Mechanism Underlying Ganoderma lucidum Polysaccharide Biosynthesis Regulation by the β-1,3-Glucosyltransferase Gene gl20535
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain and Culture Medium
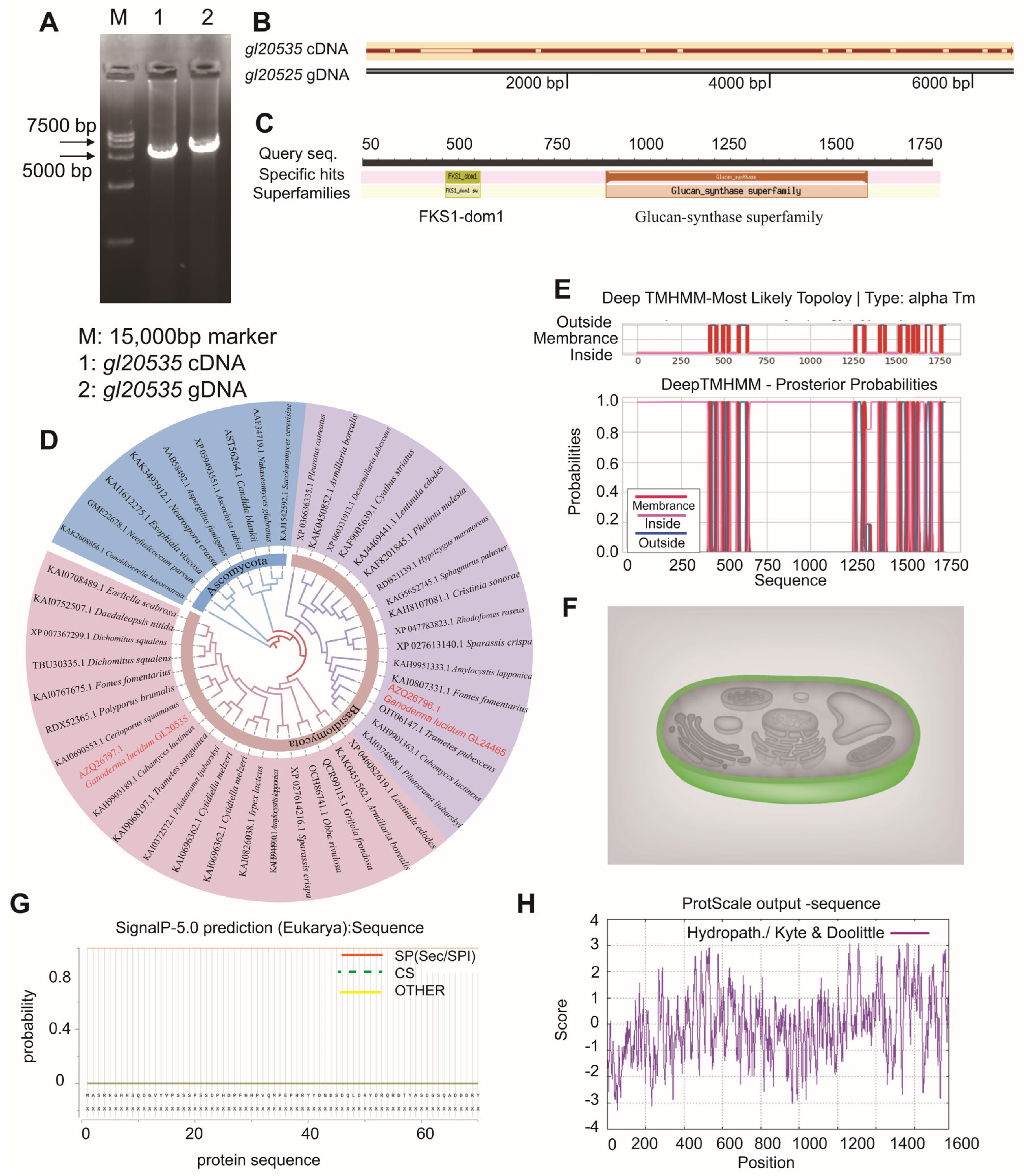
2.2. Cloning and Bioinformatic Analysis of gl20535
2.3. Construction of the Overexpression and Silenced Plasmids
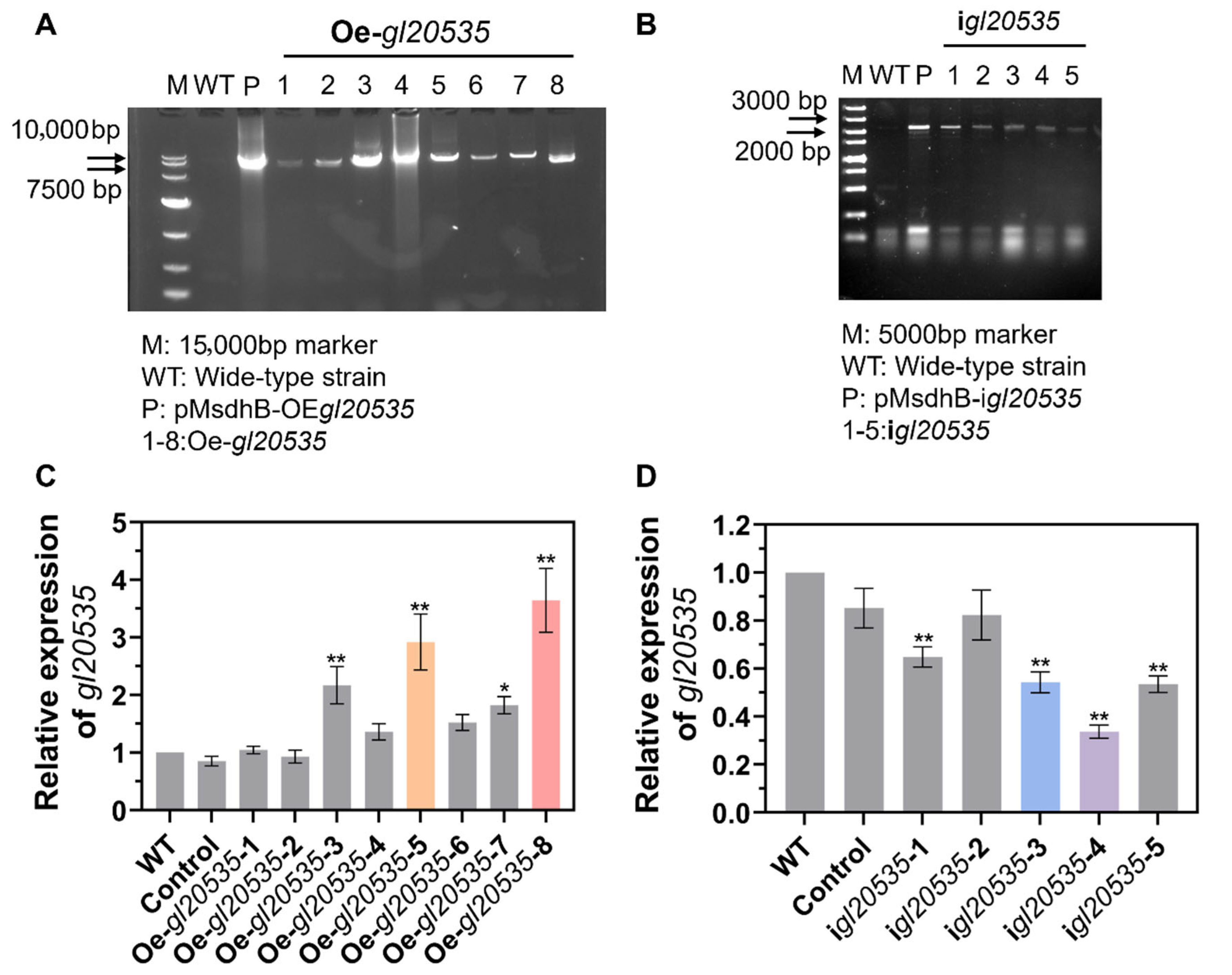
2.4. Construction and Identification of Overexpression and Silenced Strains
2.5. Real-Time Quantitative PCR (RT-qPCR) Analysis of Gene Expression
2.6. Characterization of Mycelial Morphology
2.7. Measurements of Biomass, Residual Sugars, EPS, and IPS
2.8. Comparison of Monosaccharides
2.9. Quantification of β-1,3-glucan and Chitin in the Cell Wall
2.10. Statistical Analysis
3. Results
3.1. Silencing and Overexpression of gl20535
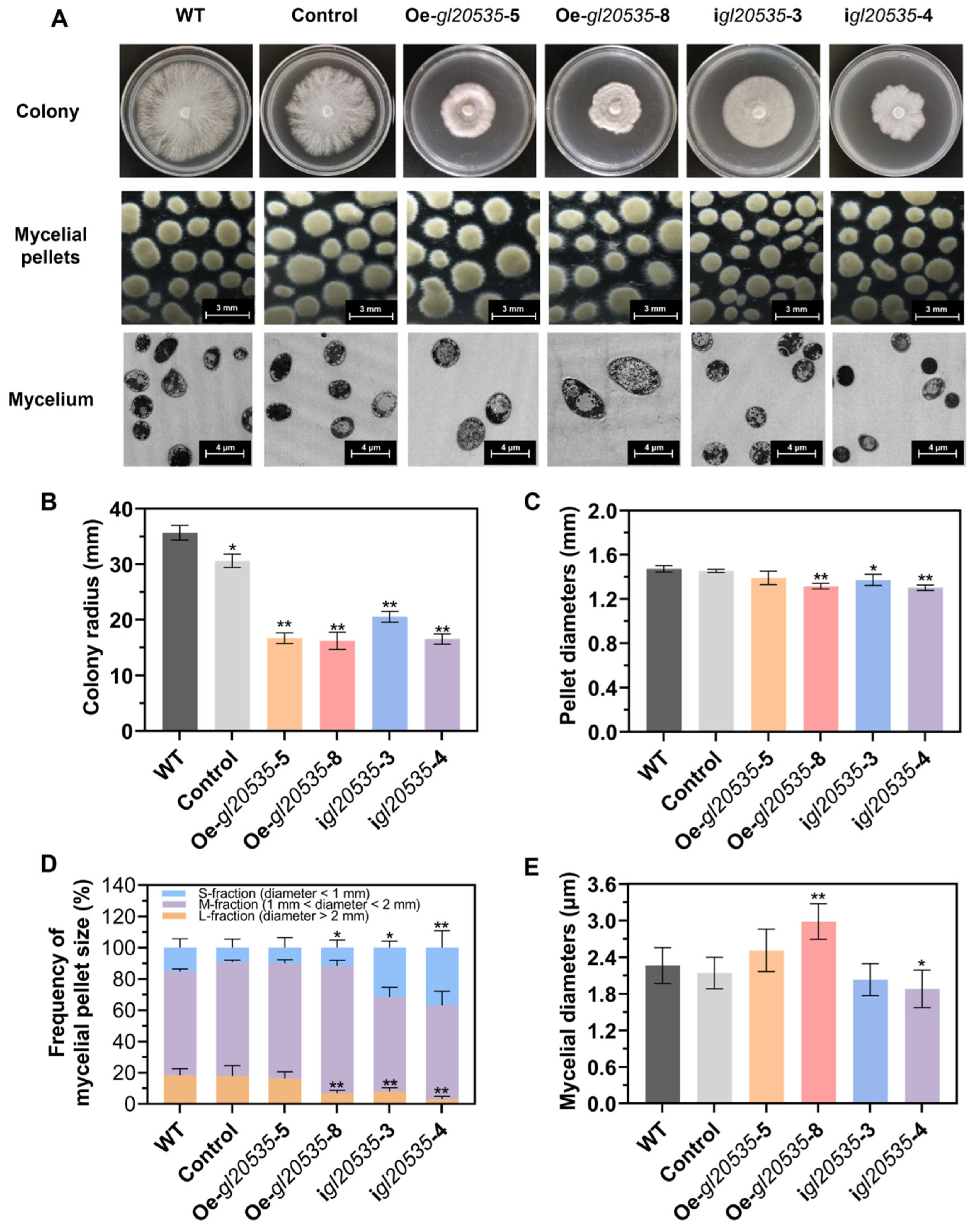
3.2. Effect of GL20535 on Mycelial Morphology and Growth Characteristics
3.2.1. Morphological Characteristics
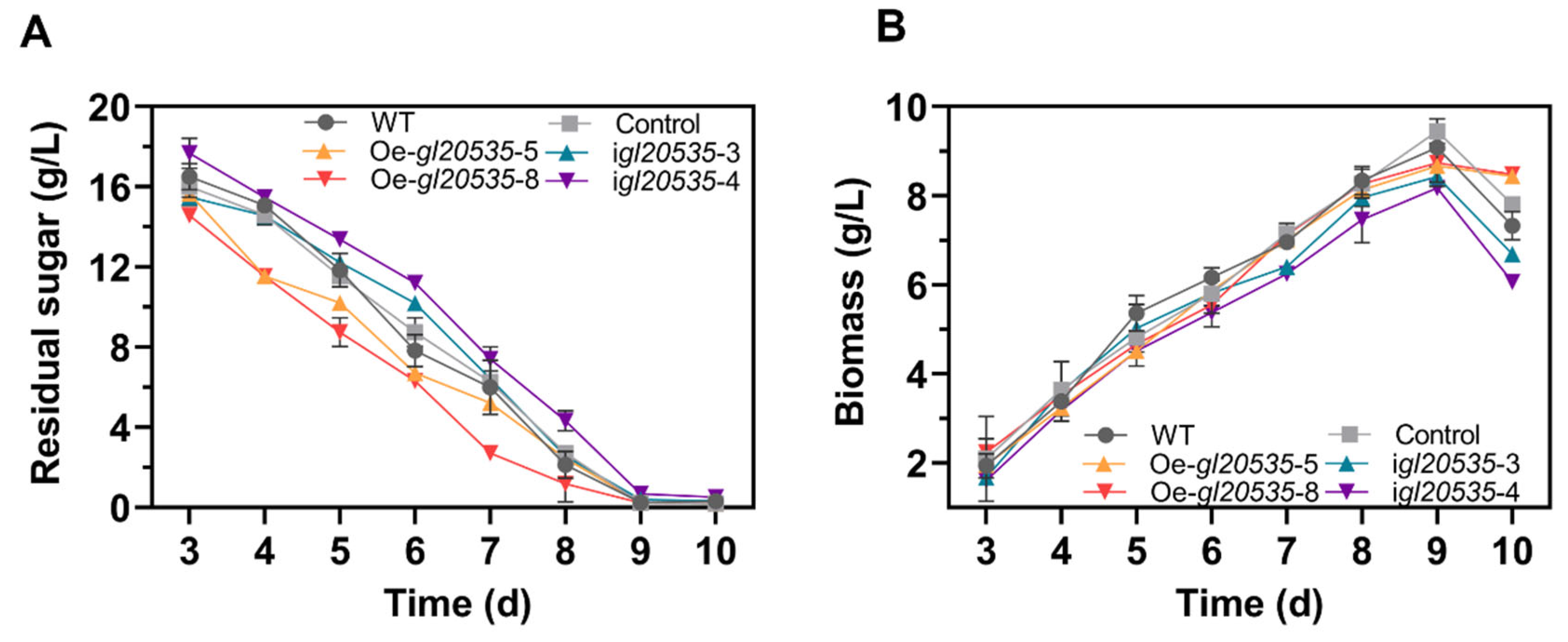
3.2.2. Growth Curve
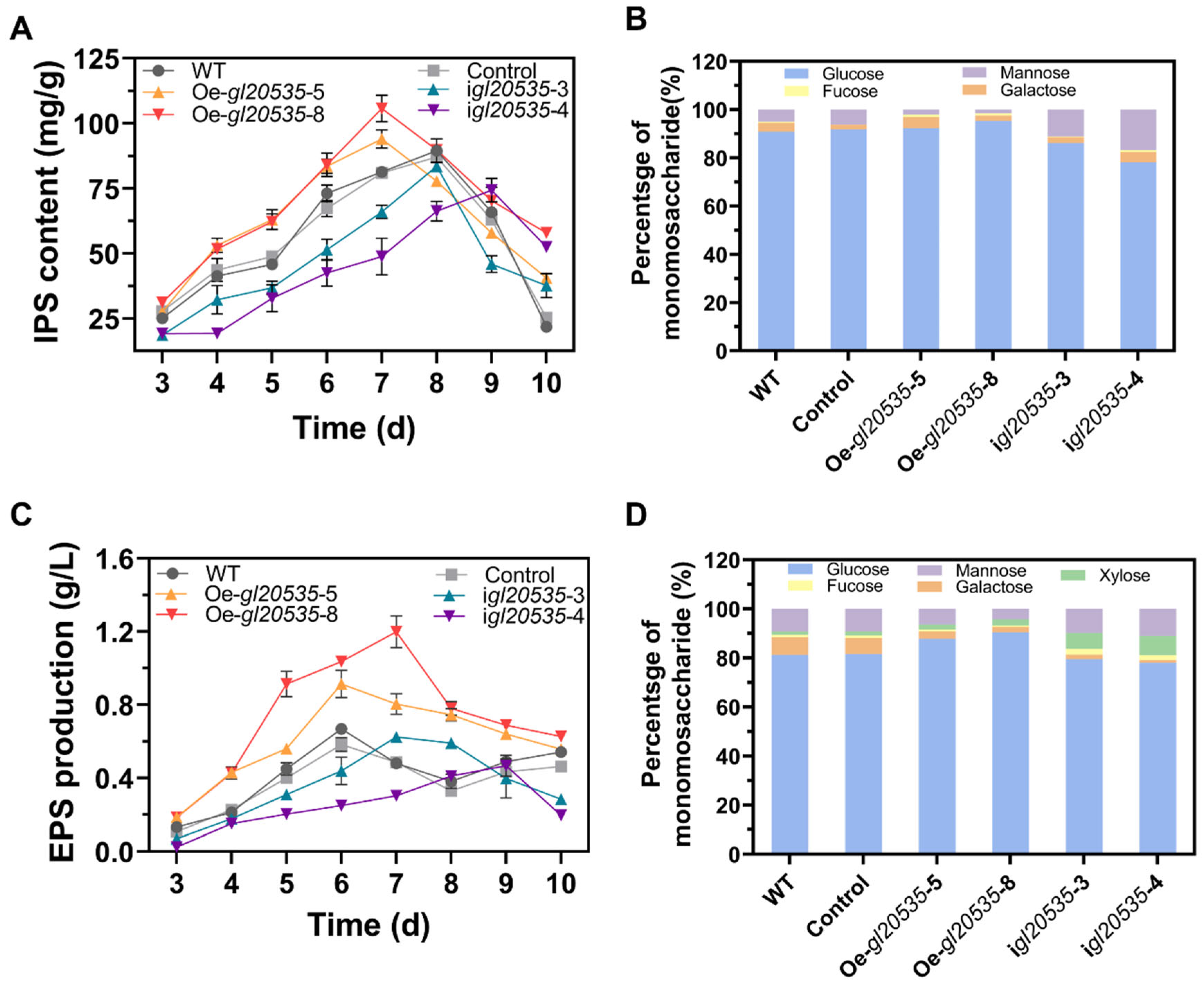
3.3. Effect of GL20535 on Polysaccharide Production and Monosaccharide Composition
3.3.1. IPS
3.3.2. EPS
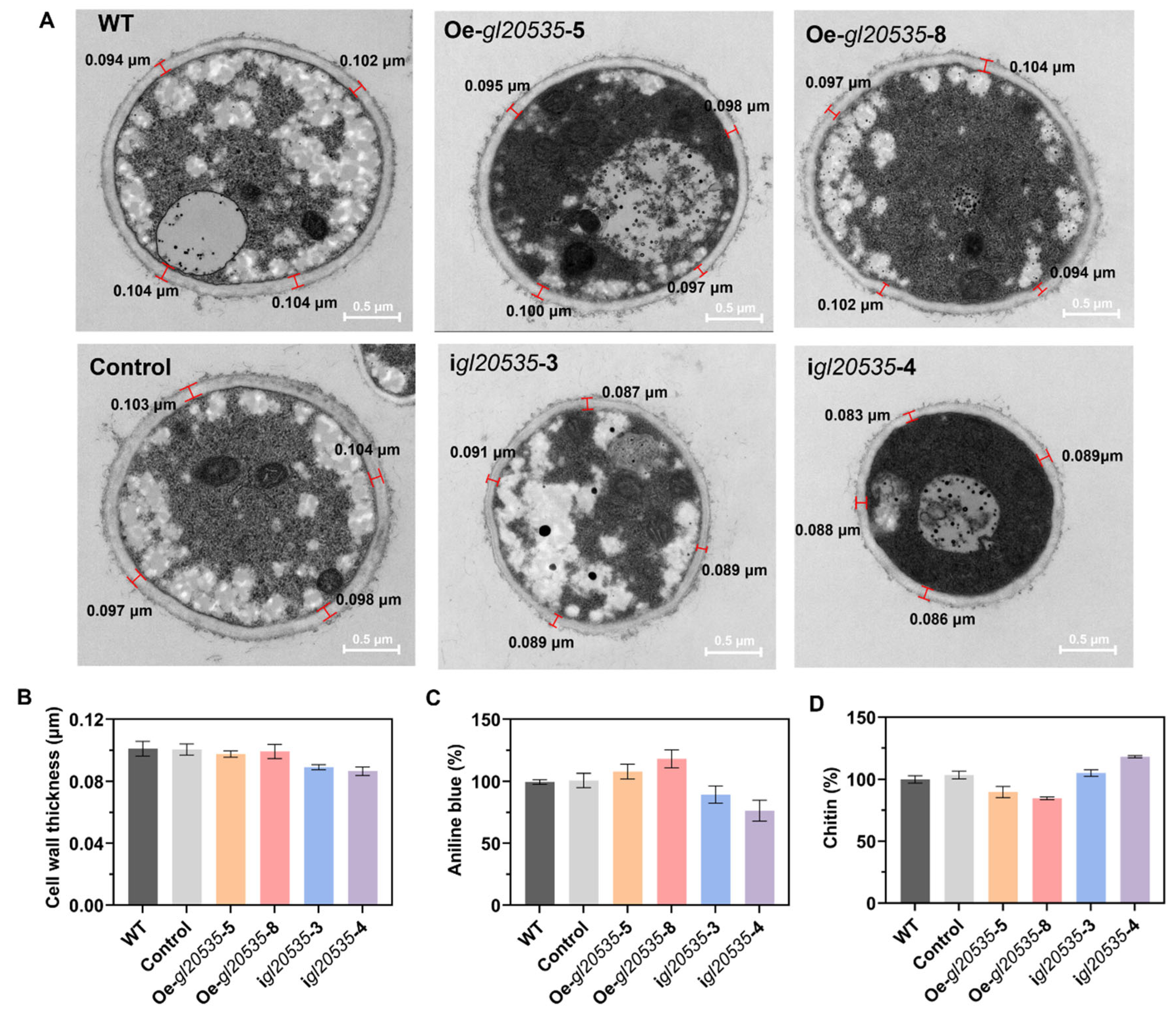
3.4. Effect of gl20535 on the Cell Wall
3.5. Transcript-Level Analysis of Genes in the Polysaccharide Biosynthesis Pathway
3.5.1. β-1,3-glucosyltransferase Isozyme Gene gl24465
3.5.2. Key Enzymes in Nucleotide Sugar Donor Biosynthesis
3.5.3. Glycoside Hydrolases
3.5.4. Chitin Synthases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sharif, M.; Bondzie-Quaye, P.; Wang, H.; Shao, C.; Hua, P.; Alrasheed, M.; Benjami, J.; Sossah, F.L.; Huang, Q. Potentialities of Ganoderma lucidum extracts as functional ingredients in food formulation. Food Res. Int. 2023, 172, 113161. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, S.; Peng, B.; Tan, D.; Wu, M.; Wei, J.; Wang, Y.; Luo, H. Ganoderma lucidum: A comprehensive review of phytochemistry, efficacy, safety and clinical study. Food Sci. Hum. Wellness 2024, 13, 568–596. [Google Scholar] [CrossRef]
- Swallah, M.S.; Bondzie-Quaye, P.; Wu, Y.; Acheampong, A.; Sossah, F.L.; Elsherbiny, S.M.M.; Huang, Q. Therapeutic potential and nutritional significance of Ganoderma lucidum—A comprehensive review from 2010 to 2022. Food Funct. 2023, 14, 1812–1838. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Pi, X.; Li, H.; Cheng, S.; Su, Y.; Zhang, Y.; Man, C.; Jiang, Y. Ganoderma spp. polysaccharides are potential prebiotics: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 909–927. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.L.; Shi, L. Methods of study on conformation of polysaccharides from natural products: A review. Int. J. Biol. Macromol. 2024, 263, 130275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cui, F.; Sun, L.; Zan, X.; Sun, W. Recent advances in Ganoderma lucidum polysaccharides: Structures/bioactivities, biosynthesis and regulation. Food Biosci. 2023, 56, 103281. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, M.; Zhang, J.; Ma, Z.; Cui, J.; Zhao, L.; Chen, L.; Shi, G.; Ding, Z. Refined regulation of polysaccharide biosynthesis in edible and medicinal fungi: From pathways to production. Carbohydr. Polym. 2025, 358, 123560. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Ye, C.; Deng, W.; Xu, M.; Wang, Q.; Liu, G.; Wang, F.; Liu, L.; Xu, Z.; Shi, G.; et al. Reconstruction and analysis of a genome-scale metabolic model of Ganoderma lucidum for improved extracellular polysaccharide production. Front. Microbiol. 2018, 9, 3076. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, T.; Gao, T.; Miao, Z.; Jiang, A.; Shi, L.; Ren, A.; Zhao, M. UDP-glucose pyrophosphorylase influences polysaccharide synthesis, cell wall components, and hyphal branching in Ganoderma lucidum via regulation of the balance between glucose-1-phosphate and UDP-glucose. Fungal Genet. Biol. 2015, 82, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, M.; Wang, S.; Yue, S.; Shi, L.; Ren, A.; Zhao, M. Ganoderma lucidum phosphoglucomutase is required for hyphal growth, polysaccharide production, and cell wall integrity. Appl. Microbiol. Biotechnol. 2018, 102, 1911–1922. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cao, Y.; Luo, Q.; Xu, Y.; Li, N.; Wang, C.; Xu, J. Overexpression of phosphomannomutase increases the production and bioactivities of Ganoderma exopolysaccharides. Carbohydr. Polym. 2022, 294, 119828. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Xu, M.; Liu, J.; Zhao, L.; Li, J.; Chen, L.; Shi, G.; Ding, Z. Carbon starvation drives biosynthesis of high mannose-composition polysaccharides in Ganoderma lucidum. Int. J. Biol. Macromol. 2024, 282, 137168. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Lei, J.; Luo, X.; Wang, S.; Tan, H.; Zhang, J.; Wu, D. Prospects of Ganoderma polysaccharides: Structural features, structure-function relationships, and quality evaluation. Int. J. Biol. Macromol. 2025, 309, 142836. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, X.; Gong, P.; Long, H.; Wang, J.; Yang, W.; Yao, W. Siraitia grosvenorii as a homologue of food and medicine: A review of biological activity, mechanisms of action, synthetic biology, and applications in future food. J. Agric. Food Chem. 2024, 72, 6850–6870. [Google Scholar] [CrossRef] [PubMed]
- Zabotina, O.A.; Zang, N.; Weerts, R. Polysaccharide biosynthesis: Glycosyltransferases and their complexes. Front. Plant Sci. 2021, 12, 625307. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, L.; You, C.; Liu, J.; Chen, L.; Gu, Z.; Shi, G.; Li, J.; Ding, Z. Roles of α-1,3-glucosyltransferase in growth and polysaccharides biosynthesis of Ganoderma lucidum. Int. J. Biol. Macromol. 2024, 276, 134031. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Wu, X.; Tao, T.; Zan, X.; Sun, W.; Mu, D.; Yang, Y.; Wu, D. Functions of a glucan synthase gene GFGLS in mycelial growth and polysaccharide production of Grifola frondosa. J. Agric. Food Chem. 2019, 67, 8875–8883. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, B.-X.; Zhang, J.; Han, N.; Liu, S.-T.; Geng, W.; Jia, S.; Li, Y.; Gan, Q.; Han, P. A newly discovered glycosyltransferase gene UGT88A1 affects growth and polysaccharide synthesis of Grifola frondosa. Appl. Microbiol. Biotechnol. 2024, 108, 12062. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, X.; Zan, X.; Fu, X.; Cui, F.; Zhu, H.; Sun, W.; Tao, T. The β-1,3-glucan synthase gene GFGLS2 plays major roles in mycelial growth and polysaccharide synthesis in Grifola frondosa. Appl. Microbiol. Biotechnol. 2022, 106, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, M.; Zhao, L.; Chen, L.; Ding, Z. Novel insights into the mechanism underlying high polysaccharide yield in submerged culture of Ganoderma lucidum revealed by transcriptome and proteome analyses. Microorganisms 2023, 11, 772. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, Y.; Wang, Z.; Xu, J. Improved Production and Rheological Properties of Exopolysaccharides by Co-Overexpression of β-1,3-glucan synthase and UDP-glucose pyrophosphorylase genes in Ganoderma lingzhi. J. Agric. Food Chem. 2025, 73, 8567–8577. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yuan, H.; Li, N.; Xiao, J.; Xu, J. Increased production and anti-senescence activity of exopolysaccharides in Ganoderma lingzhi by co-overexpression of β-1,3-glucan synthase and UDP-glucose pyrophosphorylase. Int. J. Biol. Macromol. 2023, 253, 126778. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, J.; Liu, C.; Zhu, Y.; Nelson, D.R.; Zhou, S.; Li, C.; Wang, L.; Guo, X.; Sun, Y.; et al. Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nat. Commun. 2012, 3, 1923. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, S.K.; Parsad, B.D.; Sahni, S. Rapid Method for Genomic DNA Isolation of Mungbean [Vignar adiata (L.) Wilczek]. Curr. J. Appl. Sci. Technol. 2023, 42, 10–14. [Google Scholar] [CrossRef]
- Fu, X.; Zan, X.; Sun, L.; Tan, M.; Cui, F.; Liang, Y.; Meng, L.; Sun, W. Functional characterization and structural basis of the β-1,3-glucan synthase CMGLS from mushroom Cordyceps militaris. J. Agric. Food Chem. 2022, 70, 8725–8737. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Xu, M.; Wang, Q.; Wang, F.; Zheng, H.; Gu, Z.; Li, Y.; Shi, G.; Ding, Z. Development of an efficient strategy to improve extracellular polysaccharide production of Ganoderma lucidum using L-phenylalanine as an enhancer. Front. Microbiol. 2019, 10, 2306. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Ye, G.; Li, G.; Cao, H.; Wang, Z.; Ji, S. RID serve as a more appropriate measure than phenol sulfuric acid method for natural water-soluble polysaccharides quantification. Carbohydr. Polym. 2022, 278, 118928. [Google Scholar] [CrossRef] [PubMed]
- Deshavath, N.N.; Mukherjee, G.; Goud, V.V.; Veeranki, V.D.; Sastri, C.V. Pitfalls in the 3, 5-dinitrosalicylic acid (DNS) assay for the reducing sugars: Interference of furfural and 5-hydroxymethylfurfural. Int. J. Biol. Macromol. 2020, 156, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cheung, P.C.K. Mushroom dietary fiber from the fruiting body of Pleurotus tuberregium: Fractionation and structural elucidation of nondigestible cell wall components. J. Agric. Food Chem. 2014, 62, 2891–2899. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, J.; Wang, L.; Ding, J.; Zhao, M.; Liu, R. GlPP2C1 Silencing increases the content of Ganoderma lingzhi polysaccharide (GL-PS) and enhances Slt2 phosphorylation. J. Fungi 2022, 8, 949. [Google Scholar] [CrossRef] [PubMed]
- Popolo, L.; Gilardelli, D.; Bonfante, P.; Vai, M. Increase in chitin as an essential response to defects in assembly of cell wall polymers in the ggp1delta mutant of Saccharomyces cerevisiae. J. Bacteriol. 1997, 179, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Wagener, J.; Striegler, K.; Wagener, N. α- and β-1,3-glucan synthesis and remodeling. Curr. Top. Microbiol. Immunol. 2020, 425, 53–82. [Google Scholar]
- Hu, X.; Yang, P.; Chai, C.; Liu, J.; Sun, H.; Wu, Y.; Zhang, M.; Zhang, M.; Liu, X.; Yu, H. Structural and mechanistic insights into fungal ß-1,3-glucan synthase FKS1. Nature 2023, 616, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Suwunnakorn, S.; Wakabayashi, H.; Kordalewska, M.; Perlin David, S.; Rustchenko, E. FKS2 and FKS3 genes of opportunistic human pathogen Candida albicans influence echinocandin susceptibility. Antimicrob. Agents Chemother. 2018, 62, 2299. [Google Scholar] [CrossRef] [PubMed]
- Krull, R.; Wucherpfennig, T.; Esfandabadi, M.E.; Walisko, R.; Melzer, G.; Hempel, D.C.; Kampen, I.; Kwade, A.; Wittmann, C. Characterization and control of fungal morphology for improved production performance in biotechnology. J. Biotechnol. 2013, 163, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Wan-Mohtar, W.A.A.Q.I.; Ab Kadir, S.; Saari, N. The morphology of Ganoderma lucidum mycelium in a repeated-batch fermentation for exopolysaccharide production. Biotechnol. Rep. 2016, 11, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Ost, K.J.; Student, M.; Cord-Landwehr, S.; Moerschbacher, B.M.; Ram, A.F.J.; Dirks-Hofmeister, M.E. Cell walls of filamentous fungi–challenges and opportunities for biotechnology. Appl. Microbiol. Biotechnol. 2025, 109, 125. [Google Scholar] [CrossRef] [PubMed]
- Azi, F.; Wang, Z.; Chen, W.; Lin, D.; Xu, P. Developing Ganoderma lucidum as a next-generation cell factory for food and nutraceuticals. Trends Biotechnol. 2024, 42, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Wang, J.; Long, H.; Yang, W.; Chen, X.; Li, N.; Chen, F.; Zhang, J.; Guo, Y. Edible and medicinal fungi as candidate natural antidepressants: Mechanisms and nutritional implications. Mol. Nutr. Food Res. 2025, 69, 70080. [Google Scholar] [CrossRef] [PubMed]
- Chai, R.; Qiu, C.; Liu, D.; Qi, Y.; Gao, Y.; Shen, J.; Qiu, L. β-glucan synthase gene overexpression and β-glucans overproduction in Pleurotus ostreatus Using Promoter Swapping. PLoS ONE 2013, 8, 61693. [Google Scholar] [CrossRef] [PubMed]
- Garcia Cortes, J.C.; Ramos, M.; Osumi, M.; Perez, P.; Carlos Ribas, J. The cell biology of fission yeast septation. Microbiol. Mol. Biol. Rev. 2016, 80, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Morin, E.; Kohler, A.; Baker, A.R.; Foulongne-Oriol, M.; Lombard, V.; Nagy, L.G.; Ohm, R.A.; Patyshakuliyeva, A.; Brun, A.; Aerts, A.L.; et al. Genome sequence of the button mushroom Agaricus bisporus reveals mechanisms governing adaptation to a humic-rich ecological niche. Proc. Natl. Acad. Sci. USA 2012, 109, 17501–17506. [Google Scholar] [CrossRef] [PubMed]
- Zan, X.; Wu, X.; Cui, F.; Zhu, H.; Sun, W.; Jiang, L.; Tao, T.; Zhao, X. UDP-glucose pyrophosphorylase gene affects mycelia growth and polysaccharide synthesis of Grifola frondosa. Int. J. Biol. Macromol. 2020, 161, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, C.; Pan, J.; Zhou, Y.; Li, H.; Li, W.; Zou, Y. Augmentation of exopolysaccharide synthesis and its influence on biofunctional properties of polysaccharide in Sanghuangporus vaninii via targeted overexpression of phosphoglucomutase. Int. J. Biol. Macromol. 2025, 306, 141182. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, C.M.; Lunn, J.E.; Iglesias, A.A. Nucleotide-sugar metabolism in plants: The legacy of Luis F. Leloir. J. Exp. Bot. 2021, 72, 4053–4067. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Qiao, S.; Xu, Z.; Guan, F.; Ding, Z.; Gu, Z.; Zhang, L.; Shi, G. Effects of culture conditions on monosaccharide composition of Ganoderma lucidum exopolysaccharide and on activities of related enzymes. Carbohydr. Polym. 2015, 133, 104–109. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Xu, M.; Shen, M.; Li, J.; Chen, L.; Gu, Z.; Shi, G.; Ding, Z. Mechanism Underlying Ganoderma lucidum Polysaccharide Biosynthesis Regulation by the β-1,3-Glucosyltransferase Gene gl20535. J. Fungi 2025, 11, 532. https://doi.org/10.3390/jof11070532
Liu J, Xu M, Shen M, Li J, Chen L, Gu Z, Shi G, Ding Z. Mechanism Underlying Ganoderma lucidum Polysaccharide Biosynthesis Regulation by the β-1,3-Glucosyltransferase Gene gl20535. Journal of Fungi. 2025; 11(7):532. https://doi.org/10.3390/jof11070532
Chicago/Turabian StyleLiu, Jingyun, Mengmeng Xu, Mengye Shen, Junxun Li, Lei Chen, Zhenghua Gu, Guiyang Shi, and Zhongyang Ding. 2025. "Mechanism Underlying Ganoderma lucidum Polysaccharide Biosynthesis Regulation by the β-1,3-Glucosyltransferase Gene gl20535" Journal of Fungi 11, no. 7: 532. https://doi.org/10.3390/jof11070532
APA StyleLiu, J., Xu, M., Shen, M., Li, J., Chen, L., Gu, Z., Shi, G., & Ding, Z. (2025). Mechanism Underlying Ganoderma lucidum Polysaccharide Biosynthesis Regulation by the β-1,3-Glucosyltransferase Gene gl20535. Journal of Fungi, 11(7), 532. https://doi.org/10.3390/jof11070532





