium wilt, focusing on bananas. Appl. Soil Ecol. 2018, 132, 20–33. [Google Scholar] [CrossRef]
- Yuan, J.; Wen, T.; Zhang, H.; Zhao, M.; Penton, C.R.; Thomashow, L.S.; Shen, Q. Predicting disease occurrence with high accuracy based on soil macroecological patterns of Fusarium wilt. ISME J. 2020, 14, 2936–2950. [Google Scholar] [CrossRef]
- Gordon, T.R.; Stueven, M.; Pastrana, A.M.; Henry, P.M.; Dennehy, C.M.; Kirkpatrick, S.C.; Daugovish, O. The effect of pH on spore germination, growth, and infection of strawberry roots by Fusarium oxysporum f. sp. fragariae, cause of Fusarium wilt of strawberry. Plant Dis. 2019, 103, 697–704. [Google Scholar] [CrossRef]
- Segura, R.A.; Stoorvogel, J.J.; Sandoval, J.A. The effect of soil properties on the relation between soil management and Fusarium wilt expression in Gros Michel bananas. Plant Soil 2022, 471, 89–100. [Google Scholar] [CrossRef]
- Ismaila, A.A.; Ahmad, K.; Siddique, Y.; Wahab, M.A.A.; Kutawa, A.B.; Abdullahi, A.; Zobir, S.A.M.; Abdu, A.; Abdullah, S.N.A. Fusarium wilt of banana: Current update and sustainable disease control using classical and essential oils approaches. Hortic. Plant J. 2023, 9, 1–28. [Google Scholar] [CrossRef]
- Hui, D.; Yang, X.; Deng, Q.; Liu, Q.; Wang, X.; Yang, H.; Ren, H. Soil C:N:P stoichiometry in tropical forests on Hainan Island of China: Spatial and vertical variations. Catena 2021, 201, 105228. [Google Scholar] [CrossRef]
- Li, T.; Hong, X.; Liu, S.; Wu, X.; Fu, S.; Liang, Y.; Li, J.; Li, R.; Zhang, C.; Song, X.; et al. Cropland degradation and nutrient overload on Hainan Island: A review and synthesis. Environ. Pollut. 2022, 306, 120100. [Google Scholar] [CrossRef]
- Shen, Z.Z.; Xue, C.; Penton, C.R.; Thomashow, L.S.; Zhang, N.; Wang, B.B.; Ruan, Y.; Li, R.; Shen, Q. Suppression of banana Panama disease induced by soil microbiome reconstruction through an integrated agricultural strategy. Soil Biol. Biochem. 2019, 128, 164–174. [Google Scholar] [CrossRef]
- Yang, J.; Duan, Y.; Liu, X.; Sun, M.; Wang, Y.; Liu, M.; Zhu, Z.; Shen, Z.; Gao, W.; Wang, B.; et al. Reduction of banana Fusarium wilt associated with soil microbiome reconstruction through green manure intercropping. Agric. Ecosyst. Environ. 2022, 337, 108065. [Google Scholar] [CrossRef]
- Lu, R.K. Analysis Method of Soil Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- China Soil Survey Department. Techniques of China Soil Survey; China Agricultural Press: Beijing, China, 1992. (In Chinese) [Google Scholar]
- Xi, C.F.; Zhang, S.Y. Brief introduction on achievements in national soil survey project since 1979. Acta Pedol. Sin. 1994, 31, 330–335. [Google Scholar]
- Zhou, D.; Jing, T.; Chen, Y.; Wang, F.; Qi, D.; Feng, R.; Xie, J.; Li, H. Deciphering microbial diversity associated with Fusarium wilt-diseased and disease-free banana rhizosphere soil. BMC Microbiol. 2019, 19, 161. [Google Scholar] [CrossRef]
- Carvalhais, L.C.; Henderson, J.; Rincon-Florez, V.A.; O’Dwyer, C.; Czislowski, E.; Aitken, E.A.B.; Drenth, A. Molecular Diagnostics of Banana Fusarium Wilt Targeting Secreted-in-Xylem Genes. Front. Plant Sci. 2019, 10, 547. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.L.; Li, J. Effectiveness of alkaline fertilizer on the control of banana Fusarium wilt and regulation of soil acidity in banana orchard. J. Plant Nutr. Fertil. 2014, 20, 938–946. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Zhou, D.; Wei, Y.; Feng, J.; Cai, B.; Qi, D.; Zhang, M.; Zhao, Y.; Li, K.; et al. Streptomyces-Secreted Fluvirucin B6 as a Potential Bio-Fungicide for Managing Banana Fusarium Wilt and Mycotoxins and Modulating the Soil Microbial Community Structure. J. Agric. Food Chem. 2024, 72, 17890–17902. [Google Scholar]
- Gatch, E.W.; du Toit, L.J. Limestone-mediated suppression of Fusarium wilt in spinach seed crops. Plant Dis. 2017, 101, 81–94. [Google Scholar] [CrossRef]
- Weinert, M.; Simpson, M. Sub-Tropical Banana Nutrition—Matching Nutrition Requirements to Growth Demands; NSW Department Primary Industries: Wollongbar, NSW, Australia, 2016. [Google Scholar]
- Robinson, J.C.; Galán Sauco, V. Bananas and Plantains, 2nd ed.; CABI Publishing: Wallingford, UK, 2010. [Google Scholar]
- Groenewald, S.; van den Berg, N.; Marasas, W.F.O.; Viljoen, A. Biological, physiological and pathogenic variation in a genetically homogenous population of Fusarium oxysporum f.sp. cubense. Australas. Plant Pathol. 2006, 35, 401–409. [Google Scholar] [CrossRef]
- Gupta, V.K.; Misra, A.K.; Gaur, R.K. Growth characteristics of Fusarium spp. causing wilt disease in Psidium guajava L. in India. J. Plant Prot. Res. 2010, 50, 452–462. [Google Scholar] [CrossRef]
- Wu, X.; Shan, Y.; Li, Y.; Li, Q.; Wu, C. The soil nutrient environment determines the strategy by which Bacillus velezensis HN03 suppresses Fusarium wilt in banana plants. Front. Plant Sci. 2020, 11, 599904. [Google Scholar] [CrossRef]
- Dordas, C. Role of nutrients in controlling plant diseases in sustainable agriculture. A review. Agron. Sustain. Dev. 2008, 28, 33–46. [Google Scholar] [CrossRef]
- Siddiqui, S.; Alamri, S.A.; Alrumman, S.A.; Meghvansi, M.K.; Chaudhary, K.K.; Kilany, M.; Prasad, K. Role of soil amendment with micronutrients in suppression of certain soilborne plant fungal diseases: A review. In Organic Amendments and Soil Suppressiveness in Plant Disease Management; Meghvansi, M.K., Varma, A., Eds.; Springer: Cham, Switzerland, 2015; pp. 363–380. [Google Scholar]
- Hassan, M.A.; Abo-Elyousr, K.A. Impact of compost application on Fusarium wilt disease incidence and microelements contents of basil plants. Arch. Phytopathol. Plant Prot. 2013, 46, 1904–1918. [Google Scholar] [CrossRef]
- Sun, R.; Wu, Z.; Chen, B.; Yang, C.; Qi, D.L.; Lan, G.Y. Effects of land-use change on eco-environmental quality in Hainan Island, China. Ecol. Indic. 2020, 109, 105777. [Google Scholar] [CrossRef]
- Sun, L.L.; Zha, X. Variation trend of rainfall and rainfall erosivity in 60 Years of Hainan province. J. Soil Water Conserv. 2019, 31, 1–6, (In Chinese with English Abstract). [Google Scholar]
- Borrelli, P.; Robinson, D.A.; Panagos, P.; Lugato, E.; Yang, J.E.; Alewell, C.; Wuepper, D.; Montanarella, L.; Ballabio, C. Land use and climate change impacts on global soil erosion by water (2015–2070). Proc. Natl. Acad. Sci. USA 2020, 117, 21994–22001. [Google Scholar] [CrossRef] [PubMed]
- Manlay, R.; Feller, C.; Swift, M. Historical evolution of soil organic matter concepts and their relationships with the fertility and sustainability of cropping systems. Agric. Ecosyst. Environ. 2007, 119, 217–233. [Google Scholar] [CrossRef]
- Lal, R. Restoring soil quality to mitigate soil degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef]
- Shen, Z.; Ruan, Y.; Wang, B.; Zhong, S.; Su, L.; Li, R.; Shen, Q. Effect of biofertilizer for suppressing Fusarium wilt disease of banana as well as enhancing microbial and chemical properties of soil under greenhouse trial. Appl. Soil Ecol. 2015, 93, 111–119. [Google Scholar] [CrossRef]
- Kumar, G.S.; Saini, P.K.; Deoliya, R.; Mishra, A.K.; Negi, S.K. Characterization of laterite soil and its use in construction applications: A review. Resour. Conserv. Recycl. Adv. 2022, 15, 200120. [Google Scholar] [CrossRef]
- Uexküll, H.R.V.; Mutert, E. Global extent, development and economic impact of acid soils. Plant Soil 1995, 171, 1–15. [Google Scholar] [CrossRef]
- Crusciol, C.A.C.; Artigiani, A.C.C.A.; Arf, O.; Carmeis Filho, A.C.A.; Soratto, R.P.; Nascente, A.S.; Alvarez, R.C. Soil fertility, plant nutrition, and grain yield of upland rice affected by surface application of lime, silicate, and phosphogypsum in a tropical no-till system. Catena 2016, 137, 87–99. [Google Scholar] [CrossRef]
- Ploetz, R.C.; Pegg, K.G. Tropical race 4 of Panama disease in the Middle East. Phytoparasitica 2000, 28, 283–293. [Google Scholar] [CrossRef]
- Blomme, G.; Ploetz, R.; Jones, D.; De Langhe, E.; Price, N.; Gold, C.; Geering, A.D.; Viljoen, A.; Karamura, D.E.; Pillay, M.; et al. A historical overview of the appearance and spread of Musa pests and pathogens on the African continent. Ann. Appl. Biol. 2013, 162, 4–26. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Z.; Yang, J.; Wang, H.; Cheng, N. Profile of the soil nutrients in vanilla plantations of Hainan Province. Chin. Agric. Sci. Bull. 2010, 4, 547–550. [Google Scholar]
- Tan, L.X.; Fu, C.L.; Pan, S.Q.; Liu, G.B.; Xie, L.S. Soil nutrient status at Zhayun, Qiongzhong County of Hainan Province. Chin. J. Trop. Crops 2012, 33, 816–820. [Google Scholar]
- Chai, L.; Wang, Y.; Wang, X.; Ma, L.; Cheng, Z.; Su, L. Pollution characteristics, spatial distributions, and source apportionment of heavy metals in cultivated soil in Lanzhou, China. Ecol. Indic. 2021, 125, 107507. [Google Scholar] [CrossRef]
- Fu, K.; An, M.; Song, Y.; Fu, G.; Ruan, W.; Wu, D.; Li, X.; Yuan, K.; Wan, X.; Chen, Z.; et al. Soil heavy metals in tropical coastal interface of eastern Hainan Island in China: Distribution, sources and ecological risks. Ecol. Indic. 2023, 154, 110659. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, H.; Yang, Y.; Yang, J.; Tang, H.; Deng, Y.; Ruan, Y.; Zhao, Y. Soil nutrient evaluation of pineapple orchards based on factor and cluster analysis. Chin. J. Soil Sci. 2019, 50, 137–143. [Google Scholar]
- Ko, T.H. Nature and properties of lateritic soils derived from different parent materials in Taiwan. Sci. World J. 2014, 2014, 247194. [Google Scholar] [CrossRef]
- Jain, M.S.; Kalamdhad, A.S. Soil revitalization via waste utilization: Compost effects on soil organic properties, nutritional, sorption and physical properties. Environ. Technol. Innov. 2020, 18, 100668. [Google Scholar] [CrossRef]
- Shi, C.X.; Chen, Z.; Liu, X.Y.; Wang, X.J. Soil volume water content change and its relationship with climate in Hainan Island. Guizhou Agric. Sci. 2019, 47, 142–149. [Google Scholar]
- Batista, B.D.; Wang, J.T.; Liu, H.W.; Kaur, S.; Macdonald, C.A.; Qiu, Z.G.; Trivedi, P.; Delgado-Baquerizo, M.; Xiong, C.; Liang, J.; et al. Biotic and abiotic responses to soilborne pathogens and environmental predictors of soil health. Soil Biol. Biochem. 2024, 189, 109246. [Google Scholar] [CrossRef]
- Olivares, B.O.; Rey, J.C.; Lobo, D.; Navas-Cortés, J.A.; Gómez, J.A.; Landa, B.B. Fusarium wilt of bananas: A review of agro-environmental factors in the Venezuelan production system affecting its development. Agronomy 2021, 11, 986. [Google Scholar] [CrossRef]
- Karangwa, P.; Blomme, G.; Beed, F.; Niyongere, C.; Viljoen, A. The distribution and incidence of banana Fusarium wilt in subsistence farming systems in east and central Africa. Crop Prot. 2016, 84, 132–140. [Google Scholar] [CrossRef]
- Kaushal, M.; Tumuhairwe, J.B.; Tinzaara, W.; Tripathi, L. Insights into soil microbial communities in traditional banana cropping systems of Sub-Saharan Africa. Microorganisms 2022, 10, 2341. [Google Scholar] [CrossRef]
- Shen, Z.; Penton, C.R.; Lv, N.; Xue, C.; Yuan, X.; Ruan, Y.; Li, R.; Shen, Q. Banana Fusarium wilt disease incidence is influenced by shifts of soil microbial communities under different monoculture spans. Microb. Ecol. 2018, 75, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Penton, C.R.; Shen, Z.; Zhang, R.; Huang, Q.; Li, R.; Ruan, Y.; Shen, Q. Manipulating the banana rhizosphere microbiome for biological control of Panama disease. Sci. Rep. 2015, 5, 11124. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Cheng, Q.; Jing, T.; Chen, Y.; Li, X.; Zhang, M.; Qi, D.; Feng, J.; Vafadar, F.; Wei, Y.; et al. Trichoderma virens XZ11-1 producing siderophores inhibits the infection of Fusarium oxysporum and promotes plant growth in banana plants. Microb. Cell Factories 2025, 24, 22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, X.; Pan, Y.; Qi, D.; Zhou, D.; Chen, Y.; Feng, J.; Wei, Y.; Zhao, Y.; Li, K.; et al. Biocontrol mechanism of Bacillus siamensis sp. QN2MO-1 against tomato fusarium wilt disease during fruit postharvest and planting. Microbiol. Res. 2024, 283, 127694. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, T.; Li, K.; Wang, L.; Eissa, M.A.; Cai, B.; Yun, T.; He, Y.; Baroudy, A.A.E.; Ding, Z.; Wei, Y.; et al. Acidification and Nutrient Imbalances Drive Fusarium Wilt Severity in Banana (Musa spp.) Grown on Tropical Latosols. J. Fungi 2025, 11, 611. https://doi.org/10.3390/jof11090611
Jing T, Li K, Wang L, Eissa MA, Cai B, Yun T, He Y, Baroudy AAE, Ding Z, Wei Y, et al. Acidification and Nutrient Imbalances Drive Fusarium Wilt Severity in Banana (Musa spp.) Grown on Tropical Latosols. Journal of Fungi. 2025; 11(9):611. https://doi.org/10.3390/jof11090611
Chicago/Turabian StyleJing, Tao, Kai Li, Lixia Wang, Mamdouh A. Eissa, Bingyu Cai, Tianyan Yun, Yingdui He, Ahmed A. El Baroudy, Zheli Ding, Yongzan Wei, and et al. 2025. "Acidification and Nutrient Imbalances Drive Fusarium Wilt Severity in Banana (Musa spp.) Grown on Tropical Latosols" Journal of Fungi 11, no. 9: 611. https://doi.org/10.3390/jof11090611
APA StyleJing, T., Li, K., Wang, L., Eissa, M. A., Cai, B., Yun, T., He, Y., Baroudy, A. A. E., Ding, Z., Wei, Y., Chen, Y., Wang, W., Zhou, D., Zang, X., & Xie, J. (2025). Acidification and Nutrient Imbalances Drive Fusarium Wilt Severity in Banana (Musa spp.) Grown on Tropical Latosols. Journal of Fungi, 11(9), 611. https://doi.org/10.3390/jof11090611







