Co-Culture with Two Soil Fungal Strains Enhances Growth and Secondary Metabolite Biosynthesis in Cordyceps takaomontana
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition of C. takaomontana and Its Culture Media
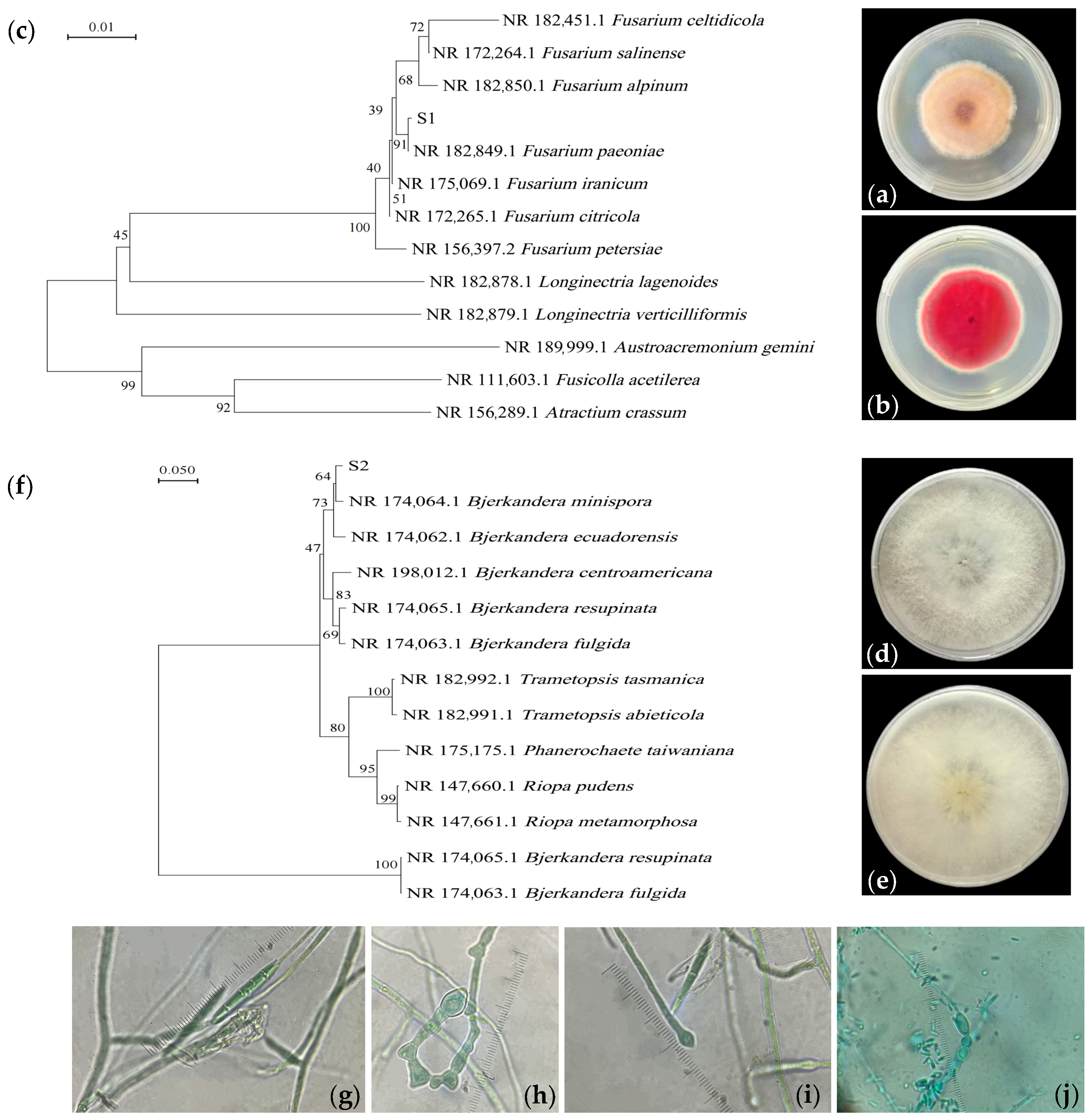
2.2. Isolation and Identification of Soil Fungi
2.3. Growth-Promoting Traits of Soil Fungi
2.4. Artificial Cultivation of C. takaomontana and Establishment of Co-Culture System
2.5. Quantification of Antioxidant Active Constituents in Cordyceps takaomontana
2.6. Antioxidant Enzyme Activity Assay of Cordyceps takaomontana
2.7. Sample Preparation and Implementation of Untargeted Metabolomics
2.8. Data Analysis
3. Results
3.1. Colony Characteristics and Species Identification of Isolated Fungi
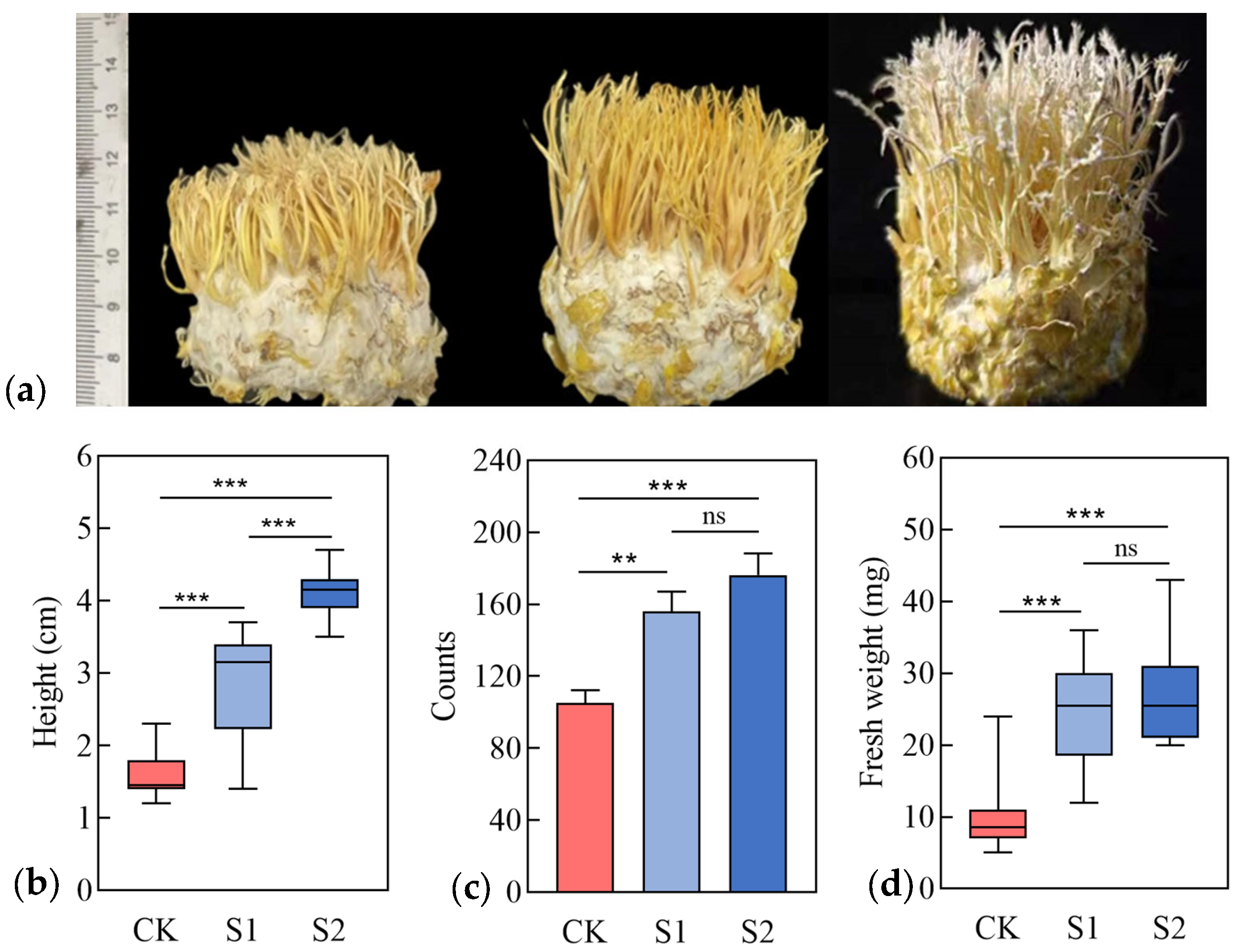
3.2. Growth-Promoting Traits of Strains
3.3. Physiological Indices of C. takaomontana
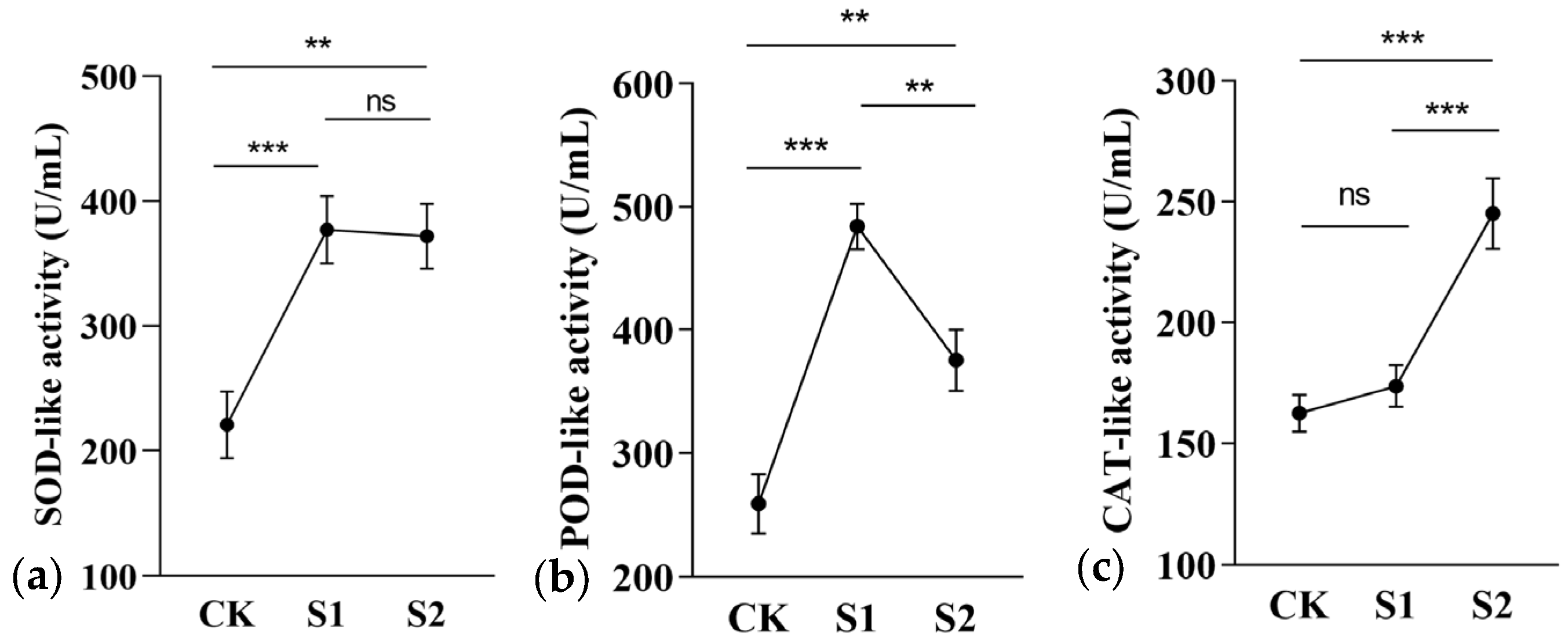
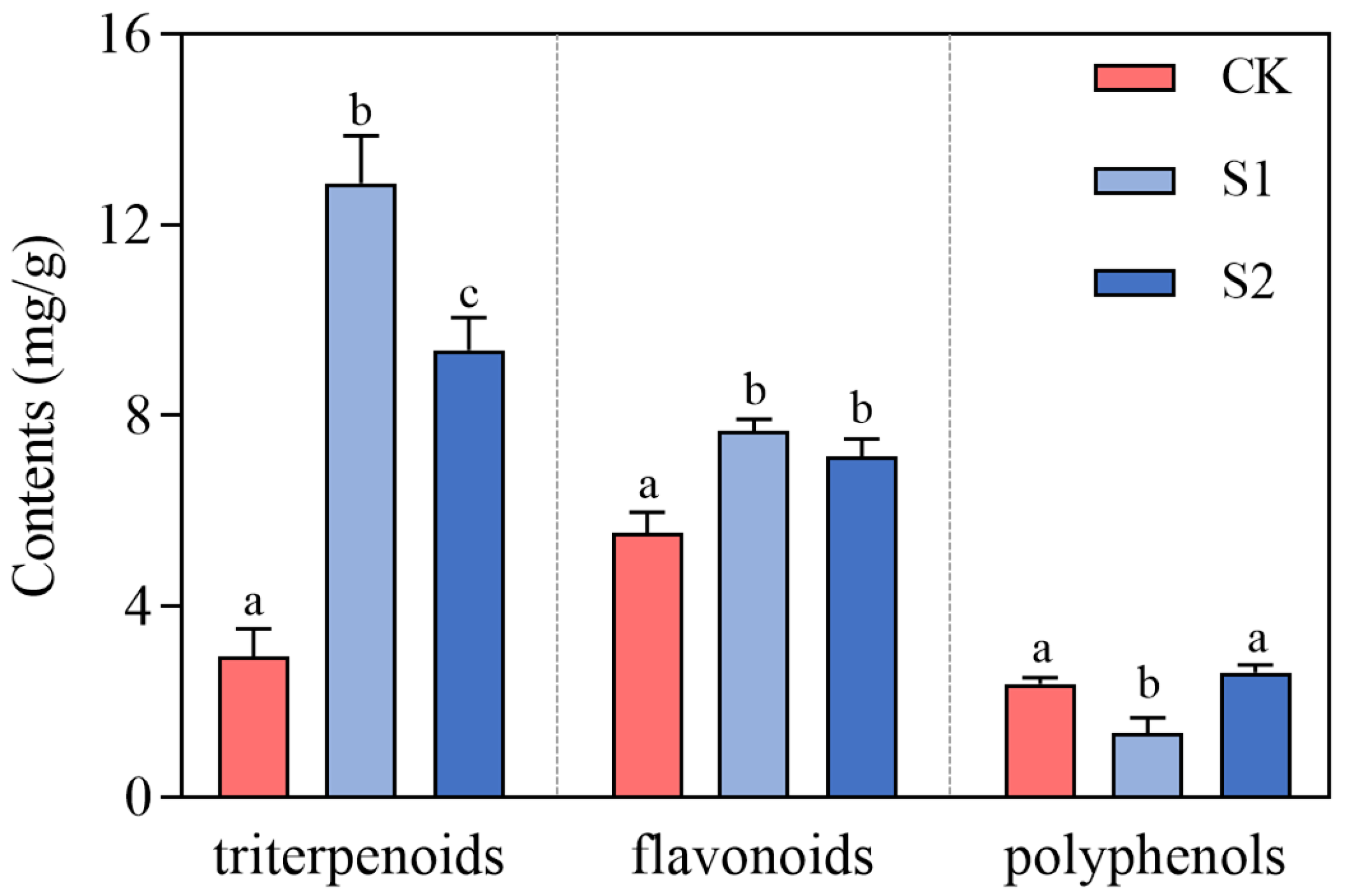
3.4. Antioxidant Activity of C. takaomontana
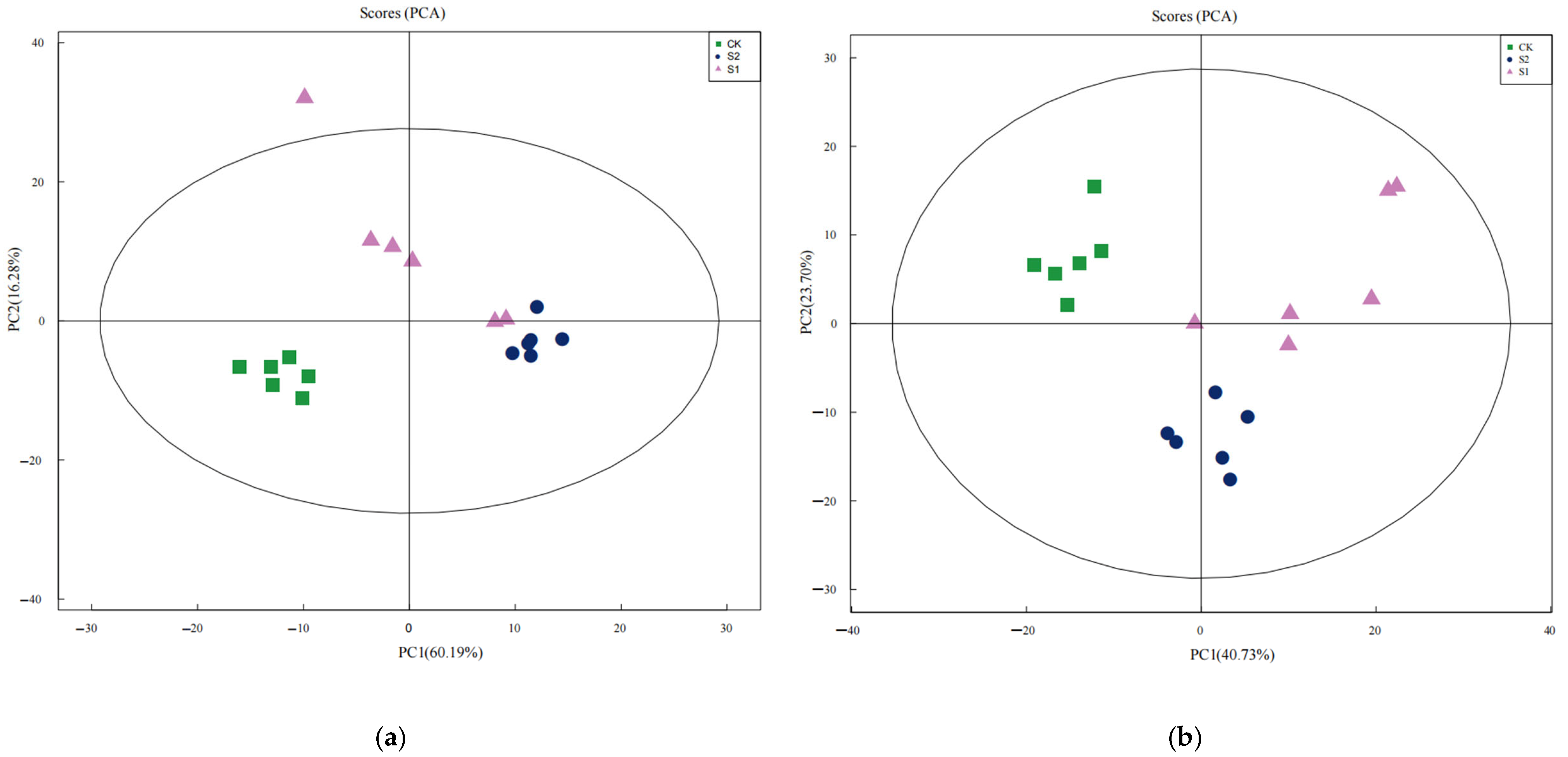
3.5. Non-Targeted Metabolomic Analysis
3.5.1. Quality Assessment of the Metabolic Model
3.5.2. Analysis of Metabolite Composition
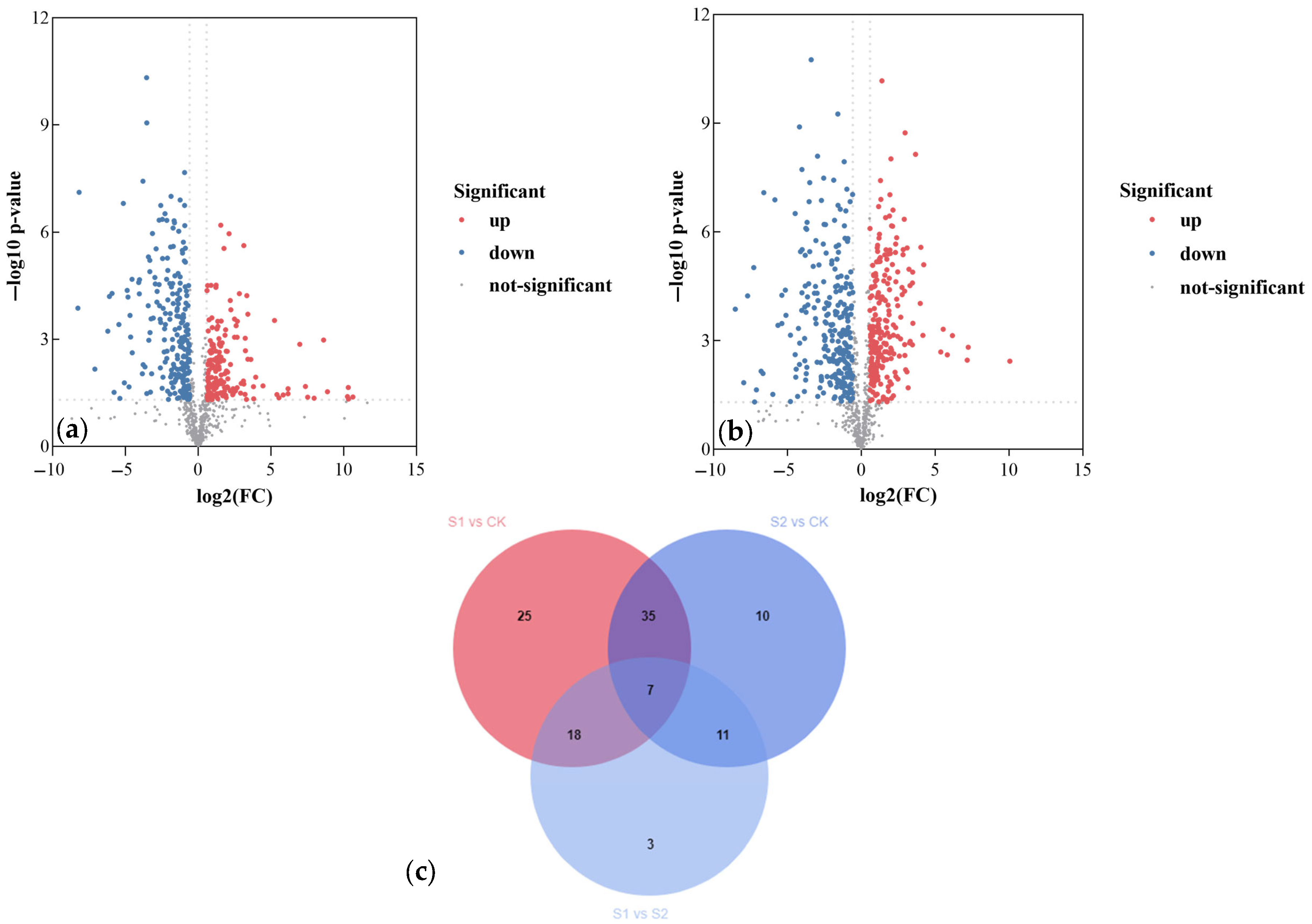
3.5.3. Analysis of Differential Metabolites
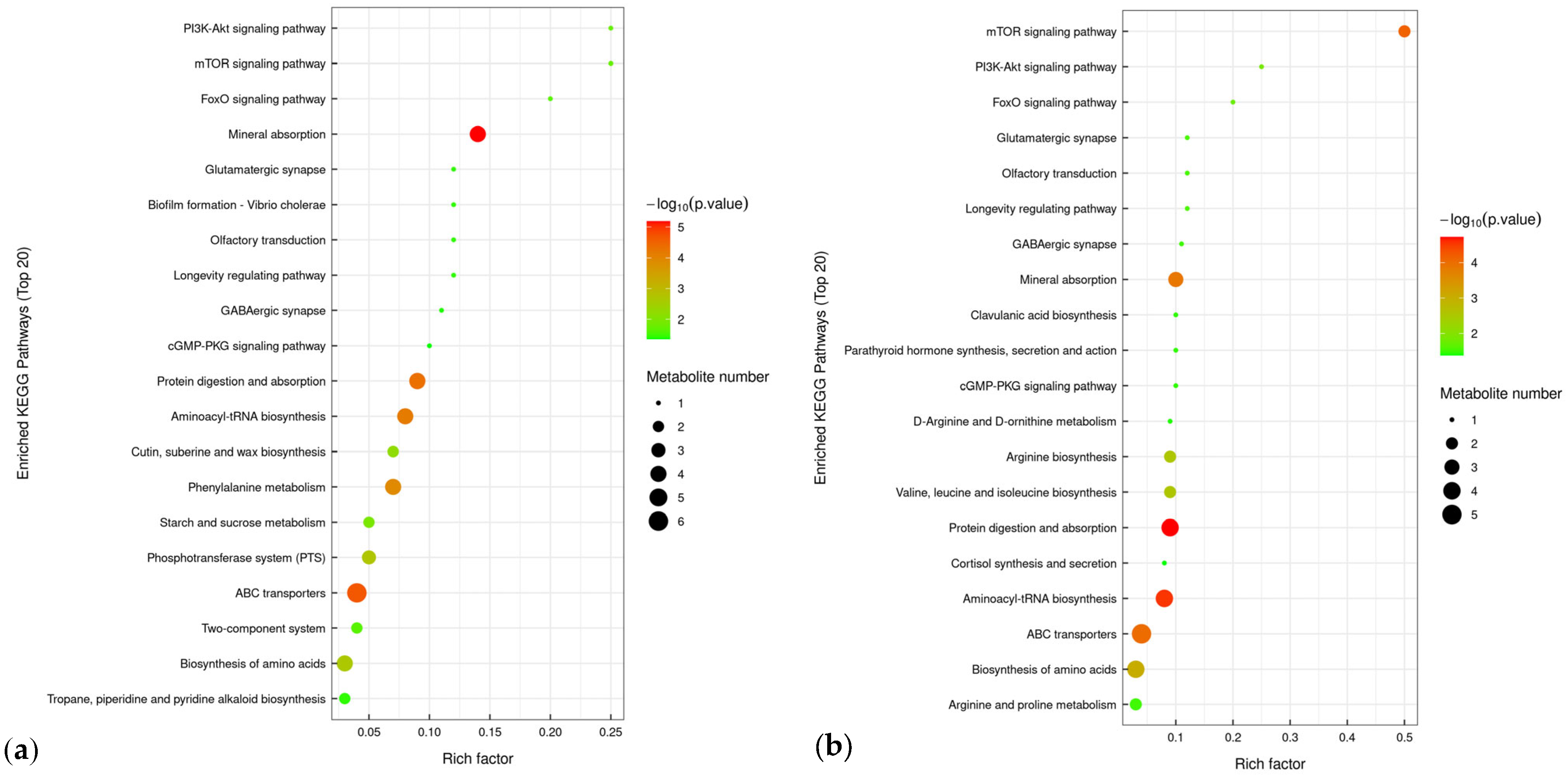
3.5.4. Analysis of Differential Metabolic Pathways
4. Discussion
4.1. Inoculation of Isolated Fungi Promoting the Growth of C. takaomontana
4.2. Differential Metabolic Pathways
4.3. Fungal-Induced Antioxidant Defense Activation
4.4. Enhancement of Beneficial Secondary Metabolites
4.5. Study Limitations and Future Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, H.; Liu, H.; Zhang, R.; Chen, Y.; Lei, L.; Qiu, C.; Xu, H. Effect of Slow-Released Biomass Alkaline Amendments Oyster Shell on Microecology in Acidic Heavy Metal Contaminated Paddy Soils. J. Environ. Manag. 2022, 319, 115683. [Google Scholar] [CrossRef]
- Xi, N.; De Long, J.R.; Davison, J.; Kardol, P.; Forero, L.E.; Zobel, M.; Semchenko, M. Plant–Soil Microbial Interactions as Modulators of Species Coexistence and Productivity. Trends Ecol. Evol. 2025, 40, 673–686. [Google Scholar] [CrossRef]
- Cao, T.; Zang, X.; Ren, J.; Liu, J.; Yang, D. Cover Crop Alters Rhizosphere Sediments to Recruit Plant Growth-Promoting Microorganisms, Enhancing Peanut Production. Appl. Soil Ecol. 2024, 203, 105620. [Google Scholar] [CrossRef]
- Yan, N.; Wang, W.; Mi, T.; Zhang, X.; Li, X.; Du, G. Enhancing Tomato Growth and Soil Fertility under Salinity Stress Using Halotolerant Plant Growth-Promoting Rhizobacteria. Plant Stress 2024, 14, 100638. [Google Scholar] [CrossRef]
- Li, X.; Yan, J.; Li, D.; Jiang, Y.; Zhang, Y.; Wang, H.; Zhang, J.; Ahmed, T.; Li, B. Isolation and Molecular Characterization of Plant-Growth-Promoting Bacteria and Their Effect on Eggplant (Solanum melongena) Growth. Agriculture 2021, 11, 1258. [Google Scholar] [CrossRef]
- Deng, Y.; Kim, B.Y.; Park, M.J.; Ok, M.; Lee, K.S.; Jin, B.R. Paecilomyces tenuipes Extracts Affect Lipid and Glucose Metabolic Parameters Similarly to Cordyceps militaris Extracts. J. Asia-Pac. Entomol. 2020, 23, 746–755. [Google Scholar] [CrossRef]
- Kim, H.C.; Choi, B.-S.; Sapkota, K.; Kim, S.; Lee, H.J.; Yoo, J.C.; Kim, S.-J. Purification and Characterization of a Novel, Highly Potent Fibrinolytic Enzyme from Paecilomyces tenuipes. Process Biochem. 2011, 46, 1545–1553. [Google Scholar] [CrossRef]
- Shim, J.-S.; Chang, H.R.; Min, E.-G.; Kim, Y.-H.; Han, Y.-H. Cytotoxicity of Paecilomyces tenuipes Against Human Carcinoma Cells, HepG2 and MCF-7 In Vitro. Mycobiology 2001, 29, 170–172. [Google Scholar] [CrossRef]
- Wu, P.; Wen, F.Y.; Wang, X.Y.; Fang, Q.M. Investigation and Analysis of Cordyceps liangshanensis Resources. Lishizhen Med. Mater. Med. Res. 2019, 30, 2252–2254. [Google Scholar] [CrossRef]
- Zhang, X.M.; Wang, X.; Wang, C.Y.; Li, M.L. Preliminary Study on the Rare Resources of Cordyceps in Shimen Mountain Forest Area of Xunyi County. Shaanxi For. Sci. Technol. 2022, 50, 29–31. [Google Scholar] [CrossRef]
- Qiu, X.H.; Cao, L.; Han, R.C. The Progress, issues and perspectives in the research of Ophiocordyceps sinensis. J. Environ. Entomol. 2016, 38, 1–23. [Google Scholar] [CrossRef]
- Yang, S.S.; Lin, Q.Y.; Zhu, L.N.; Bao, D.P.; Chen, M.J.; Li, C.H. Biological characteristics of Cordyceps tenuipes and the optimization of artificially domesticated conditions. Mycosystema 2021, 40, 1480–1497. [Google Scholar] [CrossRef]
- Liang, J.D.; Wang, Y.R.; Han, Y.F.; Liang, Z.Q. Effects of Pretreatment on the Infection Rate of Spraying Hyphal Bodies of Cordyceps takaomontana to Silkworm Pupae. J. Mt. Agric. Biol. 2013, 32, 307–309. [Google Scholar] [CrossRef]
- Lu, L.; Galappaththi, M.C.A.; Patabendige, N.M.; Feng, Y.-Z.; Yang, T.; Karunarathna, S.C.; Xie, J.-T.; Gentekaki, E.; Rapior, S.; Charria-Girón, E.; et al. Metabolomic Profiles of the Infection Pathways of Calcarisporium cordycipiticola on the Cultivated and Medicinal Mushroom, Cordyceps militaris. Fungal Biol. 2025, 129, 101561. [Google Scholar] [CrossRef]
- Jankowiak, R.; Stępniewska, H.; Bilański, P.; Hausner, G. Fusarium Species Associated with Naturally Regenerated Fagus Sylvatica Seedlings Affected by Phytophthora. Eur. J. Plant Pathol. 2025, 171, 661–681. [Google Scholar] [CrossRef]
- Zhang, J.J.; Zhu, Y.Y.; Lu, Y.F.; Li, Y. Effects of Bjerkandera adusta ‘M1’ on Gray Mold Prevention and Growth Promotion in Tomato. Acta Hortic. Sin. 2021, 48, 960–972. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Li, T.; Bao, X.; He, L.; Liu, L.; Liu, S.; Bai, J.; Zhang, H.; Niu, S.; et al. The Interplay between the Formation of Chinese Cordyceps and the Characteristics of Soil Properties and Microbial Network. Microbiol. Spectr. 2025, 13, e0327724. [Google Scholar] [CrossRef]
- Zhang, Y. Studies on the Relationship Between the Strains Related to Ophiocordyceps and Hirsutella sinensis. Master’s Thesis, Lanzhou Jiaotong University, Lanzhou, China, 2016. [Google Scholar]
- Yuan, J.-P.; Wang, J.-H.; Liu, X.; Kuang, H.-C.; Zhao, S.-Y. Simultaneous Determination of Free Ergosterol and Ergosteryl Esters in Cordyceps sinensis by HPLC. Food Chem. 2007, 105, 1755–1759. [Google Scholar] [CrossRef]
- Kim, C.-B.; Kim, J.-S.; Jang, H.-S.; Han, K.-A.; Rim, Y.-H.; Kim, J.-H. Effects of Cottonseed Powder and Perilla Oil on Cordycepin Production from Cordyceps militaris in Liquid Culture. Braz. J. Microbiol. [CrossRef]
- Liu, Y.; Xiao, K.; Wang, Z.; Wang, S.; Xu, F. Comparison of Metabolism Substances in Cordyceps sinensis and Cordyceps militaris Cultivated with Tussah Pupa Based on LC-MS. J. Food Biochem. 2021, 45, e13735. [Google Scholar] [CrossRef]
- Qu, S.-L.; Xie, J.; Wang, J.-T.; Li, G.-H.; Pan, X.-R.; Zhao, P.-J. Activities and Metabolomics of Cordyceps Gunnii under Different Culture Conditions. Front. Microbiol. 2023, 13, 1076577. [Google Scholar] [CrossRef]
- Paul, C.; Roy, T.; Singh, K.; Maitra, M.; Das, N. Study of Growth-Improving and Sporophore-Inducing Endobacteria Isolated from Pleurotus Pulmonarius. World J. Microbiol. Biotechnol. 2023, 39, 349. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, E.; Feng, B.; Xu, L.; Xue, Y.; Chen, Y. Siderophore Production Capability of Nitrogen-Fixing Bacterium (NFB) GXGL-4A Regulates Cucumber Rhizosphere Soil Microecology. Microorganisms 2025, 13, 346. [Google Scholar] [CrossRef]
- Ren, S.; Liu, Y.; Liu, Y.; Yu, H.; Xu, M. Combination of Nitrogen-Fixing Bacteria and Mycorrhizal Fungi Promotes Leymus Chinensis Growth and Bioremediation of Degraded Grasslands in Semi-Arid Regions. Plant Soil 2024, 511, 1575–1595. [Google Scholar] [CrossRef]
- Yuan, Z.-S.; Liu, F.; Zhang, G.-F. Characteristics and Biodiversity of Endophytic Phosphorus- and Potassium-Solubilizing Bacteria in Moso Bamboo (Phyllostachys edulis). Acta Biol. Hung. 2015, 66, 449–459. [Google Scholar] [CrossRef]
- Krupodorova, T.; Barshteyn, V.; Tsygankova, V.; Sevindik, M.; Blume, Y. Strain-Specific Features of Pleurotus Ostreatus Growth In Vitro and Some of Its Biological Activities. BMC Biotechnol. 2024, 24, 9. [Google Scholar] [CrossRef]
- Jain, D.; Meena, M.; Janmeda, P.; Seth, C.S.; Arora, J. Analysis of Quantitative Phytochemical Content and Antioxidant Activity of Leaf, Stem, and Bark of Gymnosporia senegalensis (Lam.) Loes. Plants 2024, 13, 1425. [Google Scholar] [CrossRef]
- Zhang, R.; Cen, Q.; Hu, W.; Chen, H.; Hui, F.; Li, J.; Zeng, X.; Qin, L. Metabolite Profiling, Antioxidant and Anti-Glycemic Activities of Tartary Buckwheat Processed by Solid-State Fermentation(SSF)with Ganoderma Lucidum. Food Chem. X 2024, 22, 101376. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, W.; Pan, Q.; Zeng, Q.; Yan, C.; Bai, X.; Liu, Y.; Zhang, L.; Li, B. Effects of Long-Term Blue Light Irradiation on Carotenoid Biosynthesis and Antioxidant Activities in Chinese Cabbage (Brassica rapa L. ssp. Pekinensis). Food Res. Int. Ott. Ont 2023, 174, 113661. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Shen, Y.; Jin, S.; Huang, S.; Cheng, X.; Wang, Z.; Li, P.; Zhao, J.; Bao, M.; Ning, G. Overexpression of Rosa Rugosa Anthocyanidin Reductase Enhances Tobacco Tolerance to Abiotic Stress through Increased ROS Scavenging and Modulation of ABA Signaling. Plant Sci. Int. J. Exp. Plant Biol. 2016, 245, 35–49. [Google Scholar] [CrossRef] [PubMed]
- García, J.E.; Pagnussat, L.A.; Amenta, M.B.; Casanovas, E.M.; Diaz, P.R.; Labarthe, M.M.; Martino, M.V.; Groppa, M.D.; Creus, C.M.; Maroniche, G.A. Maize Drought Protection by Azospirillum Argentinense Az19 Requires Bacterial Trehalose Accumulation. Appl. Microbiol. Biotechnol. 2024, 108, 543. [Google Scholar] [CrossRef] [PubMed]
- Chengfeng, W.; Xia, L.I.U.; Xiyu, Z.; Jie, D.; Shirun, Z.; Xiaomin, Y.; Wei, L.; Jiayan, L.I.U.; Zongju, L.I. Analyses of Substances Associating Termitomyces Robustus Fruiting Bodies with Termite Nests and Preliminary Studies on Growth-Promoting Effect of Amino Acids and Sugars. Mycosystema 2025, 44, 240256. [Google Scholar] [CrossRef]
- Gómez-Garrido, M.; Mora Navarro, J.; Murcia Navarro, F.J.; Faz Cano, Á. The Chelating Effect of Citric Acid, Oxalic Acid, Amino Acids and Pseudomonas Fluorescens Bacteria on Phytoremediation of Cu, Zn, and Cr from Soil Using Suaeda Vera. Int. J. Phytoremediat. 2018, 20, 1033–1042. [Google Scholar] [CrossRef]
- Perkowski, M.C.; Warpeha, K.M. Phenylalanine Roles in the Seed-to-Seedling Stage: Not Just an Amino Acid. Plant Sci. 2019, 289, 110223. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Fan, W.; Pan, X.; Luo, Y. Estimating the Risks from Phthalate Esters and Metal(Loid)s in Cultivated Edible Fungi from Jingmen, Central China. Food Chem. 2021, 348, 129065. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Fan, J.; An, Y.; Meng, G.; Ji, B.; Li, Y.; Dong, C. Quality Evaluation, Health Risk Assessment, and Geographic Origin Tracing of Ophiocordyceps sinensis through Mineral Element Analysis. Microchem. J. 2024, 201, 110512. [Google Scholar] [CrossRef]
- Chiappero, J.; Cappellari, L.d.R.; Palermo, T.B.; Giordano, W.; Khan, N.; Banchio, E. Antioxidant Status of Medicinal and Aromatic Plants under the Influence of Growth-Promoting Rhizobacteria and Osmotic Stress. Ind. Crops Prod. 2021, 167, 113541. [Google Scholar] [CrossRef]
- Nivedita, S.; Behera, S.S.; Behera, P.K.; Parwez, Z.; Giri, S.; Behera, H.T.; Ray, L. Salt-Resistant Streptomyces Consortia Promote Growth of Rice (Oryza sativa Var. Swarna) Alleviating Salinity and Drought Stress Tolerance by Enhancing Photosynthesis, Antioxidant Function, and Proline Content. The Microbe 2024, 4, 100124. [Google Scholar] [CrossRef]
- Meng, X.C.; Li, X.Y.; Yao, J.; Kong, L.; Guan, Y. Mechanism of ecological stress enhancing quality of genuine medicinal materials and its quality evaluation idea. Chin. Tradit. Herb. Drugs 2022, 53, 1587–1594. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.H.; Zhang, S.B.; Wang, X.L. Preliminary Study on Production and Antimicrobial Activity of Triterpenoids from Xuefeng Congcao (Ophiocordyceps xuefengensis) by Liquid Cell Culture. Chin. J. Tradit. Med. Sci. Technol. 2020, 27, 34–37. [Google Scholar]
- Chen, M.; Zhou, S.; Liu, L.; Wen, Y.; Chen, L. Notoginsenoside R1 Alleviates the Inflammation of Osteoarthritis by Activating the Nrf2/HO-1 Signalling Pathway In Vitro and In Vivo. J. Funct. Foods 2021, 85, 104666. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, Y.; Liu, Y.; Pei, C.; Shen, Z.; Zhao, S.; Jia, N.; Huang, D.; Wang, X.; Wu, Y.; et al. Notoginsenoside R1 Treatment Facilitated Nrf2 Nuclear Translocation to Suppress Ferroptosis via Keap1/Nrf2 Signaling Pathway to Alleviated High-Altitude Myocardial Injury. Biomed. Pharmacother. 2024, 175, 116793. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Q.; Jin, M.; Ren, J.; Sun, X.; Zhang, Z.; Luo, Y.; Sun, X. Notoginsenoside R1 Alleviates Cerebral Ischemia/Reperfusion Injury by Inhibiting the TLR4/MyD88/NF-κB Signaling Pathway through Microbiota-Gut-Brain Axis. Phytomed. Int. J. Phytother. Phytopharm. 2024, 128, 155530. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Xia, Q.; Wang, M.Q.; Wei, F.G.; Yang, S.Z.; Zhang, J.B.; Cai, Z.C.; Zhao, J. The Accumulation Differences of Sanqi Ginseng Saponins in Different Producing Areas and Their Relationships with Soil Fungal Pathogens. Soils 2025, 57, 114–123. [Google Scholar] [CrossRef]
- Roig, M.; Meca, G.; Marín, R.; Ferrer, E.; Mañes, J. Antibacterial Activity of the Emerging Fusarium Mycotoxins Enniatins A, A1, A2, B, B1, and B4 on Probiotic Microorganisms. Toxicon 2014, 85, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Saetang, P.; Rukachaisirikul, V.; Saithong, S.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J. Benzaldehyde and Azaphilone Derivatives from the Marine-Derived Fungus Penicillium Sclerotiorum PSU-AMF89. Nat. Prod. Res. 2024, 1–8. [Google Scholar] [CrossRef]
- Zhou, B.; Liang, X.; Feng, Q.; Li, J.; Pan, X.; Xie, P.; Jiang, Z.; Yang, Z. Ergosterol Peroxide Suppresses Influenza A Virus-Induced pro-Inflammatory Response and Apoptosis by Blocking RIG-I Signaling. Eur. J. Pharmacol. 2019, 860, 172543. [Google Scholar] [CrossRef]
- Huang, H.; Huang, H.-C.; Chiou, W.-C.; Lin, L.-C.; Chen, J.-C.; Liu, H.-K.; Lai, Y.-H.; Huang, C. Ergosterol Peroxide Inhibits HBV Infection by Inhibiting the Binding of the Pre-S1 Domain of LHBsAg to NTCP. Antivir. Res. 2021, 195, 105184. [Google Scholar] [CrossRef]
- Kang, J.-H.; Jang, J.-E.; Mishra, S.K.; Lee, H.-J.; Nho, C.W.; Shin, D.; Jin, M.; Kim, M.K.; Choi, C.; Oh, S.H. Ergosterol Peroxide from Chaga Mushroom (Inonotus obliquus) Exhibits Anti-Cancer Activity by down-Regulation of the β-Catenin Pathway in Colorectal Cancer. J. Ethnopharmacol. 2015, 173, 303–312. [Google Scholar] [CrossRef]
- Wang, L.; Wang, G.; Yang, D.; Guo, X.; Xu, Y.; Feng, B.; Kang, J. Euphol Arrests Breast Cancer Cells at the G1 Phase through the Modulation of Cyclin D1, P21 and P27 Expression. Mol. Med. Rep. 2013, 8, 1279–1285. [Google Scholar] [CrossRef]
- Gade, I.S.; Chadéneau, C.; Simo, R.T.; Talla, E.; Atchade, A.d.T.; Seité, P.; Vannier, B.; Laurent, S.; Henoumont, C.; Kamdje, A.H.N.; et al. Euphol from Tapinanthus Sp. Induces Apoptosis and Affects Signaling Proteins in Glioblastoma and Prostate Cancer Cells. Asian Pac. J. Cancer Prev. APJCP 2022, 23, 4205–4212. [Google Scholar] [CrossRef]
- Passos, G.F.; Medeiros, R.; Marcon, R.; Nascimento, A.F.Z.; Calixto, J.B.; Pianowski, L.F. The Role of PKC/ERK1/2 Signaling in the Anti-Inflammatory Effect of Tetracyclic Triterpene Euphol on TPA-Induced Skin Inflammation in Mice. Eur. J. Pharmacol. 2013, 698, 413–420. [Google Scholar] [CrossRef]
- Freire, R.V.M.; Dias, D.C.d.A.; Silva, J.Y.R.; do Nascimento Santos, D.K.D.; Jesus, L.T.; Freire, R.O.; Junior, S.A. Experimental and Theoretical Investigation of Phytochemical Euphol Incorporated in ZIF-8 as a Drug Delivery System for Cancer Treatment. Mater. Chem. Phys. 2024, 312, 128648. [Google Scholar] [CrossRef]
- Khamis, A.A.; Sharshar, A.H.; Mohamed, T.M.; Abdelrasoul, E.A.; Salem, M.M. Visnagin Alleviates Rheumatoid Arthritis via Its Potential Inhibitory Impact on Malate Dehydrogenase Enzyme: In Silico, In Vitro, and In Vivo Studies. Genes Nutr. 2024, 19, 20. [Google Scholar] [CrossRef]
- Yadav, P.; Singh, S.K.; Datta, S.; Verma, S.; Verma, A.; Rakshit, A.; Bali, A.; Bhatti, J.S.; Khurana, A.; Navik, U. Therapeutic Potential and Pharmacological Mechanism of Visnagin. J. Integr. Med. 2024, 22, 399–412. [Google Scholar] [CrossRef]

| Time (min) | A/% | B/% |
|---|---|---|
| 0–0.5 min | 95 | 5 |
| 0.5–12.0 min | 0 | 100 |
| 12.0–12.1 min | 95 | 5 |
| 12.1–16.0 min | 95 | 5 |
| Growth-Promoting Traits | Fungus S1 | Fungus S2 |
|---|---|---|
| IAA Production (μg/mL) | 55.739 | 46.856 |
| Potassium Solubilization | n.a. | n.a. |
| Siderophore Production | + | + |
| Nitrogen Fixation | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Ding, M.; He, D.; Zhang, D.; Wang, M.; Xiang, Y.; Liu, T. Co-Culture with Two Soil Fungal Strains Enhances Growth and Secondary Metabolite Biosynthesis in Cordyceps takaomontana. J. Fungi 2025, 11, 559. https://doi.org/10.3390/jof11080559
Chen J, Ding M, He D, Zhang D, Wang M, Xiang Y, Liu T. Co-Culture with Two Soil Fungal Strains Enhances Growth and Secondary Metabolite Biosynthesis in Cordyceps takaomontana. Journal of Fungi. 2025; 11(8):559. https://doi.org/10.3390/jof11080559
Chicago/Turabian StyleChen, Junyi, Minghao Ding, Donglan He, Dengxian Zhang, Ming Wang, Yulan Xiang, and Tianya Liu. 2025. "Co-Culture with Two Soil Fungal Strains Enhances Growth and Secondary Metabolite Biosynthesis in Cordyceps takaomontana" Journal of Fungi 11, no. 8: 559. https://doi.org/10.3390/jof11080559
APA StyleChen, J., Ding, M., He, D., Zhang, D., Wang, M., Xiang, Y., & Liu, T. (2025). Co-Culture with Two Soil Fungal Strains Enhances Growth and Secondary Metabolite Biosynthesis in Cordyceps takaomontana. Journal of Fungi, 11(8), 559. https://doi.org/10.3390/jof11080559






