Specific Primers and Nested PCR Find Trichophyton rubrum Missed by Culture of Ground Toenails from Onychomycosis in Podiatric Patients in Eastern Australia
Abstract
1. Introduction
1.1. Onychomycosis, Symptoms, and Cost
1.2. Nail Sampling and Causative Fungi
1.3. Molecular Approaches
1.4. Problem and Aims
2. Materials and Methods
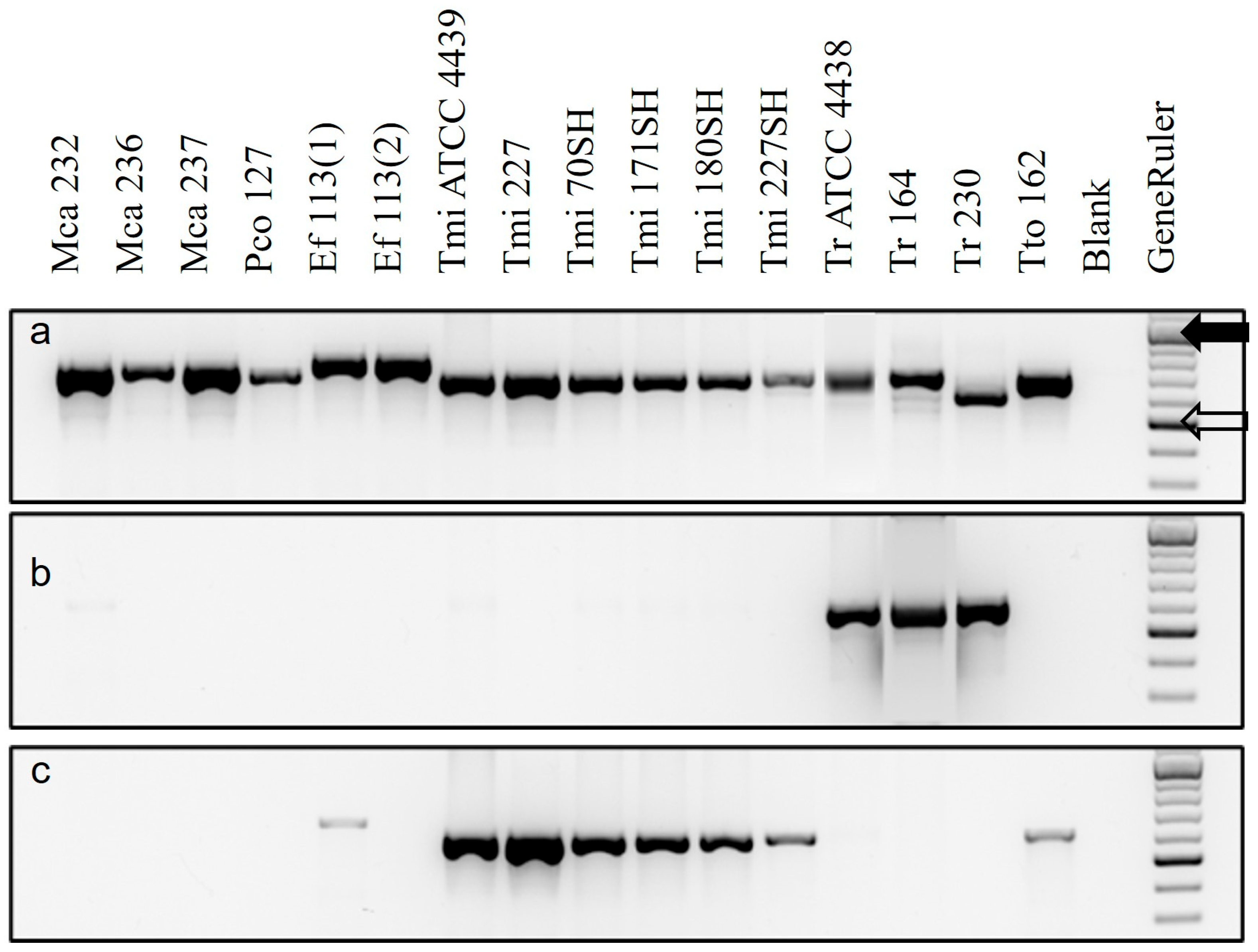
3. Results
3.1. Testing of Specific Primers with Extracts of Pure Cultures
3.2. Use of Specific Primers and Nested PCR with Ground Toenail Extracts
4. Discussion
4.1. Comparison of Methods
4.2. Problems with Trichophyton rubrum Detection
4.3. Interpretation of Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATCC | American Type Culture Collection |
| NCBI | National Center for Biotechnology Information |
| NDM | Non-dermatophyte mould |
| PCR | Polymerase chain reaction |
| RFCC | RMIT Fungal Culture Collection |
References
- CDC (Centers for Disease Control and Prevention). Impact of Fungal Diseases in the US. 2025. Data and Statistics on Fungal Diseases|Fungal Diseases|CDC. Available online: https://www.cdc.gov/fungal/data-research/facts-stats/index.html (accessed on 25 May 2025).
- Gupta, A.K.; Wang, T.; Cooper, E.A.; Lincoln, S.A.; Foreman, H.-C.; Scherer, W.P.; Bakotic, W.L. Clinical diagnosis and laboratory testing of abnormal appearing toenails: A retrospective assessment of confirmatory testing for onychomycosis in the United States, 2022–2023. J. Fungi 2024, 10, 149. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Hajjeh, R.A.; Scher, R.N.; Konnikov, N.; Gupta, A.K.; Summerbell, E.; Sullivan, S.; Daniel, R.; Krusinski, P.; Fleckman, P.; et al. A large-scale North American study of fungal isolates from nails: The frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J. Am. Acad. Dermatol. 2000, 43, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.; Jacobson, G.A.; Narkowicz, C.K.; Peterson, G.M.; Burnet, H.; Sharpe, C. Toenail onychomycosis: An important global disease burden. J. Clin. Pharm. Ther. 2010, 35, 497–519. [Google Scholar] [CrossRef]
- Gupta, A.K.; Stec, N.; Summerbell, R.C.; Shear, N.H.; Piguet, V.; Tosti, A.; Piraccini, B.M. Onychomycosis: A review. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1972–1990. [Google Scholar] [CrossRef]
- Fernández-Torres, B.; Cabañes, F.J.; Carrillo-Muñoz, A.J.; Esteban, A.; Inza, I.; Abarca, L.; Guarro, J. Collaborative evaluation of optimal antifungal susceptibility testing conditions for dermatophytes. J. Clin. Microbiol. 2002, 40, 3999–4003. [Google Scholar] [CrossRef]
- Bishnoi, A.; Vinay, K.; Dogra, S. Emergence of recalcitrant dermatophytosis in India. Lancet Infect. Dis. 2018, 18, 250–251. [Google Scholar] [CrossRef]
- Nenoff, P.; Klonowski, E.; Urlaß, S.; Verma, S.B.; Mayser, P. Clinical picture, causative agents and diagnostics of dermatomycoses. Dermatologie 2023, 74, 974–993. [Google Scholar] [CrossRef]
- English, M.P.; Atkinson, R. An improved method for the isolation of fungi in onychomycosis. Br. J. Dermatol. 1973, 88, 237–241. [Google Scholar] [CrossRef]
- Hainsworth, S.; Hubka, V.; Lawrie, A.C.; Carter, D.; Vanniasinkam, T.; Grando, D. Predominance of Trichophyton interdigitale revealed in podiatric nail dust collections in Eastern Australia. Mycopathologia 2020, 185, 175–185. [Google Scholar] [CrossRef]
- Joyce, A.; Gupta, A.K.; Koenig, L.; Wolcott, R.; Carviel, J. Fungal diversity and onychomycosis: An analysis of 8,816 toenail samples using quantitative PCR and next-generation sequencing. J. Am. Podiatr. Med. Assoc. 2019, 109, 57–63. [Google Scholar] [CrossRef]
- Hainsworth, S.; Lawrie, A.C.; Vanniasinkam, T.; Grando, D. Metagenomics of toenail onychomycosis in three Victorian regions of Australia. J. Fungi 2022, 8, 1198. [Google Scholar] [CrossRef] [PubMed]
- De Hoog, G.S.; Dukik, K.; Monod, M.; Packeu Stubbe, D.; Hendrickx Kupsch, C.; Stielow, J.B.; Freeke, F.; Gőker, M.; Rezaei-Matehkolaei, A.; Mirhendi, H.; et al. Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia 2017, 182, 5–31. [Google Scholar] [CrossRef] [PubMed]
- Nweze, E.I.; Eke, I.E. Dermatophytes and dermatophytosis in the eastern and southern parts of Africa. Med. Mycol. 2018, 56, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.; Goshen, M.; Treidgerman, O.; Ben Zion, K.; Carp, M.-J.; Maisler, N.; Binsky Ehrenreich, I.; Kimchi, A.; Lifshitz, S.; Smollan, G.; et al. Evaluation of multiplex real-time PCR for identifying dermatophytes in clinical samples—A multicentre study. Mycoses 2018, 61, 119–126. [Google Scholar] [CrossRef]
- Nilsson, K.; Friberg, M.; Rollman, O.; Tano, E. Impact of prolonged storage of clinical samples at 4 °C on the recovery of dermatophytes by culture or PCR analysis. J. Mycol. Med. 2019, 29, 1–6. [Google Scholar] [CrossRef]
- Dvořák, M.D.; Hubálek, Z.; Otčenášek, M. Survival of dermatophytes in human skin scales. Arch. Dermatol. 1968, 98, 540–542. [Google Scholar] [CrossRef]
- Sinski, J.T.; Wallis, B.M.; Kelley, L.M. Effect of storage temperature on viability of Trichophyton mentagrophytes in infected guinea pig skin scales. J. Clin. Microbiol. 1979, 10, 841–843. [Google Scholar] [CrossRef]
- Kupsch, C.; Ohst, T.; Pankewitz, F.; Nenoff, P.; Uhrla, S.; Winter, I.; Gräser, Y. The agony of choice in dermatophyte diagnostics–performance of different molecular tests and culture in the detection of Trichophyton rubrum and Trichophyton interdigitale. Clin. Microbiol. Infect. 2016, 22, 735.e11–735.e17. [Google Scholar] [CrossRef]
- Pospischil, I.; Reinhardt, C.; Bontems, O.; Salamin, K.; Fratti, M.; Blanchard, G.; Chang, Y.-T.; Wagner, H.; Hermann, P.; Monod, M.; et al. Identification of dermatophyte and non-dermatophyte agents in onychomycosis by PCR and DNA sequencing—A retrospective comparison of diagnostic tools. J. Fungi 2022, 8, 1019. [Google Scholar] [CrossRef]
- Gupta, A.K.; Cooper, E.A.; Wang, T.; Lincoln, S.A.; Bakotic, W.L. Single-point nail sampling to diagnose onychomycosis caused by non-dermatophyte molds: Utility of polymerase chain reaction (PCR) and histopathology. J. Fungi 2023, 9, 671. [Google Scholar] [CrossRef]
- Brillowska-Dąbrowska, A.; Nielsen, S.S.; Nielsen, H.V.; Arendrup, M.C. Optimized 5-hour multiplex PCR test for the detection of tinea unguium: Performance in a routine PCR laboratory. Med. Mycol. 2010, 48, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, Y.; Satoh, K.; Uchida, T.; Yamada, T.; Abe, M.; Watanabe, S.; Makimura, M.; Makimura, K. Rapid real-time diagnostic PCR for Trichophyton rubrum and Trichophyton mentagrophytes in patients with tinea unguium and tinea pedis using specific fluorescent probes. J. Dermatol. Sci. 2013, 69, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, X.; Yan, Q.; Chen, R.; Shao, L.; Li, R.; Song, Y.; Yuan, X. Detection of pan-dermatophytes and Trichophyton rubrum using recombinase polymerase amplification-lateral flow dipstick assay. Mycopathologia 2024, 190, 6. [Google Scholar] [CrossRef]
- Heckler, I.; Sabalza, M.; Bojmehrani, A.; Venkataraman, I.; Thompson, C. The need for fast and accurate detection of dermatomycosis. Med. Mycol. 2023, 61, myad037. [Google Scholar] [CrossRef]
- Mehlhorn, C.; Uhrlaß, S.; Klonowski, E.; Krueger, C.; Paasch, U.; Simon, J.C.; Nenoff, P. Conventional and molecular diagnostics in onychomycosis-part 2: Molecular identification of causative dermatophytes by polymerase chain reaction and sequence analysis of the internal transcribed spacer region of ribosomal DNA. Dermatologie 2024, 75, 238–252. [Google Scholar] [CrossRef]
- Marin-Maldonado, F.; Pacheco-Torres, A.; Gustafson, E. Comparative analysis of onychomycosis in Puerto Rico using molecular and conventional approaches. J. Med. Mycol. 2023, 33, 101412. [Google Scholar] [CrossRef]
- Uchida, T.; Makimura, K.; Ishihara, K.; Goto, H.; Taiiri, Y.; Okuma, M.; Fujisaki, R.; Uchida, K.; Abe, S.; Iijima, M. Comparative study of direct polymerase chain reaction, microscopic examination and culture-based morphological methods for detection and identification of dermatophytes in nail and skin samples. J. Dermatol. 2019, 36, 202–208. [Google Scholar] [CrossRef]
- Bergmans, A.M.C.; van der Ent, M.; Klaassen, A.; Bohm, N.; Andriesse, G.I.; Wintermans, R.G.F. Evaluation of a single-tube real-time PCR for detection and identification of 11 dermatophyte species in clinical material. Clin. Microbiol. Infect. 2010, 16, 704–710. [Google Scholar] [CrossRef]
- Ebihara, M.; Makimura, K.; Sato, K.; Abe, S.; Tsuboi, R. Molecular detection of dermatophytes and nondermatophytes in onychomycosis by nested polymerase chain reaction based on 28S ribosomal RNA gene sequences. Br. J. Dermatol. 2009, 161, 1038–1044. [Google Scholar] [CrossRef]
- Sánchez, M.J.I.; Pico, A.M.P.; Tejedor, F.M.; Sánchez, M.J.I.; Acevedo, R.M. Using a polymerase chain reaction as complementary test to improve the detection of dermatophyte fungus in nails. J. Am. Podiatr. Med. Assoc. 2014, 104, 233–237. [Google Scholar] [CrossRef]
- Taplin, D.; Zaias, N.; Rebell, G.; Blank, H. Isolation and recognition of dermatophytes on a new medium (DTM). Arch. Dermatol. 1969, 99, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Pérez, D.; Garcia-Oreja, S.; Tardáguila-Garcia, A.; León-Herce, D.; Álvaro-Afonso, F.J.; Lázaro-Martinez, J.L. Microbiological culture combined with PCR for the diagnosis of onychomycosis: Descriptive analysis of 121 patients. Mycoses 2023, 66, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Coloe, S.C.; Baird, R. Dermatophyte infections in Melbourne: Trends from 1961/64 to 2008/09. Australas J. Dermatol. 2010, 51, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Shemer, A.; Daniel, R.; Kassem, R.; Geffen, Y.; Galili, E. Cold sub-atmospheric and atmospheric pressure plasma for the treatment of Trichophyton rubrum onychomycosis: An in-vitro study. Dermatol. Ther. 2020, 33, e14084. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Stevenson, A.; Hamill, P.G.; Dijksterhuis, J.; Hallsworth, J.E. Water-, pH- and temperature relations of germination for the extreme xerophiles Xeromyces bisporus (FRR 0025, Aspergillus penicillioides (JH06THJ) and Eurotium halophilicum (FRR2471). Microb. Biotechnol. 2017, 10, 330–340. [Google Scholar] [CrossRef]
- Sneath, P.H.A.; Sokal, R.R. Numerical Taxonomy; Freeman: San Francisco, CA, USA, 1973. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Jukes, T.H.; Cantor, C.R. Evolution of protein molecules. In Mammalian Protein Metabolism; Munro, H.N., Ed.; Academic Press: New York, NY, USA, 1969; pp. 21–132. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Hainsworth, S. Advances in Studies of Australian Dermatophytes and Tinea Unguium. Ph.D. Thesis, RMIT University, Melbourne, Australia, 2022. [Google Scholar]
- Debuysschere, C.; Blairon, L.; Cupaiolo, R.; Beukinga, I.; Tré-Hardy, M. Clinical evaluation of a dermatophyte RT-PCR assay and its impact on the turn-around-time: A prospective study. Med. Mycol. 2023, 61, myad078. [Google Scholar] [CrossRef]
- Muir, D.; Pritchard, R.C.; Gregory, J.D. Dermatophytes identified at the Australian National Reference Laboratory in Medical Mycology. Pathology 1984, 16, 179–183. [Google Scholar] [CrossRef]
- Ross, I.L.; Weldhagen, G.F.; Kidd, S.E. Detection and identification of dermatophyte fungi in clinical samples using a commercial multiplex tandem PCR assay. Pathology 2020, 52, 473. [Google Scholar] [CrossRef]
- Heikkila, H. Isolation of fungi from onychomycosis-suspected nails by two methods: Clipping and drilling. Mycoses 1996, 39, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, T. Trichophyton rubrum and T. mentagrophytes studied by freeze-etching. Sabouraudia 1975, 13, 241–243. [Google Scholar] [CrossRef] [PubMed]
- Farley, D.L. The viability of ringworm fungi in dry cutaneous material. Arch. Dermatol. Syphilol. 1921, 3, 759–760. [Google Scholar] [CrossRef]
- Bontems, O.; Hauser, P.M.; Monod, M. Evaluation of a polymerase chain reaction-restriction fragment length polymorphism assay for dermatophyte and nondermatophyte identification in onychomycosis. Br. J. Dermatol. 2009, 161, 791–796. [Google Scholar] [CrossRef]
- Nowicka, D.; Nawrot, U.; Wlodarczyk, K.; Pajaczkowska, M.; Patrzalek, A.; Pecak, A.; Mozdyniewicz, P.; Fleischer, M. Detection of dermatophytes in human nail and skin dust produced during podiatric treatments in people without typical clinical signs of mycoses. Mycoses 2016, 59, 379–382. [Google Scholar] [CrossRef]
- Winter, I.; Uhrlaß, S.; Krüger, C.; Herrmann, J.; Bezold, G.; Winter, A.; Barth, S.; Simon, J.C.; Gräser, Y.; Nenoff, P. Molekularbiologischer Direktnachweis von Dermatophyten im klinischen Material bei Verdacht auf Onychomykose und tinea pedis. Hautarzt. 2013, 64, 283–289. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Lu, C. Diagnostic values of ten methods in patients with onychomycosis: A network meta-analysis. Mycoses 2024, 67, e13696. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santosh, A.C.; Grando, D.; Lawrie, A.C. Specific Primers and Nested PCR Find Trichophyton rubrum Missed by Culture of Ground Toenails from Onychomycosis in Podiatric Patients in Eastern Australia. J. Fungi 2025, 11, 520. https://doi.org/10.3390/jof11070520
Santosh AC, Grando D, Lawrie AC. Specific Primers and Nested PCR Find Trichophyton rubrum Missed by Culture of Ground Toenails from Onychomycosis in Podiatric Patients in Eastern Australia. Journal of Fungi. 2025; 11(7):520. https://doi.org/10.3390/jof11070520
Chicago/Turabian StyleSantosh, Anjana C., Danilla Grando, and Ann C. Lawrie. 2025. "Specific Primers and Nested PCR Find Trichophyton rubrum Missed by Culture of Ground Toenails from Onychomycosis in Podiatric Patients in Eastern Australia" Journal of Fungi 11, no. 7: 520. https://doi.org/10.3390/jof11070520
APA StyleSantosh, A. C., Grando, D., & Lawrie, A. C. (2025). Specific Primers and Nested PCR Find Trichophyton rubrum Missed by Culture of Ground Toenails from Onychomycosis in Podiatric Patients in Eastern Australia. Journal of Fungi, 11(7), 520. https://doi.org/10.3390/jof11070520





