Abstract
Ganoderma lucidum polysaccharides (GLPs) are natural compounds with a broad spectrum of biological activities. β-1,3-glucosyltransferase (GL20535) plays an important role in polysaccharide synthesis by catalyzing the transfer of UDP-glucose to extend sugar chains, but its underlying mechanism remains unclear. In this study, the regulatory mechanism of GL20535 in polysaccharide synthesis was elucidated by overexpressing and silencing gl20535 in G. lucidum. Overexpression of gl20535 resulted in maximum increases of 18.08%, 79.04%, and 18.01% in intracellular polysaccharide (IPS), extracellular polysaccharide (EPS), and β-1,3-glucan contents, respectively. In contrast, silencing gl20535 resulted in maximum reductions of 16.97%, 30.20%, and 23.56% in IPS, EPS, and β-1,3-glucan contents, respectively. These phenomena in the overexpression strains were attributed to gl20535-mediated promotion of UDP-glucose synthesis in the sugar donor pathway and upregulation of the expression of glycoside hydrolase genes. The opposite trend was observed in the silenced strains. In mycelial growth studies, neither overexpression nor silencing of gl20535 affected biomass and cell wall thickness. Furthermore, the GL20535 isozyme gene gl24465 remained unaffected in gl20535-overexpressed strains but was upregulated in gl20535-silenced strains, suggesting a compensatory regulatory relationship. These findings reveal the regulatory role of GL20535 on gene expression in the GLPs synthesis pathway and deepen our understanding of GL20535 function in the polysaccharide network of edible and medicinal fungi.
1. Introduction
Ganoderma lucidum, a renowned edible and medicinal fungus [1,2], exhibits a wide range of pharmacological properties, including immunoregulatory, anti-inflammatory and prebiotic activities [3,4]. G. lucidum polysaccharides (GLPs) are typically heteropolysaccharides composed of various monosaccharides linked by β-1,3-, β-1,6-, α-1,3- and α-1,4-glycosidic bonds [5]. The GLP structures are predominantly composed of β-glucan, which is characterized by a main chain of β-1,3-linked glucose units with β-1,6-linked side chains [6].
GLPs biosynthesis is a complex metabolic regulatory network involving the synthesis of nucleotide sugar donors, extension of the sugar chain, and export of polysaccharides [7]. With the advancement in metabolic modeling, the key enzymes involved in these pathways have gradually been elucidated [8]. In the initial sugar donor synthesis process, overexpression of phosphoglucomutase (PGM) and UDP-glucose pyrophosphorylase (UGP) enhances UDP-glucose synthesis, thereby increasing both polysaccharide yield and glucose content [9,10]. Similarly, phosphomannose isomerase (PMM), phosphomannomutase (PMI), and GDP-mannose pyrophosphorylase (GMP) are involved in GDP-mannose biosynthesis, affecting the mannose composition of polysaccharides [11,12]. These enzymes not only redirect carbon flux toward sugar donor biosynthesis but also influence the monosaccharide composition of GLPs [7].
In the subsequent process, sugar donors are transferred to acceptor molecules via specific glycosidic linkages, forming structurally defined polysaccharide chains [13,14]. Glycosyltransferases (GTs) play a pivotal role in this process [15]. For instance, α-1,3-glucosyltransferase [16] and β-1,3-glucosyltransferase [17], along with UGT88A1 [18], have been implicated in mushroom polysaccharide biosynthesis. In Grifola frondosa, silencing genes encoding β-1,3-glucosyltransferases, particularly GFGLS2, inhibited both mycelial growth and polysaccharide synthesis [19]. Comparatively, in G. lucidum, β-1,3-glucosyltransferases exhibited elevated expression in high polysaccharide-producing strains [20]; overexpression of genes encoding these transferases in G. lingzhi increased intracellular polysaccharide (IPS) content; and co-overexpression with ugp further enhanced total polysaccharide yield [21,22]. Although existing studies have highlighted a correlation between β-1,3-glucosyltransferase expression and polysaccharide production in Ganoderma, the precise functions and regulatory mechanisms of these genes remain unclear.
In this study, G. lucidum CGMCC 5.26 was employed to investigate the functional role of β-1,3-glucosyltransferase (GL20535) in polysaccharide biosynthesis. Overexpression and silencing strains were constructed to assess the effects of GL20535 on mycelial growth, morphology, and polysaccharide production. Furthermore, transcriptional profiling of key biosynthetic genes was performed to elucidate the underlying regulatory mechanisms. The findings could provide a deeper insight into the role of β-1,3-glucosyltransferase in G. lucidum and a theoretical foundation for further refining the polysaccharide biosynthesis pathway.
2. Materials and Methods
2.1. Strain and Culture Medium
The wild-type (WT) strain G. lucidum CGMCC 5.26 and recombinant strains were preserved on potato dextrose agar at 4 °C and incubated at 30 °C. For submerged culture, homogenized seed culture (300 mg wet-weight) was inoculated into 80 mL of liquid fermentation medium (containing 20 g/L glucose, 5 g/L tryptone, 5 g/L yeast nitrogen base without amino acids, 4.5 g/L KH2PO4, and 2 g/L MgSO4·7H2O) and cultivated at 30 °C with agitation at 150 rpm for 10 d. Transformants were screened on casein yeast extract medium (composed of 20 g/L glucose, 10 g/L maltose, 2 g/L peptone, 2 g/L yeast extract, 4.6 g/L KH2PO4, and 0.5 g/L MgSO4·7H2O). For plasmid construction, Escherichia coli JM109 served as the cloning host.
2.2. Cloning and Bioinformatic Analysis of gl20535
The β-1,3-glucosyltransferase gene gl20535 (Gene bank: AZQ26797.1) [23] was amplified from G. lucidum genomic DNA (gDNA) and complementary DNA (cDNA) using the primer pairs gl20535-F/R (Table S1). Briefly, gDNA was extracted using the CTAB method [24]. The total RNA was extracted from 6-day-old mycelial cultures using FastPure® Universal Plant Total RNA Isolation Kit (Vazyme, Nanjing, China). The cDNA template was generated by reverse transcription of total RNA using HiScript®III RT SuperMix for qPCR (+gDNA wiper) (Vazyme, Nanjing, China), following the manufacturer’s protocol. The identity of all cloned gene sequences was confirmed by sequencing (Sangon Biotech, Shanghai, China).
The deduced amino acid sequence of gl20535 was subjected to bioinformatic analyses [25]. The NCBI Conserved Domain database (https://www.ncbi.nlm.nih.gov/) was used for domain prediction, while sequence homology and phylogeny of fungal β-1,3-glucosyltransferases were assessed via NCBI Protein BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi). A phylogenetic tree was constructed using MEGA 6.06 and visualized in iTOL (https://itol.embl.de/itol.cgi). Molecular weight and isoelectric point (pI) were computed using ProtParam (https://web.expasy.org/protparam/), while hydrophobicity was analyzed using ProtScale (https://web.expasy.org/protscale/). Signal peptides and transmembrane domains were predicted using SignalP 6.0 and TMHMM (http://www.cbs.dtu.dk/services/TMHMM/), respectively.
2.3. Construction of the Overexpression and Silenced Plasmids
Following established plasmid manipulation strategies [16], the carboxin-resistance plasmids pMsdhB-OEgl20535 and pMsdhB-igl20535 were constructed (Figure S1) using the primers listed in Table S1. Mutant glsdhB (MsdhB) was cloned into pMD19-T to construct pMsdhB. Two cassettes were then ligated to pMsdhB: an overexpression cassette containing the Pglgpd promoter and TglsdhB terminator, yielding pMsdhB-OEGL, and a silencing cassette including Pglgpd, URA3-silenced sequence (ura3), and the CaMV35s promoter (P35s), producing pMsdhB-RNAiGL. The gl20535 cDNA was inserted into pMsdhB-OEGL to generate the final overexpression plasmid, pMsdhB-OEgl20535, while a conserved gl20535 fragment was ligated into pMsdhB-RNAiGL to create the silencing plasmid, pMsdhB-igl20535. All cloning steps were performed using the ClonExpress® Ultra One-Step Cloning Kit (Vazyme, Nanjing, China).
2.4. Construction and Identification of Overexpression and Silenced Strains
Transformants were generated using the PEG-mediated protoplast transformation (PMT) method [16]. Protoplasts were prepared from 4-day-old static cultures by suspending the harvested mycelia in 0.6 M mannitol containing 20% (w/v) lysing enzymes (Guangdong Microbiology Culture Center, Guangzhou, China), followed by enzymatic digestion at 30 °C and 150 rpm for 2.5 h. After filtration, 10 μg of plasmid DNA were added to 167 μL STC buffer (0.55 M sorbitol, 10 mM CaCl2, 10 mM Tris-HCl, pH 7.5) containing approximately 107 protoplasts/mL, along with 5 μL spermidine and 2 μL heparin. The mixture was incubated on ice for 10 min before sequential addition of 400 μL and then 600 μL PTC buffer (40% PEG4000, 50 mM CaCl2, 10 mM Tris-HCl, pH 7.5), followed by incubation at 28 °C for 30 min. The protoplasts were collected by centrifugation (3500× g, 10 min, 28 °C), resuspended in 1 mL CYM regeneration medium, and plated on CYM agar. After 2 days at 30 °C, 4 μg/mL carboxin in soft agar was overlaid to select transformants, which were further incubated for 10–14 days.
In addition, control (pMsdhB), overexpression (pMsdhB-OEgl20535), and RNAi silencing (pMsdhB-igl20535) plasmids were independently transfected into G. lucidum protoplasts, and the transformants were selected on CYM agar medium containing 4 mg/L carboxin. The overexpression strains were verified by amplifying the expression cassette from gDNA using Pgpd-F/sdhB-R primers (Table S1), with sequence-confirmed transformants designated as Oe-gl20535. Similarly, silenced strains were authenticated using the Pgpd-F/P35s-R primers (Table S1) and named igl20535 after sequence verification, while the control transformants retained the control designation.
2.5. Real-Time Quantitative PCR (RT-qPCR) Analysis of Gene Expression
RT-qPCR analysis was carried out using a ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) following established protocols, and the 18S rRNA gene was used as an internal control for normalization [16]. Gene expression levels were calculated using the 2−ΔΔCt comparative method [26]. Briefly, the threshold cycle (Ct) of each target gene was first normalized to that of the internal reference gene ΔCt (ΔCt = Cttarget − Ctreference) and then compared to the ΔCt of the control group to calculate ΔΔCt (ΔΔCt = ΔCtsample − ΔCtcontrol). The fold change in gene expression was determined using the formula 2−ΔΔCt.
Transcript levels were measured across WT, Control, Oe-gl20535, and igl20535 strains for key metabolic genes, including pgm, ugp, the UDP-glucose 4-epimerase gene (uge), the phosphoglucose isomerase gene (pgi), pmi, pmm, gmp, β-1,3-glucosyltransferases (gl20535 and gl24465), β-1,3-glucanohydrolases (gl24581, gl21451, gl27365, gl30087, and gl20743), and chitin synthases (gl15273, gl25613, gl28060, gl30799, and gl31550). The primer sequences are detailed in Table S2.
2.6. Characterization of Mycelial Morphology
After a 6-day cultivation at 30 °C in both liquid and plate media, mycelial morphology of WT and recombinant strains was examined using a Nikon SMZ25 camera. Colony radii and pellet diameters were quantified using ImageJ software v1.8.0 [27]. Mycelial pellet size was classified into S-fraction (diameter < 1 mm), M-fraction (1 mm < diameter < 2 mm), and L-fraction (diameter > 2 mm).
Mycelia from the liquid culture were collected, rinsed, and postfixed in 2.5% (v/v) glutaraldehyde for ultrathin sectioning. The ultrastructure of the mycelial cross-sections was subsequently examined using a transmission electron microscope (TEM, Hitachi HT-7800, Tokyo, Japan). Mycelial diameter and cell wall thickness were measured using the ImageJ software.
2.7. Measurements of Biomass, Residual Sugars, EPS, and IPS
Biomass, residual sugar, and polysaccharides were analyzed as previously described [27]. The mycelia were centrifuged, washed, and freeze-dried for dry weight measurements. IPS was extracted from the dried powder via hot-water extraction, while EPS and residual sugars were measured from the collected fermentation broth. IPS and EPS were ethanol-precipitated and quantified using the phenol–sulfuric acid method [28]. Residual sugars were determined using the DNS method [29].
2.8. Comparison of Monosaccharides
The monosaccharide composition of the polysaccharides was analyzed using ion chromatography [16]. Monosaccharides were identified by comparing their retention times, and their proportions were determined through content percentage (Figure S2).
2.9. Quantification of β-1,3-glucan and Chitin in the Cell Wall
The freeze-dried mycelium powder was pulverized and sequentially washed with aqueous solutions of NaCl (1%, 2%, and 5%), followed by eight ddH2O washes. The purified cell wall powder was freeze-dried and crushed [30].
The β-1,3-glucan in the cell wall was alkali-extracted (1 M NaOH) and quantified fluorometrically using aniline blue staining [27]. Fluorescence intensity was detected using a microplate reader (Hitachi F-7000) at 405 nm excitation and 460 nm emission wavelengths. β-1,3-glucan content (%) was quantified as fluorescence intensity per milligram of cell wall and expressed in relative units [16,31].
Chitin content was determined by measuring the glucosamine levels in the cell wall after complete enzymatic hydrolysis, following the method described by Popolo et al. [32] and Chen et al. [16]. Chitin content (%) was quantified as glucosamine content per milligram of cell wall and expressed in relative units.
2.10. Statistical Analysis
GraphPad Prism 9.5 was used for statistical analysis. Data from triplicate experiments are presented as mean ± SD (n = 3). Significant differences (* p < 0.05, ** p < 0.01) were determined by one-way ANOVA with Duncan’s multiple range test.
3. Results
3.1. Silencing and Overexpression of gl20535
The gDNA sequencing of the β-1,3-glucosyltransferase gene gl20535 from G. lucidum revealed a full-length sequence of 6408 bp (Figure 1A). A comparison with its cDNA sequence indicated the presence of 11 introns, with a total exon length of 5343 bp, encoding a 1780-amino acid polypeptide (Figure 1A,B). Bioinformatic predictions estimated the molecular weight of the encoded protein to be 204.15 kDa, with an isoelectric point (pI) of 9.14. The protein was characterized as a basic, hydrophilic, membrane-associated protein lacking signal peptides and containing 17 transmembrane helices (Figure 1E–H). Conserved domain analysis identified both a β-1,3-glucan synthase subunit FKS1 homologous domain (fks1-dom1) and a β-1,3-glucan synthase component domain (glucan synthase) (Figure 1C), which are recognized as essential catalytic regions in fungal β-1,3-glucan synthases [33,34,35]. Additionally, multiple sequence alignment showed that gl20535 shares high sequence similarity with β-1,3-glucosyltransferase genes from Cerioporus squamosus (KAI0690553.1), Grifola frondosa (QCR99115.1), Lentinula edodes (XP046082619.1), and other fungi (Figure 1D).
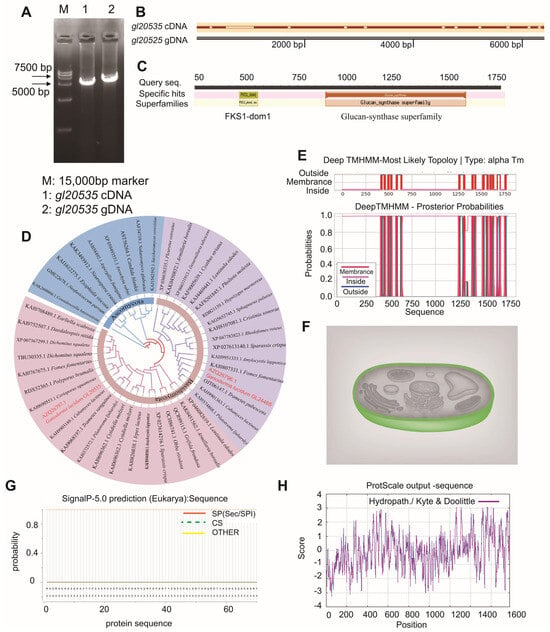
Figure 1.
Bioinformatics analysis of the β-1,3-glucosyltransferase gene gl20535. (A) Cloning of gl20535 cDNA and gDNA sequences from G. lucidum; (B) sequence comparison of gl20535 cDNA and gDNA, indicating exon–intron structure; (C) conserved domain analysis using the NCBI Conserved Domain Database; the protein harbors a β-1,3-glucan synthase subunit FKS1 homolog domain (fks-dom1) and β-1,3-glucan synthase component domain (glucan synthase); (D) phylogenetic tree of β-1,3-glucosyltransferases from G. lucidum and related fungi; (E) transmembrane domain prediction using TMHMM v2.0 with red bars indicating the locations of 17 predicted transmembrane helices; (F) subcellular localization prediction: colors indicate predicted compartments, with green representing cytoplasmic localization; (G) hydropathy plot generated using ProtScale; higher values indicate more hydrophilic regions, while lower values correspond to more hydrophobic segments of the protein; (H) signal peptide prediction using SignalP: no signal peptide was predicted.
To further analyze the function of GL20535 in G. lucidum, overexpression and silencing strains were constructed using the PMT method. Based on bioinformatics analysis, the fks1-dom1 domain (amino acid residues 261 to 372) in the gl20535 cDNA sequence was selected as the target for constructing the silenced strain igl20535 (Figure 1C). Simultaneously, gl20535 cDNA was overexpressed in G. lucidum to generate the overexpression strain Oe-gl20535. Following carboxin resistance screening and PCR verification, eight transformants were confirmed to have successfully integrated the overexpression plasmid (Figure 2A), while five transformants were verified to have successfully incorporated the silencing plasmid (Figure 2B).
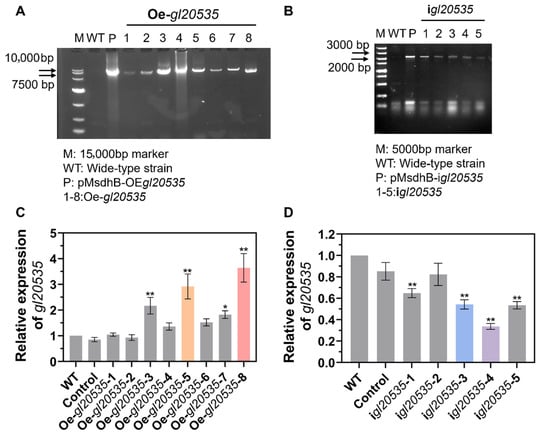
Figure 2.
Construction of gl20535 overexpression and silenced strains. PCR confirmation of (A) overexpression strains Oe-gl20535-n (n = 1–8) and (B) silenced strains igl20535-n (n = 1–5) using plasmid-specific primers. The transcription levels of gl20535 in (C) overexpression strains and (D) silenced strains compared to those in the wild type (WT). Results are expressed as mean ± standard deviation from triplicate experiments (n = 3); * (p < 0.05) and ** (p < 0.01) indicate significant differences compared to the WT.
RT-qPCR was performed to evaluate the transcript level of gl20535 in the recombinant strains. The results indicated no significant differences between the control and WT strains, suggesting that molecular manipulation did not affect the expression of gl20535 in G. lucidum. Among the transformants, strains Oe-gl20535-5 and Oe-gl20535-8 showed significant upregulation, with expression levels reaching 292.00% and 364.27% of the WT, respectively (Figure 2C). Conversely, strains igl20535-3 and igl20535-4 exhibited a reduction in gl20535 transcript levels by 48.01% and 66.32%, respectively, compared to the WT strain (Figure 2D). Therefore, four recombinant strains (igl20535-3, igl20535-4, Oe-gl20535-5, and Oe-gl20535-8) were selected for further analysis.
3.2. Effect of GL20535 on Mycelial Morphology and Growth Characteristics
3.2.1. Morphological Characteristics
On a solid medium, gl20535 expression inhibited mycelial growth and induced a distinct colony morphology (Figure 3A). The WT strain exhibited radial and uniform growth, characterized by sparse, fluffy hyphae, and smooth colony edges, with a mean colony radius of 35.7 ± 1.1 mm. The colony radius of the Oe-gl20535-5, Oe-gl20535-8, igl20535-3, and igl20535-4 strains were significantly smaller, with reductions of 55.65%, 56.48%, 42.47%, and 55.05%, respectively (p < 0.05), compared with those of the WT strain (Figure 3B). In addition, the control strain also exhibited a decrease in colony radius compared to the WT, indicating that genetic manipulation affected mycelial growth on solid medium.
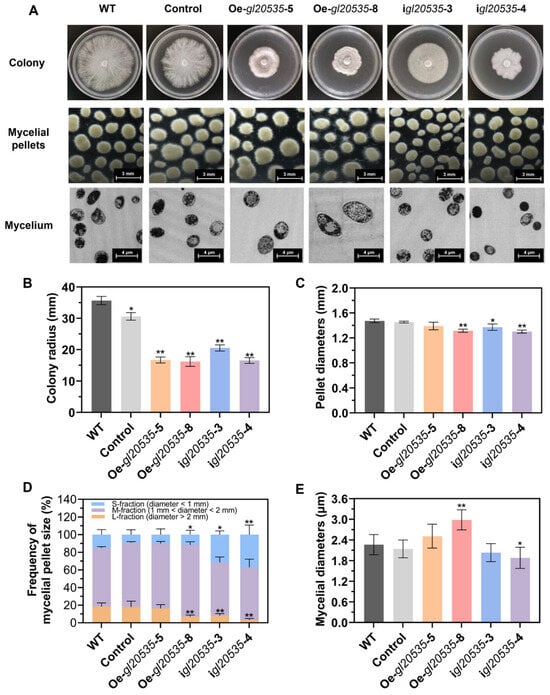
Figure 3.
Effect of GL20535 on morphological characteristics. (A) Macro-morphological analysis of the WT and recombinant strains after 6 d of culture in both plate and liquid media. (B) The mean colony radius of recombinant strains in plate culture. (C) The mean pellet diameters of recombinant strains in liquid culture. (D) The mycelial pellet size classified by diameter: S, M, L, and their calculated proportions. (E) The mean mycelial diameters of recombinant strains in liquid culture. Results are expressed as mean ± standard deviation from triplicate experiments (n = 3); * (p < 0.05) and ** (p < 0.01) indicate significant differences compared to the WT.
In submerged cultures, the morphological characteristics of mycelial pellets, particularly diameter and surface roughness, serve as indicators of mycelial growth and polysaccharide production [36]. In the WT strain, the mean pellet diameter was 1.47 ± 0.02 mm (Figure 3C), with M-fraction pellets (1 mm < diameter < 2 mm) being predominant, accounting for 66.37% (Figure 3D). Overexpression of gl20535 reduced the pellet diameter to 1.33 ± 0.01–1.39 ± 0.05 mm, accompanied by an increase in the percentage of M-fraction mycelia to 73.88–81.65% (Figure 3C,D). Morphologically, gl20535 overexpression strains exhibited ovoid and starburst-shaped pellets (Figure 3A). Conversely, the M-fraction pellets of gl20535-silenced strains showed no significant change (p > 0.05), but the S-fraction pellets (diameter < 1 mm) significantly increased to 31.47–36.90% (p < 0.05) (Figure 3D) and the diameter of the pellets decreased to 1.30 ± 0.02–1.37 ± 0.04 mm (Figure 3C). The mycelial morphology of the silenced strains changed to small and smooth. Although the pellet diameter of recombinant strains was reduced, gl20535 overexpression strains, with uniform and hairy pellets may be linked to the liberation of the second generation of pellet, while the formation of small and bead-like pellets in gl20535-silenced strains inhibited mycelium aggregation [37]. When observed by TEM, the average mycelial diameter of the WT strain was 2.26 ± 0.29 μm. In gl20535 overexpression strains, the mycelial diameter increased by 10.93–31.87% compared to that in the WT, with thicker mycelia observed in strains exhibiting higher gl20535 expression levels. Conversely, in gl20535-silenced strains, the mycelial diameter decreased by 10.25–16.89%, indicating thinning of the mycelium (Figure 3E).
3.2.2. Growth Curve
Glucose consumption and biomass accumulation during submerged fermentation by the WT and recombinant strains are shown in Figure 4. Overexpression of gl20535 accelerated glucose consumption but had no effect on biomass accumulation. Strains Oe-gl20535-5 and Oe-gl20535-8 reached maximum biomass on day 9, with values of 8.67 ± 0.11 g/L and 8.73 ± 0.10 g/L, representing a slight reduction of 3.79% and 4.57%, respectively, compared to those in the WT strain (9.08 ± 0.10 g/L). In contrast, gl20535 silencing slowed glucose consumption. Strains igl20535-3 and igl20535-4 exhibited maximal biomass levels of 8.15 ± 0.04 g/L and 7.71 ± 0.12 g/L on day 9, representing decreases of 10.28% and 15.07%, respectively, compared to those of the WT strain. These results indicate that GL20535 influenced the rate of carbon source consumption in the medium but had limited impact on biomass accumulation, except for reducing a little biomass accumulation in the silenced strains during the late fermentation phase.
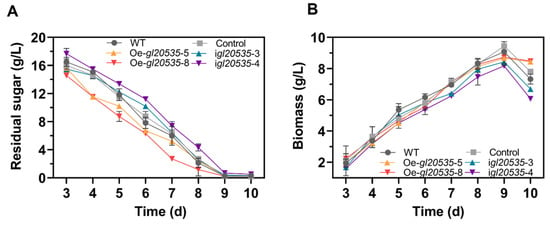
Figure 4.
Effect of GL20535 on growth in submerged culture. (A) Residual sugar. (B) Biomass. Results are expressed as mean ± standard deviation from triplicate experiments (n = 3).
3.3. Effect of GL20535 on Polysaccharide Production and Monosaccharide Composition
3.3.1. IPS
The expression of gl20535 not only affected mycelial morphology but also modulated polysaccharide synthesis in G. lucidum. Upregulation of gl20535 accelerated IPS accumulation, manifested as maximum yields of Oe-gl20535-5 and Oe-gl20535-8 strains of 94.01 ± 3.08 mg/g and 105.75 ± 3.59 mg/g, representing an improvement of 4.97% and 18.08%, respectively, compared to those of the WT. IPS accumulation in the silenced strains was lower than that in the WT, with the maximum IPS content in the igl20535-3 and igl20535-4 strains decreasing by 6.77% and 16.97%, respectively (Figure 5A).
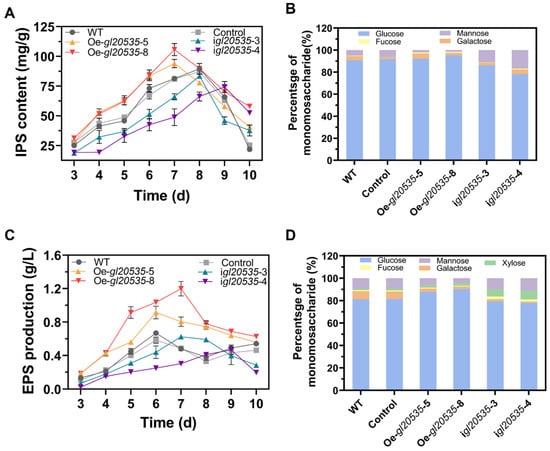
Figure 5.
Effect of gl20535 on polysaccharide production and monosaccharide composition. (A) IPS content. (B) Monosaccharide composition of IPS. (C) EPS production. (D) Monosaccharide composition of EPS. Results are expressed as mean ± standard deviation from triplicate experiments (n = 3).
Additionally, the expression of gl20535 in G. lucidum modified the monosaccharide composition of IPS. Compared to that in the WT, the glucose percentage increased by 1.34% and 4.43% in the Oe-gl20535-5 and Oe-gl20535-8 strains, respectively, while the mannose percentage decreased by 2.97% and 3.53%, respectively. Conversely, downregulation of gl20535 resulted in a decrease in glucose percentage and an increase in mannose percentage (Figure 5B). The structure of IPS is primarily composed of glucose and mannose, which may be regulated through metabolic pathways involved in the synthesis of UDP-glucose and GDP-mannose nucleotide sugar precursors.
3.3.2. EPS
The accumulation of EPS in the medium was also influenced by GL20535 (Figure 5C). Overexpression of gl20535 promoted EPS accumulation, with the maximum yields of the Oe-gl20535-5 and Oe-gl20535-8 strains reaching 0.91 ± 0.06 g/L and 1.20 ± 0.06 g/L, representing increases of 36.7% and 79.4%, respectively, compared to those in the WT. Conversely, the maximum EPS yields in the igl20535-3 and igl20535-4 strains were 0.61 ± 0.01 g/L and 0.47 ± 0.04 g/L, reflecting decreases of 7.9% and 30.2%, respectively, compared to those in the WT (Figure 5C).
For the monosaccharide composition of EPS, upregulation of gl20535 increased the glucose percentage by 6.59–9.21% and decreased the mannose and galactose percentage by 2.89–4.98% and 4.26–5.04%, respectively. Downregulation of gl20535 decreased the glucan and galactose percentages by 1.77–3.32% and 5.36–6.06%, respectively, and increased the mannose and xylose percentages by 0.52–1.82% and 5.15–6.44%, respectively (Figure 5D). Taken together, EPS production may be influenced by polysaccharide export processes, including the expression of GTs and glycoside hydrolases (GHs).
3.4. Effect of gl20535 on the Cell Wall
The cell walls play critical roles in maintaining cell morphology and supporting growth in edible and medicinal fungi [38]. Mycelial cross-sections of G. lucidum recombinant strains were analyzed using a transmission electron microscope (Figure 6A). Overexpression of gl20535 had no significant impact on cell wall thickness, with average values ranging from 0.098 μm to 0.101 μm (Figure 6B). In contrast, silencing gl20535 resulted in a slight reduction in cell wall thickness (0.087–0.089 μm), although the difference was not statistically significant (Figure 6B).
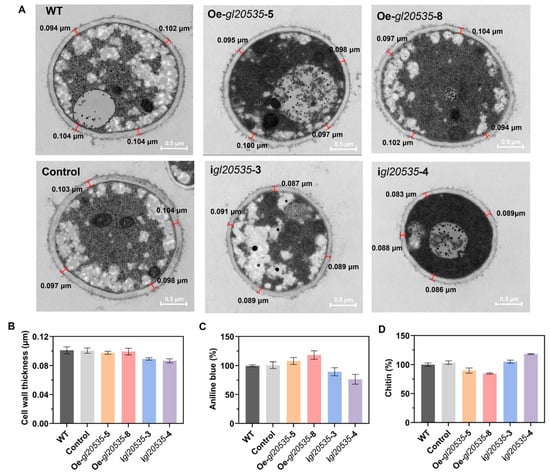
Figure 6.
Effect of gl20535 on the G. lucidum cell wall. (A) Electron microscopy of the cell wall. (B) Cell wall thickness. (C) Relative fluorescence value of β-1,3-glucan. (D) Chitin content. Results are expressed as mean ± standard deviation from triplicate experiments (n = 3).
β-1,3-glucan and chitin are the primary structural components of the G. lucidum cell wall, and their synthesis is catalyzed by β-1,3-glucosyltransferase and chitin synthase. Similar to intracellular and extracellular polysaccharides, β-1,3-glucan content in the cell walls of gl20535 overexpression strains increased by 7.83–18.01% compared to that in the WT. Conversely, gl20535 silencing reduced β-1,3-glucan content by 10.55–23.56% (Figure 6C). Meanwhile, chitin content in the cell wall decreased by 10.19–15.29% in overexpression strains, while it increased by 4.93–18.08% in silenced strains relative to those in the WT (Figure 6D). Overexpression of gl20535 increased the β-1,3-glucan content in the cell wall and reduced chitin content by inhibiting chitin synthase expression, resulting in no significant change in cell wall thickness. In contrast, in the silenced strains, the changes in cell wall composition were reversed. The increased chitin could not compensate for the reduction in β-1,3-glucan, leading to a slight thinning of the cell wall. These results demonstrated that gl20553 affects the contents of β-1,3-glucan and chitin in the cell wall and maintains the stability of the cell wall through co-regulation of their increase and decrease.
3.5. Transcript-Level Analysis of Genes in the Polysaccharide Biosynthesis Pathway
To further elucidate the regulatory role of β-1,3-glucosyltransferase gene gl20535 in polysaccharide biosynthesis, the transcript levels of associated pathway genes, including the β-1,3-glucosyltransferase isozyme gene gl24465, key genes in nucleotide sugar donor biosynthesis (pgm, ugp, pgi, pmi, pmm, gmp, uge), glycoside hydrolase genes (gl24581, gl21451, gl27365, gl30087, gl20743), and chitin synthase genes (gl15273, gl25613, gl28060, gl30799) were analyzed on days 4, 5, 6, and 7 of fermentation in recombinant strains (Figure 7 and Figure 8).
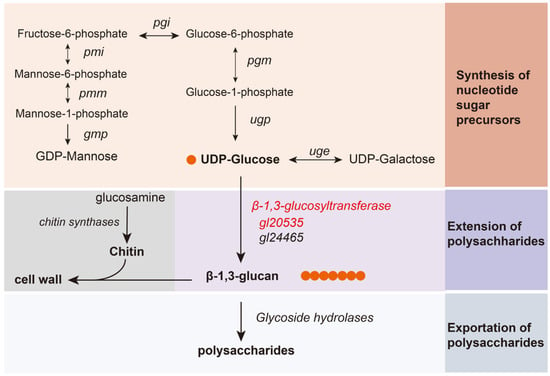
Figure 7.
Key enzymes in polysaccharide synthesis pathway. GMP, GDP-mannose pyrophosphorylase; PGI, phosphoglucose isomerase; PGM, phosphoglucomutase; UGP, UDP-glucose pyrophosphorylase; PMM, phosphomannomutase; PMI, phosphomannose isomerase.
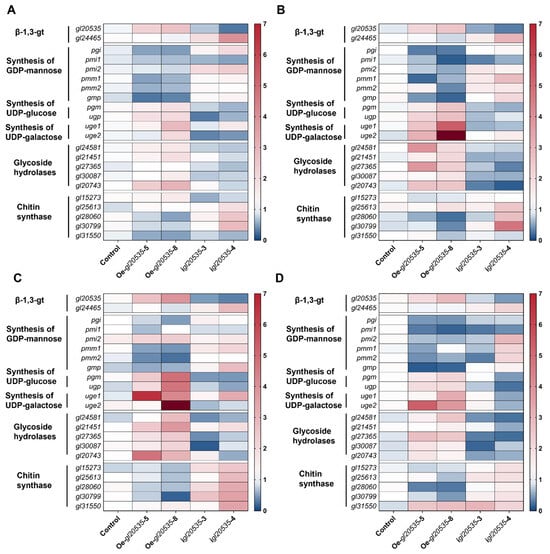
Figure 8.
Transcript-level analysis of genes in the polysaccharide synthesis pathway during fermentation. (A) Day 4. (B) Day 5. (C) Day 6. (D) Day 7. The numbers on the color axis represent the relative expression levels of the genes.
3.5.1. β-1,3-glucosyltransferase Isozyme Gene gl24465
Overexpression of gl20535 significantly elevated its transcript levels from day 4 to day 7, without notably affecting the expression of its isozyme gene gl24465. On day 6, gl20535 expression peaked in strains Oe-gl20535-5 and Oe-gl20535-8, showing increases of 165.88% and 289.22%, respectively, over those in the WT, while gl24465 expression remained unchanged (Figure 8C).
In contrast, silencing gl20535 significantly upregulated gl24465 expression, with the magnitude of compensation positively correlated with silencing efficiency. On day 6, strains igl20535-3 and igl20535-4 exhibited gl20535 expression reductions of 54.48% and 67.89%, respectively, accompanied by gl24465 upregulation of 51.04% and 186.79%, respectively (Figure 8C).
3.5.2. Key Enzymes in Nucleotide Sugar Donor Biosynthesis
In the nucleotide sugar biosynthesis pathway, gl20535 overexpression upregulated pgm, ugp, and uge, thereby promoting UDP-glucose biosynthesis. Simultaneously, it suppressed pgi, pmi, pmm, and gmp, which are involved in GDP-mannose biosynthesis. These changes suggest a metabolic flux shift favoring UDP-glucose production, consistent with the increased intracellular glucose content and reduced mannose and galactose proportions in the polysaccharide profile. Conversely, gl20535 silencing reversed these trends (Figure 8). On day 7, strain igl20535-3 exhibited an overall downregulation of genes involved in sugar donor biosynthesis (Figure 8D).
3.5.3. Glycoside Hydrolases
Glycoside hydrolases are involved in the modification and export of polysaccharides, thereby influencing exopolysaccharide accumulation [20]. In gl20535-overexpressing strains, glycoside hydrolase genes were broadly upregulated, particularly on day 6, with strain Oe-gl20535-8 showing the highest transcript levels. In contrast, gl20535 silencing downregulated these genes, with igl20535-4 exhibiting the most pronounced reduction. These results indicated a positive correlation between gl20535 expression and glycoside hydrolase activity (Figure 8).
3.5.4. Chitin Synthases
Chitin, a major component of the cell wall, is synthesized by multiple chitin synthases regulated by gl20535. Overexpression of gl20535 resulted in the progressive downregulation of chitin synthase genes during fermentation (Figure 8), contributing to reduced chitin content and unchanged cell wall thickness (Figure 6). Conversely, silencing gl20535 enhanced chitin synthase expression, with the most significant upregulation observed on day 6 (Figure 8), contributing to increased chitin content and reduced cell wall thickness (Figure 6).
4. Discussion
GLPs are widely recognized for their pharmacological potential and have become a focal point in the development of functional foods [39,40]. However, their complex biosynthetic pathways pose substantial challenges for large-scale application in the food industry [7]. Among the enzymes involved in GLP synthesis, β-1,3-glucosyltransferase plays a central role in the elongation of glucan chains [20], while the underlying metabolic regulation remains insufficiently understood [19,21,41]. In this study, we elucidated the mechanism and function of GL20535 in G. lucidum.
Previous studies have found that the interaction patterns between β-1,3-glucosyltransferase isozymes differ across fungal species [33]. In Saccharomyces cerevisiae [34] and Candida albicans [35], FKS1 is indispensable and functionally dominant, while other isozymes lack compensatory capacity. In contrast, all β-1,3-glucosyltransferase isoforms in Schizosaccharomyces pombe are functionally non-redundant [42]. In this study, gl24465 expression remained unchanged in the gl20535-overexpressing strains, consistent with the findings in G. lingzhi [21]. Silencing gl20535 induced a compensatory upregulation of gl24465, suggesting a potential supplemental mechanism in G. lucidum. The functional diversity of β-1,3-glucosyltransferases is also evident in other edible and medicinal fungi. For instance, silencing GFGLS2 in G. frondosa significantly reduces polysaccharide synthesis, with no compensatory effect observed from its isoenzymes [19]. In Agaricus bisporus, the expression patterns of isozymes are largely determined by the developmental stages [43]. These phenomena suggest that β-1,3-glucosyltransferase exhibits species-specific regulation, as observed in Ascomycota and Basidiomycota.
In the sugar donor synthesis pathway, gl20535 influences the expression of key genes. Its overexpression upregulates pgm and ugp, thereby enhancing UDP-glucose biosynthesis. This likely reflects a feedback mechanism in which increased consumption of UDP-glucose for sugar chain extension stimulates metabolic flux. Notably, previous studies have shown that overexpression of pgm [10,44] and ugp [9,45] can enhance β-1,3-glucosyltransferase expression levels, suggesting a feedback regulation between β-1,3-glucosyltransferase and UDP-glucose synthesis. Similar feedback mechanisms have been documented in plant starch and cellulose metabolism [46]. Furthermore, overexpression of gl20535 suppressed the expression of pmi, pmm, and gmp, thereby shifting the carbon flux toward UDP-glucose, and increased glucose proportion in GLPs. According to genome-scale metabolic network model analysis, the activities of pgm and pgi can regulate the flow of glucose-6-phosphate to GDP-mannose or UDP-glucose synthesis, resulting in changes in monosaccharide composition [8,47]. This is consistent with previous findings in G. frondosa, in which ugp silencing upregulated gmp [44]. In contrast, gl20535 silencing induced the opposite effect. Interestingly, in other edible and medicinal fungi, such as G. lingzhi [21,22] and G. frondosa [17,19], β-1,3-glucosyltransferases showed limited impact on sugar donor biosynthesis, reflecting their specificity among different species.
GHs contribute to polysaccharide modification and export by catalyzing the hydrolysis of glycosidic bonds. In gl20535-overexpressing strains, multiple GH genes (gl21451, gl24581, gl30087, gl24039, and gl27365) were significantly upregulated, consistent with the higher EPS production. This transcriptional activation was consistent with the observed increase in polysaccharide yield, particularly the more pronounced elevation of EPS compared to IPS. These enzymes were also highly expressed in high polysaccharide-producing G. lucidum strains, underscoring their contribution to EPS accumulation [20].
Our findings indicate that gl20535 coordinates multiple layers of the polysaccharide biosynthesis pathway, from sugar precursor synthesis to polysaccharide hydrolysis, and regulates GLP production without mycelial biomass. Overexpression of gl20535 significantly enhanced polysaccharide yield, echoing the results of similar overexpression strategies in Pleurotus ostreatus [41] and G. lingzhi [21,22]. Conversely, its silencing led to a marked reduction in yield, similar to observations in G. frondosa [17,19]. Collectively, these results highlight β-1,3-glucosyltransferase as a key regulatory enzyme in polysaccharide production in edible and medicinal fungi.
GTs also influence the cell wall due to their involvement in cell wall polysaccharide synthesis. For instance, in G. frondosa, silencing β-1,3-glucosyltransferase genes led to thinner mycelium [17,19], and overexpression of glucosyltransferase gene UGT88A1 increased sensitivity to cell wall stress [18]. In G. lucidum, overexpression of α-glucosyltransferase reduced β-1,3-glucan content in the cell wall without altering chitin levels [16]. Unlike previous reports, GL20535 influenced chitin content by regulating the expression of chitin synthase genes in response to changes in β-1,3-glucan levels, resulting in no significant alteration in cell wall thickness. These findings elucidate the differential effects of glucosyltransferases on cell wall synthesis in edible and medicinal fungi.
The regulatory mechanism of GL20535 in GLP biosynthesis has been basically clarified, while the interaction and feedback mechanisms of its isozyme (GL24465) require further investigations. This will facilitate a comprehensive elucidation of the mechanism of action of β-1,3-glucosyltransferase and enhance the in-depth understanding of the polysaccharide biosynthesis process.
5. Conclusions
β-1,3-glucosyltransferase (GL20535) plays an important role in polysaccharide synthesis. GL20535 influences the synthesis of UDP-glucose and GDP-mannose in the sugar donor biosynthesis pathway, thereby enhancing polysaccharide yield and altering monosaccharide composition. During sugar chain extension, β-1,3-glucosyltransferase isozyme gene gl24465 exhibited compensatory upregulation in response to gl20535 silencing. Furthermore, gl20535 regulated the transcription of several glycoside hydrolase genes (gl24681, gl27365, gl30087, and gl20743) associated with EPS accumulation. These findings highlight the functional role of GL20535 in G. lucidum and promote the application of GLPs in the food production industry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof11070532/s1, Figure S1: Construction of the gl20535-silencing plasmid pMsdhB-igl20535 and gl20535-overexpression plasmid pMsdhB-OEgl20535; Figure S2: Ion chromatogram of product separation. (A) The standard solution contains fucose, galactose, glucose, xylose and mannose. (B) Ion chromatogram of IPS samples. (C) Ion chromatogram of EPS samples; Figures S3–S14: Full, uncropped TEM images; Table S1: Primers used to clone genes; Table S2: Primers used to RT-qPCR.
Author Contributions
Conceptualization, J.L. (Jingyun Liu) and Z.D.; methodology, J.L. (Jingyun Liu), M.X. and M.S.; validation, J.L. (Jingyun Liu), J.L. (Junxun Li) and Z.D.; formal analysis, J.L. (Jingyun Liu); investigation, M.S.; resources, Z.G.; writing—original draft preparation, J.L. (Jingyun Liu).; writing—review and editing, M.X., J.L. (Junxun Li), L.C. and Z.D.; visualization, J.L. (Jingyun Liu) and L.C.; supervision, Z.G. and G.S.; funding acquisition, M.X. and Z.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32272283); the Postdoctoral Fellowship Program of CPSF (GZC20230988), and the China Postdoctoral Science Foundation (Certificate Number: 2024M751158).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
Author Junxun Li was employed by the company Shandong Taishan Shengliyuan Group Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
EPS, extracellular polysaccharides; F-6-P, fructose-6-phosphate; GLPs, Ganoderma lucidum polysaccharides; GT, glucosyltransferase; GH, glycoside hydrolases; G-6-P, glucose-6-phosphate; G-1-P, glucose-1-phosphate; GMP, GDP-mannose pyrophosphorylase; IPS, intracellular polysaccharides; M-1-P, mannose-1-phophate; M-6-P, mannose-6-phosphate; PGI, phosphoglucose isomerase; PGM, phosphoglucomutase; UGP, UDP-glucose pyrophosphorylase; PMM, phosphomannomutase; PMI, phosphomannose isomerase.
References
- Sharif, M.; Bondzie-Quaye, P.; Wang, H.; Shao, C.; Hua, P.; Alrasheed, M.; Benjami, J.; Sossah, F.L.; Huang, Q. Potentialities of Ganoderma lucidum extracts as functional ingredients in food formulation. Food Res. Int. 2023, 172, 113161. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhang, S.; Peng, B.; Tan, D.; Wu, M.; Wei, J.; Wang, Y.; Luo, H. Ganoderma lucidum: A comprehensive review of phytochemistry, efficacy, safety and clinical study. Food Sci. Hum. Wellness 2024, 13, 568–596. [Google Scholar] [CrossRef]
- Swallah, M.S.; Bondzie-Quaye, P.; Wu, Y.; Acheampong, A.; Sossah, F.L.; Elsherbiny, S.M.M.; Huang, Q. Therapeutic potential and nutritional significance of Ganoderma lucidum—A comprehensive review from 2010 to 2022. Food Funct. 2023, 14, 1812–1838. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Pi, X.; Li, H.; Cheng, S.; Su, Y.; Zhang, Y.; Man, C.; Jiang, Y. Ganoderma spp. polysaccharides are potential prebiotics: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 909–927. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.L.; Shi, L. Methods of study on conformation of polysaccharides from natural products: A review. Int. J. Biol. Macromol. 2024, 263, 130275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Cui, F.; Sun, L.; Zan, X.; Sun, W. Recent advances in Ganoderma lucidum polysaccharides: Structures/bioactivities, biosynthesis and regulation. Food Biosci. 2023, 56, 103281. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, M.; Zhang, J.; Ma, Z.; Cui, J.; Zhao, L.; Chen, L.; Shi, G.; Ding, Z. Refined regulation of polysaccharide biosynthesis in edible and medicinal fungi: From pathways to production. Carbohydr. Polym. 2025, 358, 123560. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Ye, C.; Deng, W.; Xu, M.; Wang, Q.; Liu, G.; Wang, F.; Liu, L.; Xu, Z.; Shi, G.; et al. Reconstruction and analysis of a genome-scale metabolic model of Ganoderma lucidum for improved extracellular polysaccharide production. Front. Microbiol. 2018, 9, 3076. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, T.; Gao, T.; Miao, Z.; Jiang, A.; Shi, L.; Ren, A.; Zhao, M. UDP-glucose pyrophosphorylase influences polysaccharide synthesis, cell wall components, and hyphal branching in Ganoderma lucidum via regulation of the balance between glucose-1-phosphate and UDP-glucose. Fungal Genet. Biol. 2015, 82, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, M.; Wang, S.; Yue, S.; Shi, L.; Ren, A.; Zhao, M. Ganoderma lucidum phosphoglucomutase is required for hyphal growth, polysaccharide production, and cell wall integrity. Appl. Microbiol. Biotechnol. 2018, 102, 1911–1922. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cao, Y.; Luo, Q.; Xu, Y.; Li, N.; Wang, C.; Xu, J. Overexpression of phosphomannomutase increases the production and bioactivities of Ganoderma exopolysaccharides. Carbohydr. Polym. 2022, 294, 119828. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Xu, M.; Liu, J.; Zhao, L.; Li, J.; Chen, L.; Shi, G.; Ding, Z. Carbon starvation drives biosynthesis of high mannose-composition polysaccharides in Ganoderma lucidum. Int. J. Biol. Macromol. 2024, 282, 137168. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Lei, J.; Luo, X.; Wang, S.; Tan, H.; Zhang, J.; Wu, D. Prospects of Ganoderma polysaccharides: Structural features, structure-function relationships, and quality evaluation. Int. J. Biol. Macromol. 2025, 309, 142836. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, X.; Gong, P.; Long, H.; Wang, J.; Yang, W.; Yao, W. Siraitia grosvenorii as a homologue of food and medicine: A review of biological activity, mechanisms of action, synthetic biology, and applications in future food. J. Agric. Food Chem. 2024, 72, 6850–6870. [Google Scholar] [CrossRef] [PubMed]
- Zabotina, O.A.; Zang, N.; Weerts, R. Polysaccharide biosynthesis: Glycosyltransferases and their complexes. Front. Plant Sci. 2021, 12, 625307. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, L.; You, C.; Liu, J.; Chen, L.; Gu, Z.; Shi, G.; Li, J.; Ding, Z. Roles of α-1,3-glucosyltransferase in growth and polysaccharides biosynthesis of Ganoderma lucidum. Int. J. Biol. Macromol. 2024, 276, 134031. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Wu, X.; Tao, T.; Zan, X.; Sun, W.; Mu, D.; Yang, Y.; Wu, D. Functions of a glucan synthase gene GFGLS in mycelial growth and polysaccharide production of Grifola frondosa. J. Agric. Food Chem. 2019, 67, 8875–8883. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, B.-X.; Zhang, J.; Han, N.; Liu, S.-T.; Geng, W.; Jia, S.; Li, Y.; Gan, Q.; Han, P. A newly discovered glycosyltransferase gene UGT88A1 affects growth and polysaccharide synthesis of Grifola frondosa. Appl. Microbiol. Biotechnol. 2024, 108, 12062. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, X.; Zan, X.; Fu, X.; Cui, F.; Zhu, H.; Sun, W.; Tao, T. The β-1,3-glucan synthase gene GFGLS2 plays major roles in mycelial growth and polysaccharide synthesis in Grifola frondosa. Appl. Microbiol. Biotechnol. 2022, 106, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xu, M.; Zhao, L.; Chen, L.; Ding, Z. Novel insights into the mechanism underlying high polysaccharide yield in submerged culture of Ganoderma lucidum revealed by transcriptome and proteome analyses. Microorganisms 2023, 11, 772. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, Y.; Wang, Z.; Xu, J. Improved Production and Rheological Properties of Exopolysaccharides by Co-Overexpression of β-1,3-glucan synthase and UDP-glucose pyrophosphorylase genes in Ganoderma lingzhi. J. Agric. Food Chem. 2025, 73, 8567–8577. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yuan, H.; Li, N.; Xiao, J.; Xu, J. Increased production and anti-senescence activity of exopolysaccharides in Ganoderma lingzhi by co-overexpression of β-1,3-glucan synthase and UDP-glucose pyrophosphorylase. Int. J. Biol. Macromol. 2023, 253, 126778. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, J.; Liu, C.; Zhu, Y.; Nelson, D.R.; Zhou, S.; Li, C.; Wang, L.; Guo, X.; Sun, Y.; et al. Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nat. Commun. 2012, 3, 1923. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, S.K.; Parsad, B.D.; Sahni, S. Rapid Method for Genomic DNA Isolation of Mungbean [Vignar adiata (L.) Wilczek]. Curr. J. Appl. Sci. Technol. 2023, 42, 10–14. [Google Scholar] [CrossRef]
- Fu, X.; Zan, X.; Sun, L.; Tan, M.; Cui, F.; Liang, Y.; Meng, L.; Sun, W. Functional characterization and structural basis of the β-1,3-glucan synthase CMGLS from mushroom Cordyceps militaris. J. Agric. Food Chem. 2022, 70, 8725–8737. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Xu, M.; Wang, Q.; Wang, F.; Zheng, H.; Gu, Z.; Li, Y.; Shi, G.; Ding, Z. Development of an efficient strategy to improve extracellular polysaccharide production of Ganoderma lucidum using L-phenylalanine as an enhancer. Front. Microbiol. 2019, 10, 2306. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Ye, G.; Li, G.; Cao, H.; Wang, Z.; Ji, S. RID serve as a more appropriate measure than phenol sulfuric acid method for natural water-soluble polysaccharides quantification. Carbohydr. Polym. 2022, 278, 118928. [Google Scholar] [CrossRef] [PubMed]
- Deshavath, N.N.; Mukherjee, G.; Goud, V.V.; Veeranki, V.D.; Sastri, C.V. Pitfalls in the 3, 5-dinitrosalicylic acid (DNS) assay for the reducing sugars: Interference of furfural and 5-hydroxymethylfurfural. Int. J. Biol. Macromol. 2020, 156, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cheung, P.C.K. Mushroom dietary fiber from the fruiting body of Pleurotus tuberregium: Fractionation and structural elucidation of nondigestible cell wall components. J. Agric. Food Chem. 2014, 62, 2891–2899. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, J.; Wang, L.; Ding, J.; Zhao, M.; Liu, R. GlPP2C1 Silencing increases the content of Ganoderma lingzhi polysaccharide (GL-PS) and enhances Slt2 phosphorylation. J. Fungi 2022, 8, 949. [Google Scholar] [CrossRef] [PubMed]
- Popolo, L.; Gilardelli, D.; Bonfante, P.; Vai, M. Increase in chitin as an essential response to defects in assembly of cell wall polymers in the ggp1delta mutant of Saccharomyces cerevisiae. J. Bacteriol. 1997, 179, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Wagener, J.; Striegler, K.; Wagener, N. α- and β-1,3-glucan synthesis and remodeling. Curr. Top. Microbiol. Immunol. 2020, 425, 53–82. [Google Scholar]
- Hu, X.; Yang, P.; Chai, C.; Liu, J.; Sun, H.; Wu, Y.; Zhang, M.; Zhang, M.; Liu, X.; Yu, H. Structural and mechanistic insights into fungal ß-1,3-glucan synthase FKS1. Nature 2023, 616, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Suwunnakorn, S.; Wakabayashi, H.; Kordalewska, M.; Perlin David, S.; Rustchenko, E. FKS2 and FKS3 genes of opportunistic human pathogen Candida albicans influence echinocandin susceptibility. Antimicrob. Agents Chemother. 2018, 62, 2299. [Google Scholar] [CrossRef] [PubMed]
- Krull, R.; Wucherpfennig, T.; Esfandabadi, M.E.; Walisko, R.; Melzer, G.; Hempel, D.C.; Kampen, I.; Kwade, A.; Wittmann, C. Characterization and control of fungal morphology for improved production performance in biotechnology. J. Biotechnol. 2013, 163, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Wan-Mohtar, W.A.A.Q.I.; Ab Kadir, S.; Saari, N. The morphology of Ganoderma lucidum mycelium in a repeated-batch fermentation for exopolysaccharide production. Biotechnol. Rep. 2016, 11, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Ost, K.J.; Student, M.; Cord-Landwehr, S.; Moerschbacher, B.M.; Ram, A.F.J.; Dirks-Hofmeister, M.E. Cell walls of filamentous fungi–challenges and opportunities for biotechnology. Appl. Microbiol. Biotechnol. 2025, 109, 125. [Google Scholar] [CrossRef] [PubMed]
- Azi, F.; Wang, Z.; Chen, W.; Lin, D.; Xu, P. Developing Ganoderma lucidum as a next-generation cell factory for food and nutraceuticals. Trends Biotechnol. 2024, 42, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Wang, J.; Long, H.; Yang, W.; Chen, X.; Li, N.; Chen, F.; Zhang, J.; Guo, Y. Edible and medicinal fungi as candidate natural antidepressants: Mechanisms and nutritional implications. Mol. Nutr. Food Res. 2025, 69, 70080. [Google Scholar] [CrossRef] [PubMed]
- Chai, R.; Qiu, C.; Liu, D.; Qi, Y.; Gao, Y.; Shen, J.; Qiu, L. β-glucan synthase gene overexpression and β-glucans overproduction in Pleurotus ostreatus Using Promoter Swapping. PLoS ONE 2013, 8, 61693. [Google Scholar] [CrossRef] [PubMed]
- Garcia Cortes, J.C.; Ramos, M.; Osumi, M.; Perez, P.; Carlos Ribas, J. The cell biology of fission yeast septation. Microbiol. Mol. Biol. Rev. 2016, 80, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Morin, E.; Kohler, A.; Baker, A.R.; Foulongne-Oriol, M.; Lombard, V.; Nagy, L.G.; Ohm, R.A.; Patyshakuliyeva, A.; Brun, A.; Aerts, A.L.; et al. Genome sequence of the button mushroom Agaricus bisporus reveals mechanisms governing adaptation to a humic-rich ecological niche. Proc. Natl. Acad. Sci. USA 2012, 109, 17501–17506. [Google Scholar] [CrossRef] [PubMed]
- Zan, X.; Wu, X.; Cui, F.; Zhu, H.; Sun, W.; Jiang, L.; Tao, T.; Zhao, X. UDP-glucose pyrophosphorylase gene affects mycelia growth and polysaccharide synthesis of Grifola frondosa. Int. J. Biol. Macromol. 2020, 161, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, C.; Pan, J.; Zhou, Y.; Li, H.; Li, W.; Zou, Y. Augmentation of exopolysaccharide synthesis and its influence on biofunctional properties of polysaccharide in Sanghuangporus vaninii via targeted overexpression of phosphoglucomutase. Int. J. Biol. Macromol. 2025, 306, 141182. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, C.M.; Lunn, J.E.; Iglesias, A.A. Nucleotide-sugar metabolism in plants: The legacy of Luis F. Leloir. J. Exp. Bot. 2021, 72, 4053–4067. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Qiao, S.; Xu, Z.; Guan, F.; Ding, Z.; Gu, Z.; Zhang, L.; Shi, G. Effects of culture conditions on monosaccharide composition of Ganoderma lucidum exopolysaccharide and on activities of related enzymes. Carbohydr. Polym. 2015, 133, 104–109. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).