Identification, Characterization, Pathogenicity, and Fungicide Sensitivity of Postharvest Fungal Diseases in Culinary Melon from Northern Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Fungal Isolation
2.3. Fungal Identification
2.3.1. Morphological Study
2.3.2. DNA Extraction, PCR Amplification, and Sequencing
2.3.3. Sequence Alignment and Phylogenetic Analyses
2.4. Pathogenicity Tests
2.5. Commercial Fungicide Sensitivity Tests
2.6. Statistical Analysis
3. Results
3.1. Disease Symptoms
3.2. Fungal Isolation and Morphological Study
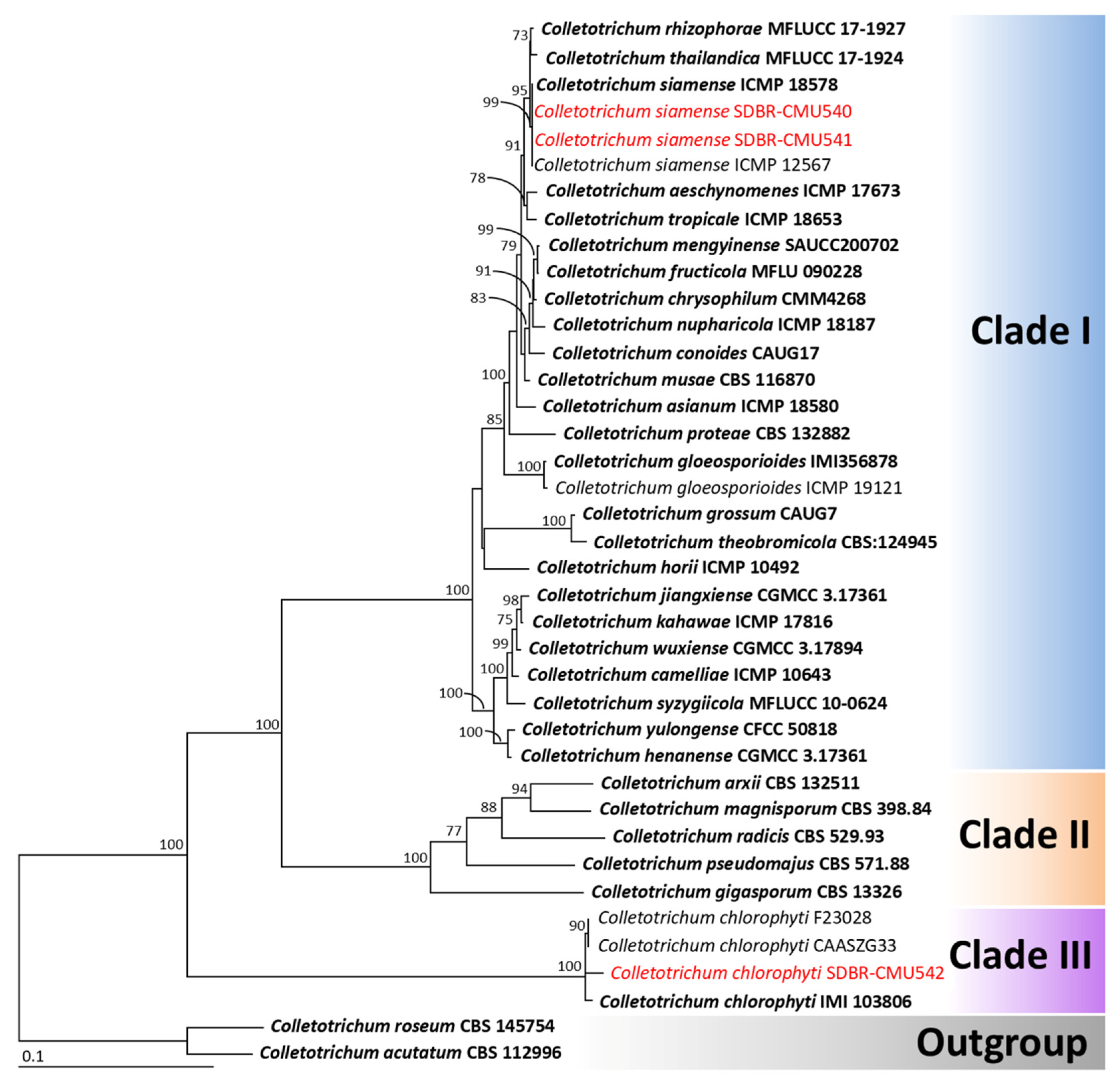
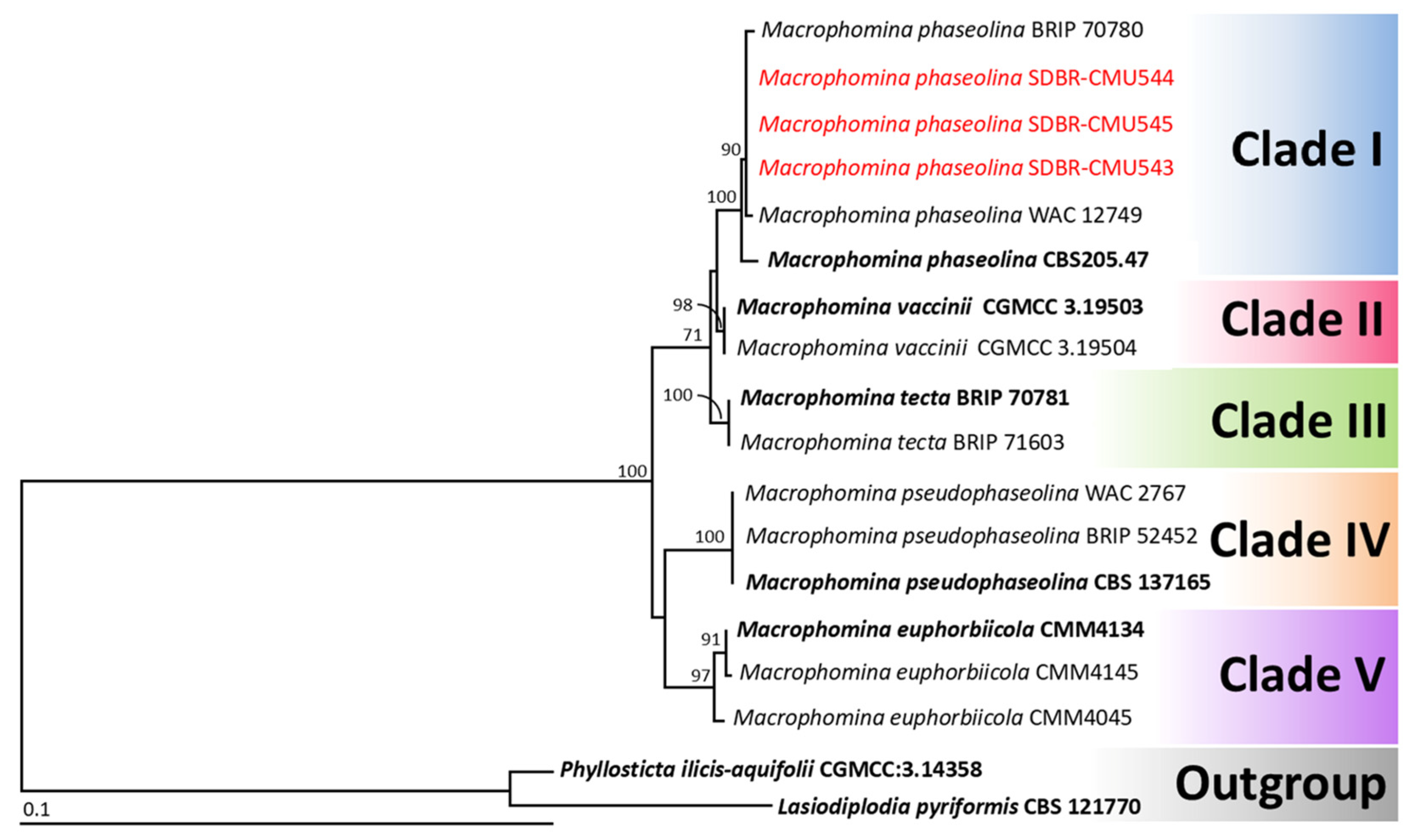
3.3. Phylogenetic Analysis
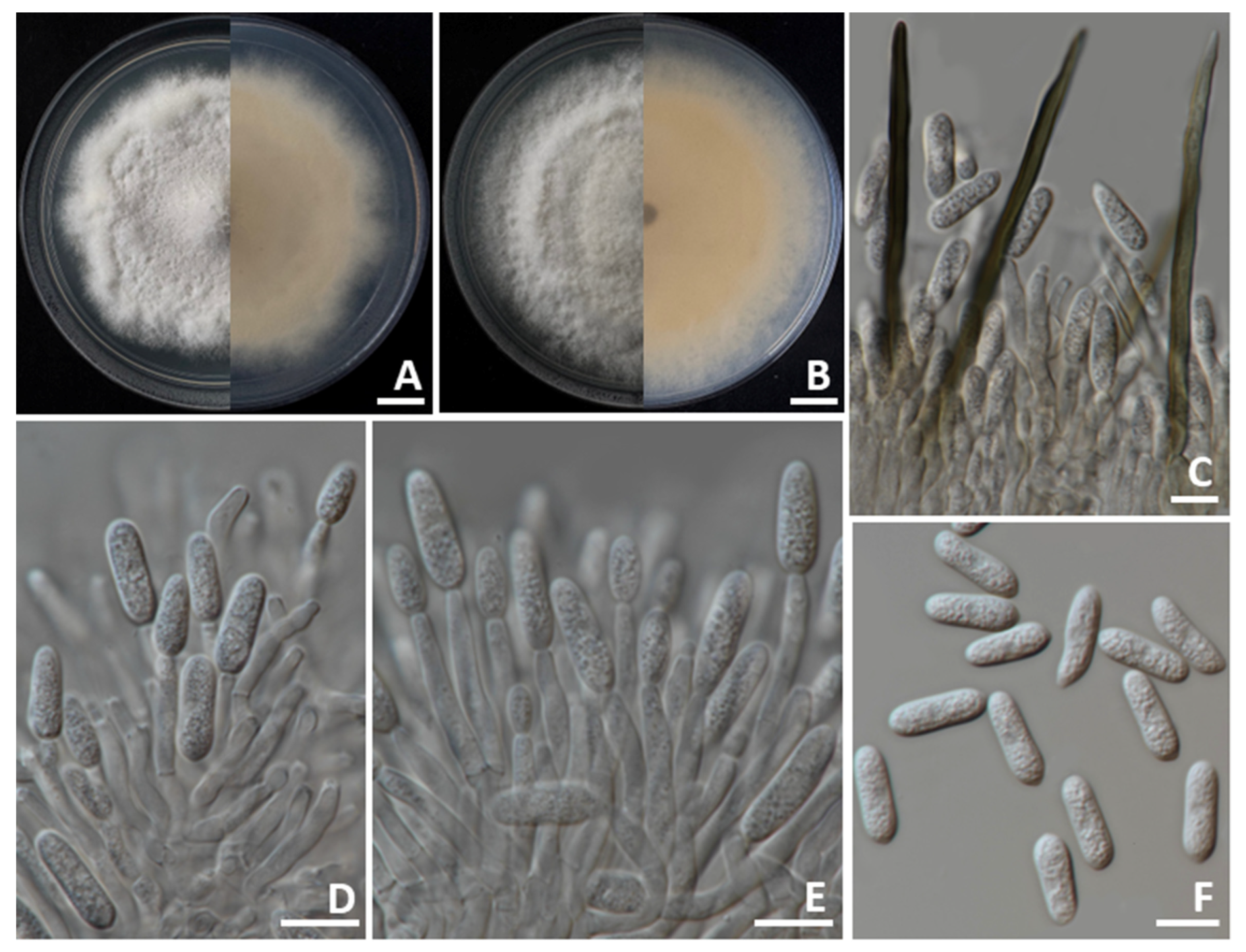
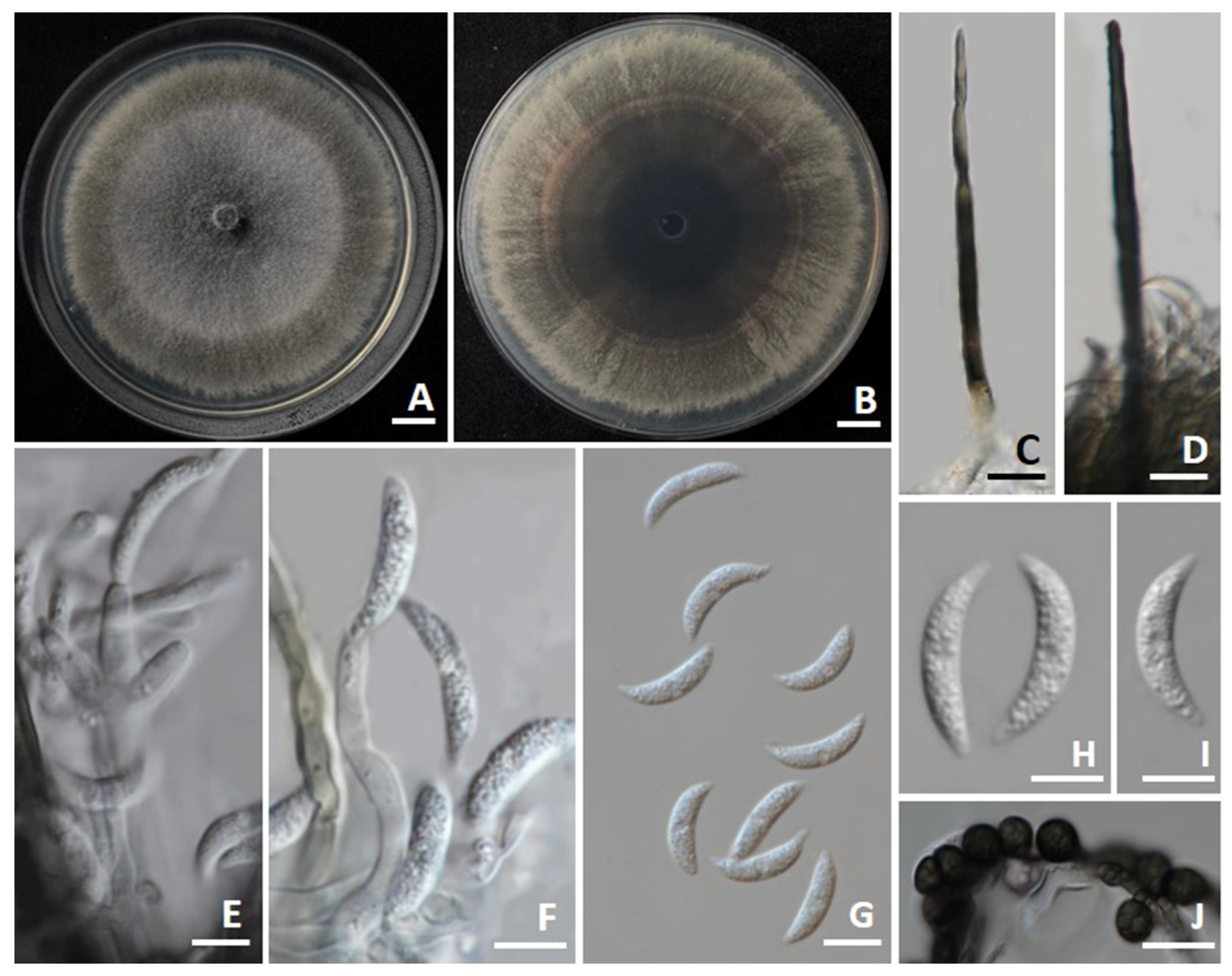
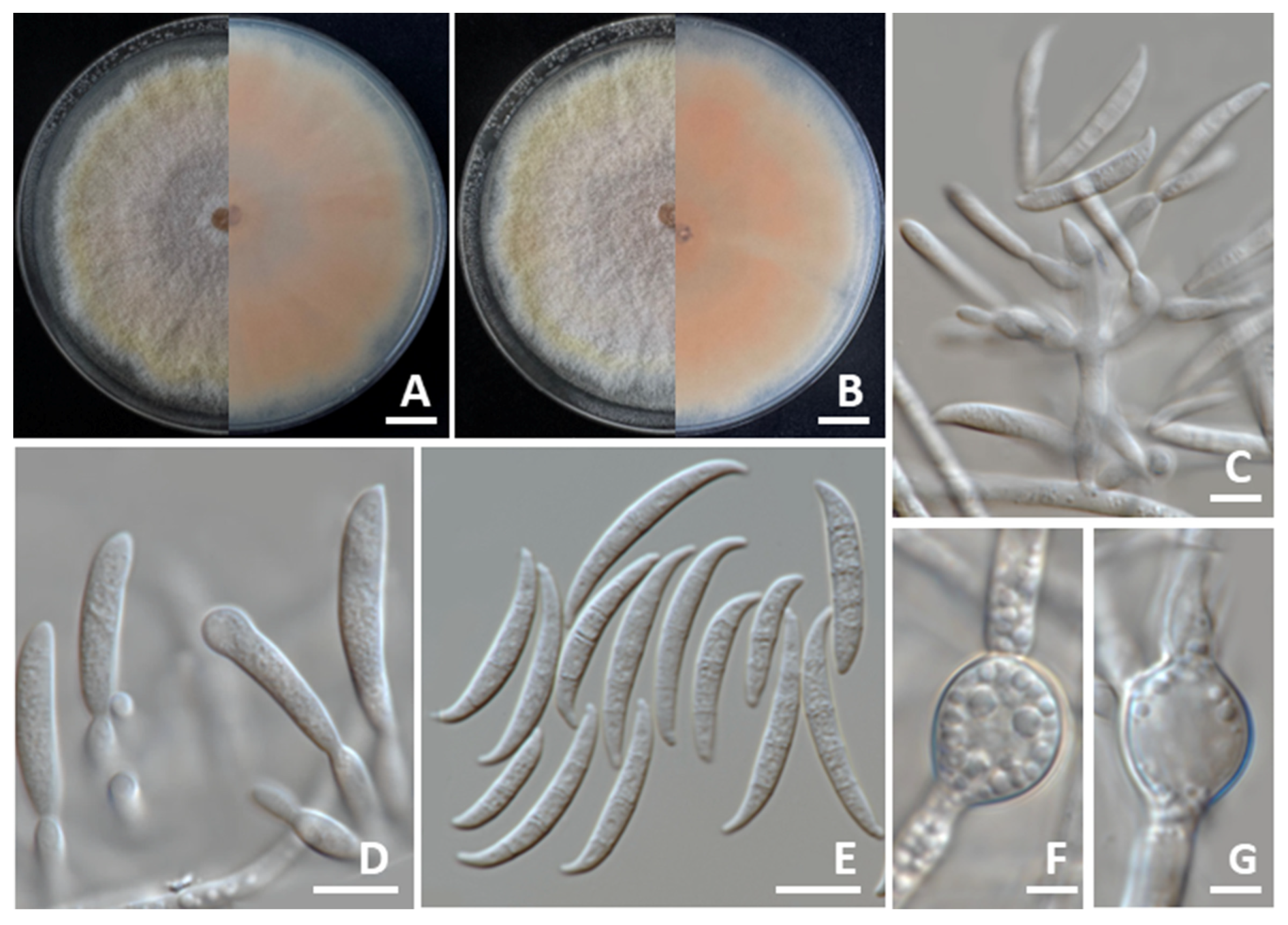
3.4. Morphological Description
3.4.1. Colletotrichum siamense
3.4.2. Colletotrichum chlorophyti
3.4.3. Fusarium sulawesiense
3.4.4. Macrophomina phaseolina
3.5. Pathogenicity Test
3.6. Response of Fungal Pathogens to Commercial Fungicides
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/faostat/en/#home (accessed on 15 May 2025).
- Ohki, T.; Sako, I.; Kanda, A.; Mochizuki, T.; Honda, Y.; Tsuda, S. A new strain of melon necrotic spot virus that is unable to systemically infect Cucumis melo. Phytopathology 2008, 98, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.L.; Harata, K.; Ogawa, M.; Shirota, K.; Sasaki, A.; Nakamura, T.; Okamato, S.; Park, E.Y.; Sato, K.; Nakamura, Y.; et al. Multiple Colletotrichum species cause anthracnose disease on Japanese pickling melon var. Katsura-uri (Cucumis melo var. conomon). J. Gen. Plant Pathol. 2023, 89, 249–259. [Google Scholar] [CrossRef]
- Lima, E.N.; Oster, A.H.; Bordallo, P.N.; Araújo, A.A.C.; Silva, D.E.M.; Lima, C.S. A novel lineage in the Fusarium incarnatum-equiseti species complex is one of the causal agents of fusarium rot on melon fruits in Northeast Brazil. Plant Pathol. 2021, 70, 133–143. [Google Scholar] [CrossRef]
- Khuna, S.; Kumla, J.; Thitla, T.; Nuangmek, W.; Lumyong, S.; Suwannarach, N. Morphology, molecular identification, and pathogenicity of two novel Fusarium species associated with postharvest fruit rot of cucurbits in northern Thailand. J. Fungi 2022, 8, 1135. [Google Scholar] [CrossRef] [PubMed]
- Ezrari, S.; Lahlali, R.; Radouane, N.; Tahiri, A.; Lazraq, A. First report of Fusarium equiseti causing pre- and postharvest fruit rot on zucchini in Morocco. J. Plant Pathol. 2020, 102, 251. [Google Scholar] [CrossRef]
- Suwannarach, N.; Khuna, S.; Thitla, T.; Senwanna, C.; Nuangmek, W.; Kumla, J.; Lumyong, S. Morpho-phylogenetic identification and characterization of new causal agents of Fusarium species for postharvest fruit rot disease of muskmelon in northern Thailand and their sensitivity to fungicides. Front. Plant Sci. 2024, 15, e1459759. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Tsukamoto, T.; Saito, J.; Sugimoto, S. Alternaria fruit rot of melon caused by Alternaria alternata. Res. Bull. Pl. Prot. Jpn. 2004, 40, 153–155. [Google Scholar]
- Broge, M.; Howard, A.; Biles, C.L.; Udayanga, D.; Taff, H.; Dudley, L.; Bruton, B.D. First report of Diaporthe fruit rot of melons caused by D. pterocarpi in Costa Rica. Plant Dis. 2020, 104, e1550. [Google Scholar] [CrossRef]
- Suwannarach, N.; Khuna, S.; Kumla, J.; Tanruean, K.; Lumyong, S. First report of Lasiodiplodia theobromae causing fruit rot disease on melon (Cucumis melo) in Thailand. Plant Dis. 2019, 104, e280. [Google Scholar] [CrossRef]
- Mirtalebi, M.; Sabahi, F.; Banihashemi, Z. Fruit rot caused by Neoscytalidium hyalinum on melon in Iran. Australas. Plant Dis. Notes 2019, 14, e8. [Google Scholar] [CrossRef]
- Huo, J.F.; Ren, Y.X.; Yao, Y.R.; Ben, H.Y.; Wen, X.Y.; Ren, W.L.; Yang, L.J.; Wang, W.L.; Hao, Y.J. First report of fruit rot caused by Paramyrothecium foliicola on muskmelon in China. J. Plant Pathol. 2023, 105, 629–630. [Google Scholar] [CrossRef]
- Pornsuriya, C.; Chitphithak, I. Blue mold caused by Penicillium oxalicum on muskmelon (Cucumis melo) in Thailand. Australas. Plant Dis. Notes 2018, 13, e46. [Google Scholar] [CrossRef]
- Kwon, J.H.; Kim, J.H.; Lee, Y.H.; Shim, H.S. Soft rot on Cucumis melo var. makuwa caused by Rhizopus oryzae. Mycobiology 2010, 38, 336–338. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Chi, T.T.P.; Park, C.S. Occurrence of fruit rot of melon caused by Sclerotium rolfsii in Korea. Mycobiology 2009, 37, 158–159. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Kim, M.G.; Kang, M.G.; Kang, I.K.; Lee, S.Y.; Jung, H.Y. First report of Stagonosporopsis cucumeris causing internal fruit rot on oriental melon (Cucumis melo) in Korea. Plant Dis. 2023, 107, e2846. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, J.; Yan, W.; You, H.; Wang, X.; Han, W.; Wu, J.; Li, Y. Identification, Pathogenic Mechanism and Control of Stagonosporopsis cucurbitacearum Causing Post-Harvest Fruit Rot in Melon. Pest Manag. Sci. 2025; online ahead of print. [Google Scholar] [CrossRef]
- Cohen, R.; Elkabets, M.; Paris, H.S.; Freeman, S.; Gur, A. Charcoal rot (Macrophomina phaseolina) across melon diversity: Evaluating the interaction between the pathogen, plant age and environmental conditions as a step towards breeding for resistance. Eur. J. Plant Pathol. 2022, 163, 601–613. [Google Scholar] [CrossRef]
- Marquez, N.; Giachero, M.L.; Declerck, S.; Ducasse, D.A. Macrophomina phaseolina: General characteristics of pathogenicity and methods of control. Front. Plant Sci. 2021, 12, e634397. [Google Scholar] [CrossRef] [PubMed]
- Jacob, C.J.; Krarup, C.; Díaz, G.A.; Latorre, B.A. A severe outbreak of charcoal rot in cantaloupe melon caused by Macrophomina phaseolina in Chile. Plant Dis. 2013, 97, 141. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Luo, C.X.; Wu, H.J.; Peng, B.; Kang, B.S.; Liu, L.M.; Zhang, M.; Gu, Q.S. Colletotrichum species associated with anthracnose disease of watermelon (Citrullus lanatus) in China. J. Fungi 2022, 8, 790. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Du, C.J.; Xie, H.; Yang, D.; Li, G.; Jiang, S.; Qin, S.; Hong, R.; He, Y.; Liu, T.; et al. First report of Colletotrichum truncatum causing anthracnose on melon (Cucumis melo L.) in China. Plant Dis. 2025, 109, 1173. [Google Scholar] [CrossRef]
- Swamy, K.R.M. Origin, distribution and systematics of culinary cucumber (Cucumis melo subsp. agrestis var. conomon). J. Hortl. Sci. 2017, 12, 1–22. [Google Scholar] [CrossRef]
- Chomicki, G.; Schaefer, H.; Renner, S.S. Origin and domestication of Cucurbitaceae crops: Insights from phylogenies, genomics and archaeology. New Phytol. 2020, 226, 1240–1255. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.N.; Park, S.Y.; Paek, K.Y. Bioactive compounds of culinary melon (Cucumis melo subsp. agrestis var. conomon). In Bioactive Compounds in Underutilized Vegetables and Legumes; Murthy, H.N., Park, S.Y., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 321–339. [Google Scholar]
- Swamy, K.R.M.; Nath, P. Culinary melon of South India: A review. Veg. Sci. 2020, 47, 157–175. [Google Scholar] [CrossRef]
- Nakamura, Y.; Watanabe, S.; Kageyama, M.; Shirota, K.; Shirota, K.; Amano, H.; Ksahimoto, T.; Matsuo, T.; Okamoto, S.; Park, E.Y.; et al. Antimutagenic, differentiation inducing and antioxidative effects of fragrant ingredients in Katsura-uri (Japanese pickling melon, Cucumis melo var. conomon). Mutat. Res. 2010, 703, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Nuangmek, W.; Aiduang, W.; Suwannarach, N.; Kumla, J.; Kiatsiriroat, T.; Lumyong, S. First report of fruit rot on cantaloupe caused by Fusarium equiseti in Thailand. J. Gen. Plant Pathol. 2019, 85, 295–300. [Google Scholar] [CrossRef]
- Wonglom, P.; Sunpapao, A. Fusarium incarnatum is associated with postharvest fruit rot of muskmelon (Cucumis melo). J. Phytopathol. 2020, 168, 204–210. [Google Scholar] [CrossRef]
- Li, W.; Jiang, Y.; Hu, C.; Liu, G.; Li, Y.; Wang, S. Identification, pathogenic mechanism and control of Rhizopus oryzae causing postharvest fruit rot in pumpkin. Postharvest Biol. Technol. 2023, 204, e112460. [Google Scholar] [CrossRef]
- Crous, P.W.; Lombard, L.; Sandoval-Denis, M.; Seifert, K.A.; Schroers, H.-J.; Chaverri, P.; Gene, J.; Guarro, J.; Hirooka, Y.; Bensch, K.; et al. Fusarium: More than a node or a foot-shaped basal cell. Stud. Mycol. 2021, 98, e100116. [Google Scholar] [CrossRef] [PubMed]
- Norphanphoun, C.; Hyde, K.D. First Report of Colletotrichum fructicola, C. rhizophorae sp. nov. and C. thailandica sp. nov. on mangrove in Thailand. Pathogens 2023, 12, 1436. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Hyde, K.D.; Taylor, P.W.J.; Weir, B.S.; Waller, J.; Abang, M.M.; Zhang, J.Z.; Yang, Y.L.; Phoulivong, S.; Liu, Z.Y.; et al. A polyphasic approach for studying Colletotrichum. Fungal Divers. 2009, 39, 183–204. [Google Scholar]
- Wang, M.M.; Crous, P.W.; Sandoval-Denis, M.; Han, S.L.; Liu, F.; Liang, J.M.; Duan, W.J.; Cai, L. Fusarium and allied genera from China: Species diversity and distribution. Persoonia 2022, 48, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Bhunjun, C.S.; Phukhamsakda, C.; Jayawardena, R.S.; Jeewon, R.; Promputtha, I.; Hyde, K.D. Investigating species boundaries in Colletotrichum. Fungal Divers. 2021, 107, 107–127. [Google Scholar] [CrossRef]
- Sarr, M.P.; Ndiaye, M.; Groenewald, J.Z.; Crous, P.W. Genetic diversity in Macrophomina phaseolina, the causal agent of charcoal rot. Phytopathol. Mediterr. 2014, 53, 250–268. [Google Scholar]
- Poudel, B.; Shivas, R.G.; Adorada, D.L.; Barbetti, M.J.; Bithell, S.L.; Kelly, L.A.; Moore, N.; Sparks, A.H.; Tan, Y.P.; Thomas, G.; et al. Hidden diversity of Macrophomina associated with broadacre and horticultural crops in Australia. Eur. J. Plant Pathol. 2021, 161, 1–23. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Hall, T. Bioedit Version 6.0.7. 2004. Available online: https://thalljiscience.github.io/ (accessed on 15 May 2025).
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688–2690. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree Tree Figure Drawing Tool Version 131; Institute of Evolutionary 623 Biology, University of Edinburgh: Edinburgh, Scotland, UK, 2019. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 15 May 2025).
- Meng, S.; Xiong, M.; Cheng, L.; Wang, L.; Chen, Y.; Luo, C.; Chao, S. CaEch1-mediated mitophagy regulates vegetative growth, conidiation, appressorium formation, and pathogenicity in Colletotrichum camelliae. Phytopathol. Res. 2025, 7, e20. [Google Scholar] [CrossRef]
- Nuangmek, W.; Kumla, J.; Khuna, S.; Lumyong, S.; Suwannarach, N. Identification and characterization of Fusarium species causing watermelon fruit rot in northern Thailand. Plants 2023, 12, 956. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Hubballi, M.; Sharma, H.K.; Ramesh, R.; Roy, S.; Dinesh, K.; Babu, A. Molecular delineation and genetic diversity of Fusarium species complex causing tea dieback in India and their sensitivity to fungicides. Crop. Prot. 2024, 181, e106707. [Google Scholar] [CrossRef]
- Xia, J.W.; Sandoval-Denis, M.; Crous, P.W.; Zhang, X.G.; Lombard, L. Numbers to names—Restyling the Fusarium incarnatum-equiseti species complex. Persoonia 2019, 43, 186–221. [Google Scholar] [CrossRef] [PubMed]
- Prihastuti, H.; Cai, L.; Chen, H.; McKenzie, E.H.C.; Hyde, K.D. Characterization of Colletotrichum species associated with coffee berries in northern Thailand. Fungal Divers. 2009, 39, 89–109. [Google Scholar]
- Li, M.; Feng, W.; Yang, J.; Gao, Z.; Zhang, Z.; Zhang, W.; Wang, S.; Wang, W.; Gong, D.; Hu, M. First report of anthracnose caused by Colletotrichum siamense on avocado fruits in China. Crop Prot. 2022, 155, e105922. [Google Scholar] [CrossRef]
- Damm, U.; Woudenberg, J.H.C.; Cannon, P.F.; Crous, P.W. Colletotrichum species with curved conidia from herbaceous hosts. Fungal Divers. 2009, 39, 45–87. [Google Scholar]
- Maryani, N.; Sandoval-Denis, M.; Lombard, L.; Crous, P.W.; Kema, G.H.J. New endemic Fusarium species hitch-hiking with pathogenic Fusarium strains causing Panama disease in small-holder banana plots in Indonesia. Persoonia 2019, 43, 48–69. [Google Scholar] [CrossRef] [PubMed]
- Yi, R.H.; Lian, T.; Su, J.J.; Chen, J. First report of internal black rot on Carica papaya fruit caused by Fusarium sulawesiense in China. Plant Dis. 2022, 106, e319. [Google Scholar] [CrossRef]
- Shih, P.T.; Chang, T.D.; Liu, H.H.; Chang, H.X.; Lin, Y.H. Macrophomina phaseolina causing charcoal rot on soybean (Glycine max) in Taiwan. Australas. Plant Dis. Notes 2022, 17, e11. [Google Scholar] [CrossRef]
- Avinash, T.S.; Pillai, H.P.J.S.; Biradar, M.; Shinde, V.M. A review on fungal diseases of Cucurbitaceae and their management. Int. J. Curr. Microbiol. App. Sci. 2021, 10, 653–672. [Google Scholar]
- Nama, C.P.; Lal, J.; Ranawat, J.S.; Meena, R.K. Diseases of cucurbits and their management. Marumegh 2016, 1, 8–16. [Google Scholar]
- Talhinhas, P.; Baroncelli, R. Hosts of Colletotrichum. Mycosphere 2023, 14, 158–261. [Google Scholar] [CrossRef]
- Zakaria, L. Diversity of Colletotrichum species associated with anthracnose disease in tropical fruit crops—A review. Agriculture 2021, 11, 297. [Google Scholar] [CrossRef]
- Hindorf, H. Colletotrichum spp. causing anthracnose of tropical crops. Acta Hortic. 2020, 531, 275–282. [Google Scholar] [CrossRef]
- Talhinhas, P.; Baroncelli, R. Colletotrichum species and complexes: Geographic distribution, host range and conservation status. Fungal Divers. 2021, 110, 109–198. [Google Scholar] [CrossRef]
- Meng, S.; Chao, S.; Xiong, M.; Cheng, L.; Sun, Y.; Wang, L.; Chen, Y.; Jane, S.J.; Luo, C.; Chen, J. CaSun1, a SUN family protein, governs the pathogenicity of Colletotrichum camelliae by recruiting CaAtg8 to promote mitophagy. Hortic. Res. 2025, 12, uhaf121. [Google Scholar] [CrossRef] [PubMed]
- Nuangmek, W.; Suwannarach, N.; Sukyai, S.; Khitka, B.; Kumla, J. Fungal pathogens causing postharvest anthracnose and fruit rot in Indian jujube (Ziziphus mauritiana) from northern Thailand and their fungicide response profiles. Front. Plant Sci. 2025, 16, e1634557. [Google Scholar] [CrossRef]
- Li, Y.G.; Zhang, R.; Meng, L.; Ali, E.; Ji, P.; Zhang, Q.F.; Cui, G.W. Occurrence of fruit rot of cantaloupe caused by Fusarium equiseti in China. Plant Dis. 2019, 103, e2683. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, H.J. Fusarium fruit rot of posthavest oriental melon (Cucumis melo L. var. makuwa Mak.) caused by Fusarium spp. Res. Plant Dis. 2004, 10, 260–267. [Google Scholar] [CrossRef]
- de Almeida Nogueira, G.; Costa Conrado, V.S.; Luiz de Almeida Freires, A.; Ferreira de Souza, J.J.; Figueiredo, F.R.; Barroso, K.A.; Araujo, M.B.M.; Nascimento, L.V.; de Lima, J.S.S.; Neto, F.B.; et al. Aggressivity of different Fusarium Species causing fruit rot in melons in Brazil. Plant Dis. 2023, 107, 886–892. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, M.D.; Junior, R.D.L.; da Silva, F.F.E.; Inokuti, E.M.; Oster, A.H.; Zampieri, D.; Lima, C.S.; Fill, T.P.; de Lemos, T.L.G. Unraveling the antifungal composition of bitter orange decoction against the melon pathogen Fusarium jinanense. Food Chem. 2024, 455, e139769. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.B.; Moreira, G.M.; Nascimento, L.V.; Nogueira, G.A.; Nascimento, S.R.C.; Pfenning, L.H.; Ambrosio, P.M.M.Q. Fusarium rot of melon is caused by several Fusarium species. Plant Pathol. 2021, 70, 712–721. [Google Scholar] [CrossRef]
- Wang, M.M.; Chen, Q.; Diao, Y.Z.; Duan, W.J.; Cai, L. Fusarium incarnatum-equiseti complex from China. Persoonia 2019, 43, 70–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.P.; Cao, X.D.; Dang, Q.Q.; Liu, Y.G.; Zhu, X.P.; Xia, J.W. First report of fruit rot caused by Fusarium luffae in muskmelon in China. Plant Dis. 2022, 106, e1763. [Google Scholar] [CrossRef]
- Zhang, X.P.; Xia, J.W.; Liu, J.K.; Zhao, D.; Kong, L.G.; Zhu, X.P. First report of Fusarium pernambucanum causing fruit rot of muskmelon in China. Plant Dis. 2022, 106, e1997. [Google Scholar] [CrossRef]
- Zhang, X.P.; Dang, Q.Q.; Cao, X.D.; Liu, Y.G.; Bi, Z.B.; Zhu, X.P.; Xia, J.W. First report of muskmelon fruit rot caused by Fusarium nanum in China. Plant Dis. 2023, 107, e226. [Google Scholar] [CrossRef]
- Liu, Y.G.; Zhang, X.P.; Liu, S.M.; Zhu, X.P.; Xia, J.W. First report of muskmelon fruit rot caused by Fusarium sulawesiense in China. Plant Dis. 2023, 107, e3313. [Google Scholar] [CrossRef]
- Hao, F.; Zang, Q.; Ding, W.; Ma, E.; Huang, Y.; Wang, Y. First report of fruit rot of melon caused by Fusarium asiaticum in China. Plant Dis. 2021, 105, e1225. [Google Scholar] [CrossRef]
- Champaco, E.R.; Martyn, R.D. Comparison of Fusarium solani and F. oxysporum as causal agents of fruit rot and root rot of muskmelon. Hortic. Sci. 1993, 28, 1174–1177. [Google Scholar] [CrossRef]
- Parra, M.Á.; Gómez, J.; Aguilar, F.W.; Martínez, J.A. Fusarium annulatum causes Fusarium rot of cantaloupe melons in Spain. Phytopathol. Mediterr. 2022, 61, 269–277. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Shi, C.; Shi, Z.; Luan, F. First report of fruit rot disease on melon (Cucumis melo) caused by Fusarium ipomoeae in China. Plant Dis. 2024, 108, e2923. [Google Scholar] [CrossRef]
- Cohen, R.; Elkabetz, M.; Edelstein, M. Variation in the responses of melon and watermelon to Macrophomina phaseolina. Crop Prot. 2016, 85, 46–51. [Google Scholar] [CrossRef]
- Wu, H.; Li, C.; Chakraborti, P.; Guo, Z.; Peng, B.; Gu, W.; Kang, B.; Gu, Q. First report of watermelon charcoal rot (Macrophomina phaseolina) in China. Plant Dis. 2022, 160, e1521. [Google Scholar] [CrossRef] [PubMed]
- Egel, D.S.; Adkins, S.T.; Wintermantel, W.M.; Keinath, A.P.; D’Arcangelo, K.; Parada-Rojas, C.H.; Rennberger, G.; Toporek, S.M.; Hausbeck, M.K.; Quesada-Ocampo, L.M. Diseases of cucumbers, melons, pumpkins, squash, and watermelons. In Handbook of Vegetable and Herb Diseases; Elmer, W.H., McGrath, M., McGovern, R.J., Eds.; Springer Nature: Cham, Switzerland, 2022; pp. 1–105. [Google Scholar]
- Li, P.; Dai, X.; Wang, S.; Luo, Q.; Tang, Q.; Xu, Z.; Zhao, W.; Wu, F. Biological characteristics and fungicide screening of Colletotrichum fructicola causing mulberry anthracnose. Microorganisms 2024, 12, 2386. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.M.; Usman, H.M.; Tan, Q.; Hu, J.J.; Fan, F.; Hussain, R.; Luo, C.X. Fungicide resistance in Colletotrichum fructicola and Colletotrichum siamense causing peach anthracnose in China. Pestic. Biochem. Physiol. 2024, 203, e106006. [Google Scholar] [CrossRef] [PubMed]
- Kongtragoul, P.; Imamota, K.; Ishii, H. Resistance to quinone-outside inhibitor (qoi) fungicides in Colletotrichum species isolated from anthracnose disease occurring in Thailand. Curr. Appl. Sci. Technol. 2020, 20, 79–89. [Google Scholar]
- Apithanasakulngeon, P.; Suwannarat, S.; Tongsri, V. Fungicide resistance in Colletotrichum species causing durian anthracnose in eastern Thailand. Agr. Nat. Resour. 2025, 59, 1–10. [Google Scholar]
- Usman, H.M.; Tan, Q.; Karim, M.M.; Adnan, M.; Yin, W.X.; Zhu, F.X.; Luo, C.X. Sensitivity of Colletotrichum fructicola and Colletotrichum siamense of peach in China to multiple classes of fungicides and characterization of pyraclostrobin-resistant isolates. Plant Dis. 2021, 105, 3459–3465. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, J.; Chen, Q.; Liu, Z.; Sun, J.; Yan, Y.; Zhang, H.; Bi, Y. identification, pathogenicity, and sensitivity to fungicide of Colletotrichum species that causes walnut anthracnose in Beijing. Agronomy 2023, 13, 214. [Google Scholar] [CrossRef]
- Maniçoba, F.E.; Negreiros, A.M.P.; Cavalcante, A.L.A.; Santos Alves, C.P.d.S.; Nascimento, M.T.d.A.e.; Ambrósio, M.M.d.Q.; Sales Júnior, R. Effect of environmental factors, fungicide sensitivity, andpathogenicity of Fusarium spp. associated with fruit rot of melon. J. Phytopathol. 2023, 171, 504–516. [Google Scholar] [CrossRef]
- Cohen, R.; Omari, N.; Porat, A.; Edelstein, M. Management of Macrophomina phaseolina in melons using grafting or fungicide soil application: Pathological, horticultural and economical aspects. Crop Prot. 2012, 35, 58–63. [Google Scholar] [CrossRef]
- Tonin, R.F.B.; Avozani, A.; Durante Danelli, A.L.; Reis, E.M.; Zoldan, S.M.; Garcés-Fiallos, F.R. In vitro mycelial sensitivity of Macrophomina phaseolina to fungicides. Pesqui. Agropecu. Trop. 2013, 43, 460–466. [Google Scholar] [CrossRef]
- Chamorro, M.; Miranda, L.; Domínguez, P.; Medina, J.J.; Soria, C.; Romero, F.; López Aranda, J.M.; De los Santos, B. Evaluation of biosolarization for the control of charcoal rot disease (Macrophomina phaseolina) in strawberry. Crop Prot. 2015, 67, 279–286. [Google Scholar] [CrossRef]
- Parmar, H.V.; Kapadiya, H.J.; Bhaliya, C.M. Efficacy of different fungicides against Macrophomina phaseolina (Tassi) Goid causing castor root rot. Int. J. Chem. Stud. 2017, 5, 1807–1809. [Google Scholar]
- Lokesh, R.; Rakholiya, K.B.; Thesiya, M.R. Evaluation of different fungicides against Macrophomina phaseolina (Tassi) goid. causing dry root rot of chickpea (Cicer arietinum L.) in vitro. Artic. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 901–911. [Google Scholar] [CrossRef]
- Iqbal, U.; Mukhtar, T. Inhibitory effects of some fungicides against Macrophomina phaseolina causing charcoal rot. Pak. J. Zool. 2020, 52, 709–715. [Google Scholar] [CrossRef]
- FRAC. Fungal Control Agents Sorted by Cross Resistance Pattern and Mode of Action. Available online: https://www.frac.info (accessed on 10 May 2025).
- Yin, Y.; Miao, J.; Shao, W.; Liu, X.; Zhao, Y.; Ma, Z. Fungicide Resistance: Progress in understanding mechanism, monitoring, and management. Phytopathology 2023, 113, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Corkley, I.; Fraaije, B.; Hawkins, N. Fungicide resistance management: Maximizing the effective life of plant protection products. Plant Pathol. 2021, 71, 150–169. [Google Scholar] [CrossRef]
- Davies, C.R.; Wohlgemuth, F.; Young, T.; Violet, J.; Dickinson, M.; Sanders, J.-W.; Vallieres, C.; Avery, S.V. Evolving challenges and strategies for fungal control in the food supply chain. Fungal. Biol. Rev. 2021, 36, 15–26. [Google Scholar] [CrossRef] [PubMed]





| Target Gene | Primer Name | Direction | Primer Sequence |
|---|---|---|---|
| ITS | ITS5 | Forward | 5′-GGAAGTAAAAGTCGTAACAAGG-3′ |
| ITS4 | Reverse | 5′-TCCTCCGCTTATTGATATGC-3′ | |
| act | ACT512F | Forward | 5′-ATG TGCAAGGCCGGTTTCGC-3′ |
| ACT738R | Reverse | 5′-TACGAGTCCTTCTGGCCCAT-3′ | |
| tub | T1 | Forward | 5′-AACATGCGTGAGATTGTAAGT-3′ |
| T2 | Reverse | 5′-TAGTGACCCTTGGCCCAGTTG-3′ | |
| T22 | Reverse | 5′-TCTGGATGTTGTTGGGAATCC-3′ | |
| cal | CL1C | Forward | 5′-GAATTCAAGGAGGCCTTCTC-3′ |
| CL2C | Reverse | 5′-CTTCTGCATCATGAGCTGGAC-3′ | |
| CAL-228F | Forward | 5′-GAGTTCAAGGAGGCCTTCTCCC-3′ | |
| CAL-737R | Reverse | 5′-CATCTTTCTGGCCATCATGG-3′ | |
| gapdh | GDF1 | Forward | 5′-GCCGTCAACGACCCCTTCATTGA-3′ |
| GPDHR2 | Reverse | 5′-CTCRGMRGCRGCCTTGATGG-3′ | |
| rpb2 | RPB2-5F2 | Forward | 5′-GGGGWGAYCAGAAGAAGGC-3′ |
| RPB2-7cR | Reverse | 5′-CCCATRGCTTGYTTRCCCAT-3′ | |
| tef1-α | EF1 | Forward | 5′-ATGGGTAAGGARGACAAGAC-3′ |
| EF2 | Reverse | 5′-GGARGTACCAGTSATCATG-3′ | |
| EF1-728F | Forward | 5′-CATCGAGAAGTTCGAGAAGG-3′ | |
| EF1-986R | Reverse | 5′-TACTTGAAGGAACCCTTACC-3′ |
| Fungal Strain | Gene | GenBank Accession Number | The Closely Related Ex-Type Strain/ Similarity Value (%) |
|---|---|---|---|
| SDBR-CMU540 | ITS | PV789656 | Colletotrichum siamense ICMP 18578/99.82 |
| gapdh | PV796144 | Colletotrichum siamense ICMP 18578/99.55 | |
| cal | PV796147 | Colletotrichum siamense ICMP 18578/99.56 | |
| act | PV796150 | Colletotrichum siamense ICMP 18578/100.00 | |
| tub | PV796152 | Colletotrichum siamense ICMP 18578/99.57 | |
| SDBR-CMU541 | ITS | PV789657 | Colletotrichum siamense ICMP 18578/99.82 |
| gapdh | PV796145 | Colletotrichum siamense ICMP 18578/99.55 | |
| cal | PV796148 | Colletotrichum siamense ICMP 18578/99.56 | |
| act | PV796151 | Colletotrichum siamense ICMP 18578/100.00 | |
| tub | PV796153 | Colletotrichum siamense ICMP 18578/99.54 | |
| SDBR-CMU542 | ITS | PV789658 | Colletotrichum chlorophyti IMI 103806/99.81 |
| gapdh | PV796146 | Colletotrichum chlorophyti IMI 103806/100 | |
| act | PV796149 | Colletotrichum chlorophyti IMI 103806/99.01 | |
| tub | PV796154 | Colletotrichum chlorophyti IMI 103806/99.60 | |
| SDBR-CMU543 | ITS | PV789659 | Macrophomina phaseolina CBS 205.47/100.00 |
| act | PV796155 | Macrophomina phaseolina CBS 205.47/99.62 | |
| cal | PV796158 | Macrophomina phaseolina CBS 205.47/99.64 | |
| tef1-α | PV796161 | Macrophomina phaseolina CBS 205.47/98.57 | |
| SDBR-CMU544 | ITS | PV789660 | Macrophomina phaseolina CBS 205.47/100.00 |
| act | PV796156 | Macrophomina phaseolina CBS 205.47/99.62 | |
| cal | PV796159 | Macrophomina phaseolina CBS 205.47/99.64 | |
| tef1-α | PV796162 | Macrophomina phaseolina CBS 205.47/98.58 | |
| SDBR-CMU545 | ITS | PV789661 | Macrophomina phaseolina CBS 205.47/100.00 |
| act | PV796157 | Macrophomina phaseolina CBS 205.47/99.62 | |
| cal | PV796160 | Macrophomina phaseolina CBS 205.47/99.64 | |
| tef1-α | PV796163 | Macrophomina phaseolina CBS 205.47/98.58 | |
| SDBR-CMU546 | tef1-α | PV796164 | Fusarium mianyangense LC15879/99.10 |
| cal | PV796166 | Fusarium sulawesiense InaCC F940/100.00 | |
| rpb2 | PV796168 | Fusarium sulawesiense InaCC F940/100.00 | |
| SDBR-CMU547 | tef1-α | PV796165 | Fusarium mianyangense LC15879/99.10 |
| cal | PV796167 | Fusarium sulawesiense InaCC F940/100.00 | |
| rpb2 | PV796169 | Fusarium sulawesiense InaCC F940/100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suwannarach, N.; Wongsa, K.; Senwanna, C.; Nuangmek, W.; Kumla, J. Identification, Characterization, Pathogenicity, and Fungicide Sensitivity of Postharvest Fungal Diseases in Culinary Melon from Northern Thailand. J. Fungi 2025, 11, 540. https://doi.org/10.3390/jof11070540
Suwannarach N, Wongsa K, Senwanna C, Nuangmek W, Kumla J. Identification, Characterization, Pathogenicity, and Fungicide Sensitivity of Postharvest Fungal Diseases in Culinary Melon from Northern Thailand. Journal of Fungi. 2025; 11(7):540. https://doi.org/10.3390/jof11070540
Chicago/Turabian StyleSuwannarach, Nakarin, Karnthida Wongsa, Chanokned Senwanna, Wipornpan Nuangmek, and Jaturong Kumla. 2025. "Identification, Characterization, Pathogenicity, and Fungicide Sensitivity of Postharvest Fungal Diseases in Culinary Melon from Northern Thailand" Journal of Fungi 11, no. 7: 540. https://doi.org/10.3390/jof11070540
APA StyleSuwannarach, N., Wongsa, K., Senwanna, C., Nuangmek, W., & Kumla, J. (2025). Identification, Characterization, Pathogenicity, and Fungicide Sensitivity of Postharvest Fungal Diseases in Culinary Melon from Northern Thailand. Journal of Fungi, 11(7), 540. https://doi.org/10.3390/jof11070540







