Impact of Malt Bagasse Silage on Fungal Diversity, Fusarium Species, and Mycotoxin Contamination Under a Circular Economy Approach to Climate Change Mitigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Silage Conditions
2.2. Morphological and Molecular Identification
2.3. Mycotoxin Quantification
2.4. Statistical Analysis
3. Results
3.1. Climatic Conditions
3.2. Fungal Diversity and Mycotoxin Contamination
3.3. Fusarium Species and Fungal Correlation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-ADON | 3-acetyl deoxynivalenol |
| 15-ADON | 15-acetyl deoxynivalenol |
| AF | aflatoxin |
| aw | water activity |
| CFU | colony forming units |
| CO2 | carbon dioxide |
| FB1 | fumonisin B1 |
| FB2 | fumonisin B2 |
| FDA | Food and Drug Administration |
| DAS | diacetocyscirpenol |
| DON | deoxynivalenol |
| ENSO | El Niño Southern Oscillation |
| MB-PRE | malt bagasse pre-silage treatment |
| MB-POST | malt bagasse post-silage treatment |
| MB + AA-POST | malt bagasse and alfalfa pellets post-silage treatment |
| NIV | nivalenol |
| O2 | oxygen |
| OTA | ochratoxin-A |
| PA | Penicillic acid |
| T-2 | toxin T-2 |
| ZEA | zearalenone |
References
- Gaitán, J.J.; Maestre, F.T.; Bran, D.E.; Buono, G.G.; Dougill, A.J.; Garcia Martinez, G.; Ferrante, D.; Guuroh, R.T.; Linstädter, A.; Massara, V.; et al. Biotic and abiotic drivers of topsoil organic carbon concentration in drylands have similar effects at regional and global scales. Ecosystems 2019, 22, 1445–1456. [Google Scholar] [CrossRef]
- Busso, C.A.; Fernández, O.A. Arid and semiarid rangelands of Argentina. In Climate Variability Impacts on Land Use and Livelihoods in Drylands; Springer: Berlin/Heidelberg, Germany, 2018; pp. 261–291. [Google Scholar]
- Squires, V.R.; Dengler, J.; Feng, H.; Hua, L. Grasslands of the World. Diversity, Management and Conservation; CRC Press: Boca Raton, FL, USA; Taylor&Francis: Abingdon, UK, 2018. [Google Scholar]
- Sun, J.; Wang, Y.; Piao, S.; Liu, M.; Han, G.; Li, J.; Liang, E.; Lee, T.M.; Liu, G.; Wilkes, A.; et al. Toward a sustainable grassland ecosystem worldwide. Innovation 2022, 3, 100265. [Google Scholar] [CrossRef] [PubMed]
- Franciski, M.A.; Peres, E.C.; Godinho, M.; Perondi, D.; Foletto, E.L.; Collazzo, G.C.; Dotto, G.L. Development of CO2 activated biochar from solid wastes of a beer industry and its application for methylene blue adsorption. Waste Manag. 2018, 78, 630–638. [Google Scholar] [CrossRef]
- Silveira-Neto, A.L.S.; Pimentel-Almeida, W.; Niero, G.; Wanderlind, E.H.; Radetski, C.M.; Almerindo, G.I. Application of a biochar produced from malt bagasse as a residue of brewery industry in fixed-bed column adsorption of paracetamol. Chem. Eng. Res. Des. 2023, 194, 779–786. [Google Scholar] [CrossRef]
- Gonçalves, G.D.C.; Nakamura, P.K.; Furtado, D.F.; Veit, M.T. Utilisation of brewery residues to produces granular activated carbon and bio-oil. J. Clean. Prod. 2017, 168, 908–916. [Google Scholar] [CrossRef]
- Conway, J. Statista. Beer Production Worldwide From 1998 to 2022. 2022. Available online: https://es.statista.com/estadisticas/600571/produccion-de-cerveza-a-nivel-mundial-1998/ (accessed on 3 July 2025).
- Ravanal, M.C.; Doussoulin, J.P.; Mougenot, B. Does sustainability matter in the global beer industry? Bibliometrics trends in recycling and the circular economy. Front. Sustain. Food Syst. 2024, 8, 1437910. [Google Scholar] [CrossRef]
- Sugiura, K.; Yamatani, S.; Watahara, M.; Onodera, T. Ecofeed, animal feed produced from recycled food waste. Vet. Ital. 2009, 45, 397–404. [Google Scholar]
- Said, P.P.; Pradhan, C.R. Food grain storage practices: A review. J. Grain Process. Storage 2014, 1, 1–5. [Google Scholar]
- Bartosik, R.; Cardoso, L.; Urcola, H. Silo Bag Storage. In Control and Management of Pests in Stored Products; CRC Press: Boca Raton, FL, USA, 2024; pp. 121–152. [Google Scholar]
- El-Kholy, M.M.; Kamel, R.M. Performance analysis and quality evaluation of wheat storage in horizontal silo bags. Int. J. Food Sci. 2021, 2021, 1248391. [Google Scholar] [CrossRef]
- Garg, M.K.; Sharma, D.K.; Singh, V.K.; Pawar, K.; Kumar, S. Comparative evaluation of quality changes in stored wheat (Triticum aestivum) in hermetic bags and conventional storage methods. In Proceedings of the 10th International Conference on Controlled Atmosphere and Fumigation in Stored Products (CAF2016), New Delhi, India, 6–11 November 2016; pp. 280–286. [Google Scholar]
- Hadidi, M.; Palacios, J.C.O.; McClements, D.J.; Mahfouzi, M.; Moreno, A. Alfalfa as a sustainable source of plant-based food proteins. Trends Food Sci. Technol. 2023, 135, 202–214. [Google Scholar] [CrossRef]
- Srisaikham, S. A comparison of nutritional values, bioactive compounds, amino acids, and antioxidant activities of alfalfa (Medicago sativa) plant and Pellet for use as beneficial material ruminant feed. Walailak J. Sci. Technol. WJST 2021, 18, 10312–10316. [Google Scholar] [CrossRef]
- Trevizán, J.A.C.; Bido, G.D.S.; Ferrari, A.; Felipe, D.F. Nutritional composition of malted barley residue from brewery. J. Manag. Sustain. 2021, 11, 27. [Google Scholar] [CrossRef]
- Magan, N.; Aldred, D. Post-harvest control strategies: Minimising mycotoxins in the food chain. Int. J. Food Microbiol. 2007, 119, 131–139. [Google Scholar] [CrossRef]
- Castellari, C.C.; Marcos Valle, F.J.; Pacin, A.M. Observación de interacciones entre hifas de Fusarium verticillioides, Aspergillus flavus y Talaromyces funiculosus en microcultivos desarrollados en ambientes herméticos. Rev. Argent. De Microbiol. 2019, 51, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Joshaghani, H.; Namjoo, M.; Rostami, M.; Kohsar, F.; Niknejad, F. Mycoflora of fungal contamination in wheat storage (silos) in Golestan Province, North of Iran. Jundishapur J. Microbiol. 2013, 6, 6334. [Google Scholar] [CrossRef]
- Magan, N.; Hope, R.; Cairns, V.; Aldred, D. Post-harvest fungal ecology: Impact of fungal growth and mycotoxin accumulation in stored grain. In Epidemiology of Mycotoxin Producing Fungi: Under the Aegis of COST Action 835 ‘Agriculturally Important Toxigenic Fungi 1998–2003’, EU Project (QLK 1-CT-1998–01380); Springer: Berlin/Heidelberg, Germany, 2003; pp. 723–730. [Google Scholar]
- World Health Organization. Food Safety. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 12 March 2025).
- Desjardins, A.E. Fusarium Mycotoxins: Chemistry, Genetics, and Biology; APS: St. Paul, MN, USA, 2006. [Google Scholar]
- Gregori, R.; Meriggi, P.; Pietri, A.; Formenti, S.; Baccarini, G.; Battilani, P. Dynamics of fungi and related mycotoxins during cereal storage in silo bags. Food Control 2013, 30, 280–287. [Google Scholar] [CrossRef]
- Barreto, A.A.; Abalone, R.; Gastón, A.; Bartosik, R. Analysis of storage conditions of a wheat silo-bag for different weather conditions by computer simulation. Biosyst. Eng. 2013, 116, 497–508. [Google Scholar] [CrossRef]
- SIGA. Sistema de Información y Gestión Agrometereológica del INTA. 2025. Available online: https://siga.inta.gob.ar/#/ (accessed on 12 March 2025).
- ONI. The Oceanic Niño Index. National Weather Service, Climate Prediction Center. 2025. Available online: https://origin.cpc.ncep.noaa.gov/products/analysis_monitoring/ensostuff/ONI_v5.php (accessed on 12 March 2025).
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Atoui, A.; El Khoury, A.; Kallassy, M.; Lebrihi, A. Quantification of Fusarium graminearum and Fusarium culmorum by real-time PCR system and zearalenone assessment in maise. Int. J. Food Microbiol. 2012, 154, 59–65. [Google Scholar] [CrossRef]
- Nogueira, M.S. Fusarium: Producción de Toxinas en Granos de Cebada y su Interacción con Brachypodium. Doctoral Dissertation, Universidad Nacional de La Plata, La Plata, Argentina, 2020. [Google Scholar]
- Patiño, B.; Mirete, S.; González-Jaén, M.T.; Mulé, G.; Rodríguez, M.T.; Vázquez, C. PCR detection assay of fumonisin-producing Fusarium verticillioides strains. J. Food Prot. 2004, 67, 1278–1283. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025; Available online: https://www.R-project.org/ (accessed on 3 July 2025).
- Ripley, B. MASS: Support functions and datasets for Venables and Ripley’s MASS. R Package Version 2011, 7, 3–29. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Christensen, R.H.B.; Singmann, H.; Dai, B.; Grothendieck, G.; Green, P.; Bolker, M.B. Package ‘lme4’. Convergence 2015, 12, 2. [Google Scholar]
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. Performance: An R package for assessment, comparison and testing of statistical models. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Paruelo, J.M.; Beltrán, A.; Jobbágy, E.; Sala, O.E.; Golluscio, R.A. The climate of Patagonia general patterns and controls on biotic processes. Ecol. Austral 1998, 8, 85–101. [Google Scholar]
- Agrios, G.N. Plant Pathology; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Bebber, D.P.; Chaloner, T.M. Specialists, generalists and the shape of the ecological niche in fungi. New Phytol. 2022, 234, 345. [Google Scholar] [CrossRef]
- Blonder, B.; Lamanna, C.; Violle, C.; Enquist, B.J. The n-dimensional hypervolume. Glob. Ecol. Biogeogr. 2014, 23, 595–609. [Google Scholar] [CrossRef]
- Kaur, M.; Bowman, J.P.; Stewart, D.C.; Evans, D.E. The fungal community structure of barley malts from diverse geographical regions correlates with malt quality parameters. Int. J. Food Microbiol. 2015, 215, 71–78. [Google Scholar] [CrossRef]
- Justé, A.; Malfliet, S.; Lenaerts, M.; De Cooman, L.; Aerts, G.; Willems, K.A.; Lievens, B. Microflora during malting of barley: Overview and impact on malt quality. Brew. Sci. 2011, 64, 22–31. [Google Scholar]
- Van Nierop, S.N.E.; Rautenbach, M.; Axcell, B.C.; Cantrell, I.C. The impact of microorganisms on barley and malt quality—A review. J. Am. Soc. Brew. Chem. 2006, 64, 69–78. [Google Scholar] [CrossRef]
- Gonzalez Pereyra, M.L.; Rosa, C.A.R.; Dalcero, A.M.; Cavaglieri, L.R. Mycobiota and mycotoxins in malted barley and brewer’s spent grain from Argentinean breweries. Lett. Appl. Microbiol. 2011, 53, 649–655. [Google Scholar] [CrossRef]
- Ogórek, R.; Lejman, A.; Pusz, W.; Miłuch, A.; Miodyńska, P. Characteristics and taxonomy of Cladosporium fungi. Mikol. Lek. 2012, 19, 80–85. [Google Scholar]
- Walther, G.; Wagner, L.; Kurzai, O. Updates on the taxonomy of Mucorales with an emphasis on clinically important taxa. J. Fungi 2019, 5, 106. [Google Scholar] [CrossRef] [PubMed]
- Briggs, D.E.; McGuinness, G. Microbes on barley grains. J. Inst. Brew. 1993, 99, 249–255. [Google Scholar] [CrossRef]
- Nielsen, L.K.; Cook, D.J.; Edwards, S.G.; Ray, R.V. The prevalence and impact of Fusarium head blight pathogens and mycotoxins on malting barley quality in UK. Int. J. Food Microbiol. 2014, 179, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, K.C.; Běláková, S.; Benešová, K.; Pernica, M.; Savi, G.D.; Rocha, L.O.; Hartman, I.; Čáslavský, J.; Corrêa, B. Fusarium mycotoxins stability during the malting and brewing processes. Toxins 2019, 11, 257. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, M.; Marin, S.; Sanchis, V.; Ramos, A.J. Fusarium mycotoxins in total mixed rations for dairy cows. Mycotoxin Res. 2020, 36, 277–286. [Google Scholar] [CrossRef]
- Perrone, G.; Susca, A. Penicillium species and their associated mycotoxins. Mycotoxigenic Fungi Methods Protoc. 2017, 1542, 107–119. [Google Scholar]
- Ivic, D.; Kovacevik, B.; Vasilj, V.; Idzakovic, N. Occurrence of potentially toxigenic Fusarium verticillioides and low fumonisin B 1 content on barley grain in Bosnia and Herzegovina. J. Appl. Bot. Food Qual. 2012, 84, 121. [Google Scholar]
- Nesci, A.; Barros, G.; Castillo, C.; Etcheverry, M. Soil fungal population in preharvest maise ecosystem in different tillage practices in Argentina. Soil Tillage Res. 2006, 91, 143–149. [Google Scholar] [CrossRef]
- Nogueira, M.S.; Decundo, J.; Martinez, M.; Dieguez, S.N.; Moreyra, F.; Moreno, M.V.; Stenglein, S.A. Natural contamination with mycotoxins produced by Fusarium graminearum and Fusarium poae in malting barley in Argentina. Toxins 2018, 10, 78. [Google Scholar] [CrossRef]
- Tran, M.T.; Ameye, M.; Phan, L.T.K.; Devlieghere, F.; De Saeger, S.; Eeckhout, M.; Audenaert, K. Impact of ethnic pre-harvest practices on the occurrence of Fusarium verticillioides and fumonisin B1 in maise fields from Vietnam. Food Control 2021, 120, 107567. [Google Scholar] [CrossRef]
- Arata, A.F.; Martínez, M.; Castañares, E.; Galizio, R.I.; Fernández, M.D.; Dinolfo, M.I. The richness of Fusarium species in maise tassels and their relationship with Fusarium stalk rot. Eur. J. Plant Pathol. 2024, 168, 351–362. [Google Scholar] [CrossRef]
- Dinolfo, M.I.; Martínez, M.; Castañares, E.; Arata, A.F. Fusarium in maise during harvest and storage: A review of species involved, mycotoxins, and management strategies to reduce contamination. Eur. J. Plant Pathol. 2022, 164, 151–166. [Google Scholar] [CrossRef]
- Martínez, M.; Arata, A.F.; Fernández, M.D.; Stenglein, S.A.; Dinolfo, M.I. Fusarium species richness in mono-and dicotyledonous weeds and their ability to infect barley and wheat. Mycol. Prog. 2021, 20, 1203–1216. [Google Scholar] [CrossRef]
- Jedidi, I.; Mateo, E.M.; Marín, P.; Jiménez, M.; Said, S.; González-Jaén, M.T. Contamination of wheat, barley, and maise seeds with toxigenic Fusarium species and their mycotoxins in Tunisia. J. AOAC Int. 2021, 104, 959–967. [Google Scholar] [CrossRef]
- Marin, S.; Sanchis, V.; Magan, N. Water activity, temperature, and pH effects on growth of Fusarium moniliforme and Fusarium proliferatum isolates from maise. Can. J. Microbiol. 1995, 41, 1063–1070. [Google Scholar] [CrossRef]
- Reid, L.M.; Nicol, R.W.; Ouellet, T.; Savard, M.; Miller, J.D.; Young, J.C.; Stewart, D.W.; Schaafsma, A.W. Interaction of Fusarium graminearum and F. moniliforme in maise ears: Disease progress, fungal biomass, and mycotoxin accumulation. Phytopathology 1999, 89, 1028–1037. [Google Scholar] [CrossRef]
- Barbosa-Cánovas, G.V.; Fontana, A.J., Jr.; Schmidt, S.J.; Labuza, T.P. (Eds.) Water Activity in Foods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- RSA Re de Seguridad Alimentaria-CONICET. Recomendaciones Para la Inclusión del Bagazo Cervecero Seco en el Código Alimentario Argentino (CAA); RSA: Buenos Aires, Argentina, 2021. [Google Scholar]
- Magnoli, A.P.; Poloni, V.L.; Cavaglieri, L. Impact of mycotoxin contamination in the animal feed industry. Curr. Opin. Food Sci. 2019, 29, 99–108. [Google Scholar] [CrossRef]
- Fumagalli, F.; Ottoboni, M.; Pinotti, L.; Cheli, F. Integrated mycotoxin management system in the feed supply chain: Innovative approaches. Toxins 2021, 13, 572. [Google Scholar] [CrossRef]
- Santos Pereira, C.; Cunha, S.C.; Fernandes, J.O. Prevalent mycotoxins in animal feed: Occurrence and analytical methods. Toxins 2019, 11, 290. [Google Scholar] [CrossRef]
- Carlson, M.P.; Biehler, C. Use of Mycotoxin-Contaminated Feed for Animals; Extension bulletin; University of Nebraska, Lincoln: St. Lincoln, NE, USA, 2023. [Google Scholar]
- Asurmendi, P.; Barberis, C.; Dalcero, A.; Pascual, L.; Barberis, L. Survey of Aspergillus section Flavi and aflatoxin B 1 in brewer’s grain used as pig feedstuff in Córdoba, Argentina. Mycotoxin Res. 2013, 29, 3–7. [Google Scholar] [CrossRef]
- Chulze, S.N. Strategies to reduce mycotoxin levels in maize during storage: A review. Food Addit. Contam. 2010, 27, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Hesseltine, C.W. Conditions leading to mycotoxin contamination of foods and feeds. In Mycotoxins and Other Fungal Related Food Problems; ACS Publications: Washington, DC, USA, 1976. [Google Scholar]
- Alonso, V.A.; Pereyra, C.M.; Keller, L.A.M.; Dalcero, A.M.; Rosa, C.A.R.; Chiacchiera, S.M.; Cavaglieri, L.R. Fungi and mycotoxins in silage: An overview. J. Appl. Microbiol. 2013, 115, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Wambacq, E.; Vanhoutte, I.; Audenaert, K.; De Gelder, L.; Haesaert, G. Occurrence, prevention and remediation of toxigenic fungi and mycotoxins in silage: A review. J. Sci. Food Agric. 2016, 96, 2284–2302. [Google Scholar] [CrossRef]
- Conti Taguali, S.; Riolo, M.; Dopazo, V.; Meca, G.; Cacciola, S.O. Characterization of mycotoxins produced by two Fusarium species responsible for postharvest rot of banana fruit. J. Plant Pathol. 2024, 106, 1785–1800. [Google Scholar] [CrossRef]
- Lofgren, L.A.; LeBlanc, N.R.; Certano, A.K.; Nachtigall, J.; LaBine, K.M.; Riddle, J.; Broz, K.; Dong, Y.; Bethan, B.; Kafer, C.W.; et al. Fusarium graminearum: Pathogen or endophyte of North American grasses? New Phytol. 2018, 217, 1203–1212. [Google Scholar] [CrossRef]
- Qu, Z.; Ren, X.; Du, Z.; Hou, J.; Li, Y.; Yao, Y.; An, Y. Fusarium mycotoxins: The major food contaminants. mLife 2024, 3, 176–206. [Google Scholar] [CrossRef]
- Johns, L.E.; Bebber, D.P.; Gurr, S.J.; Brown, N.A. Emerging health threat and cost of Fusarium mycotoxins in European wheat. Nat. Food 2022, 3, 1014–1019. [Google Scholar] [CrossRef]
- Doerr, J.A. A little fresh air: Fungal toxins and silage. In Proceedings of the California Alfalfa and Forage Symposium and Corn/Cereal Silage Mini-Symposium, Visalia, CA, USA, 1–2 December 2010; University of California: Davis, CA, USA, 2010; pp. 117–124. [Google Scholar]
- Laraba, I.; McCormick, S.P.; Vaughan, M.M.; Geiser, D.M.; O’Donnell, K. Phylogenetic diversity, trichothecene potential, and pathogenicity within Fusarium sambucinum species complex. PLoS ONE 2021, 16, e0245037. [Google Scholar] [CrossRef]
- Abalone, R.; Gastón, A.; Bartosik, R.; Cardoso, L.; Rodríguez, J. Gas concentration in the interstitial atmosphere of a wheat silo-bag. Part I: Model development and validation. J. Stored Prod. Res. 2011, 47, 268–275. [Google Scholar] [CrossRef]
- Undi, M.; Wittenberg, K.M.; Holliday, N.J. Occurrence of fungal species in stored alfalfa forage as influenced by moisture content at baling and temperature during storage. Can. J. Anim. Sci. 1997, 77, 95–103. [Google Scholar] [CrossRef]

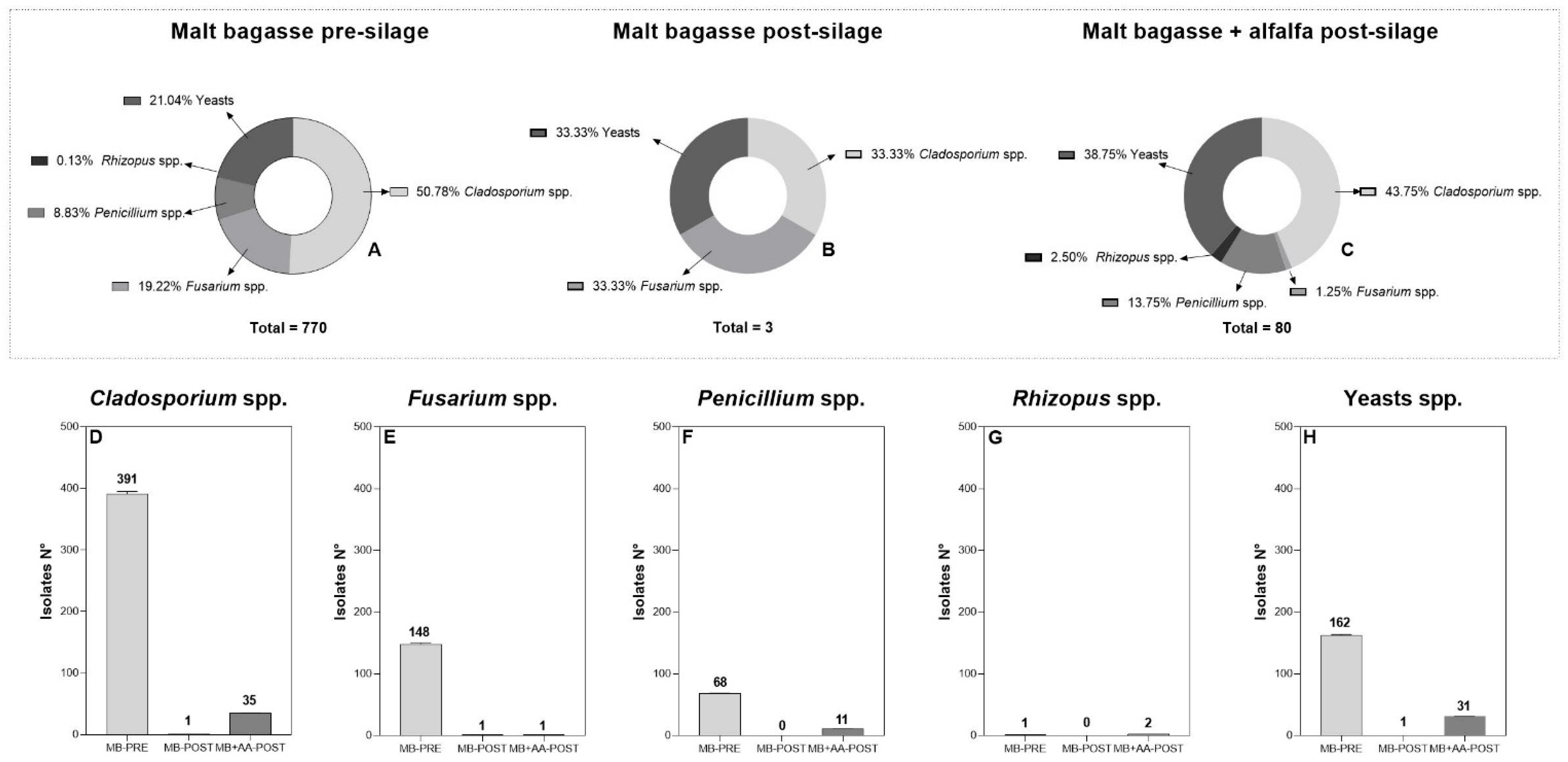
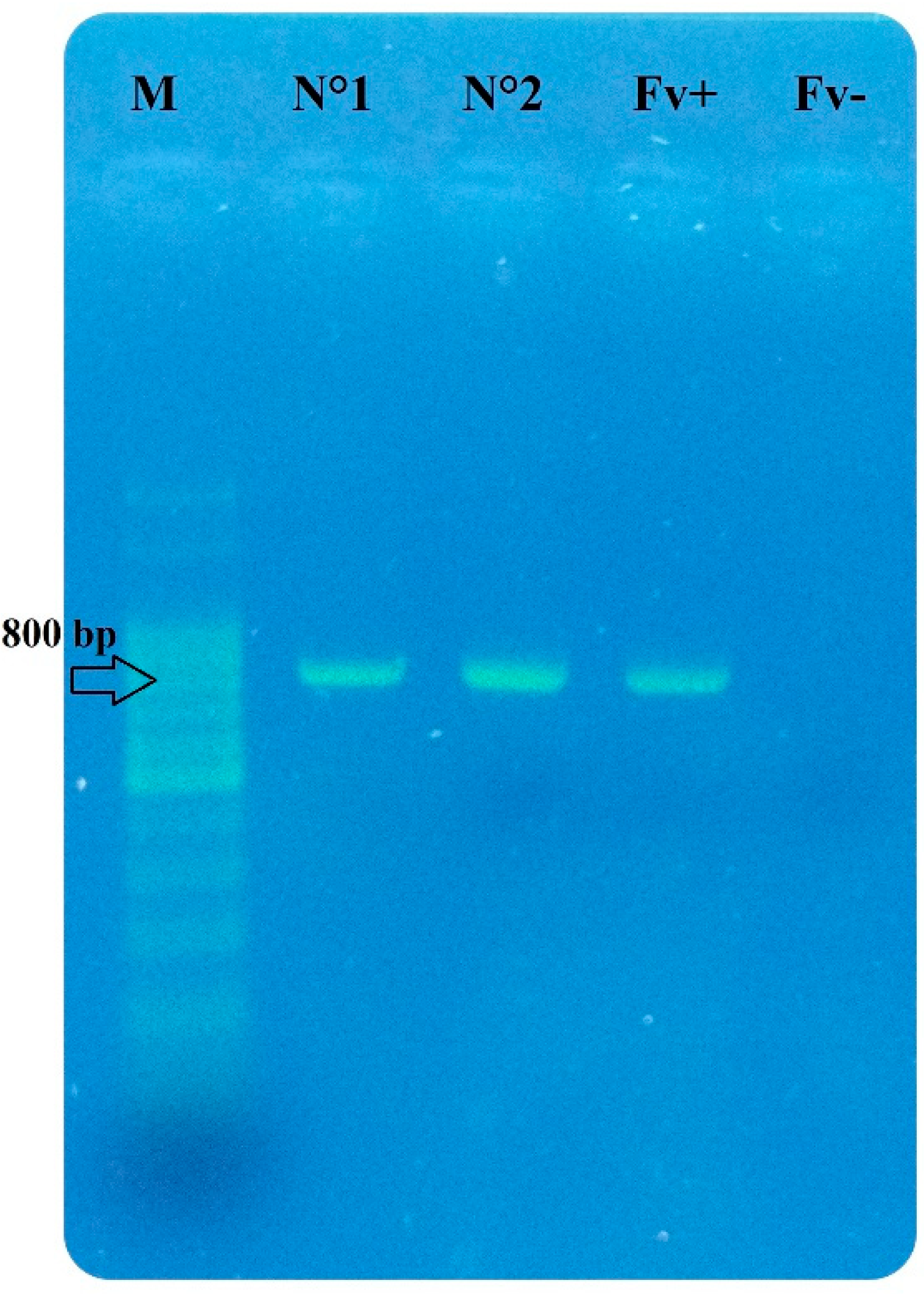
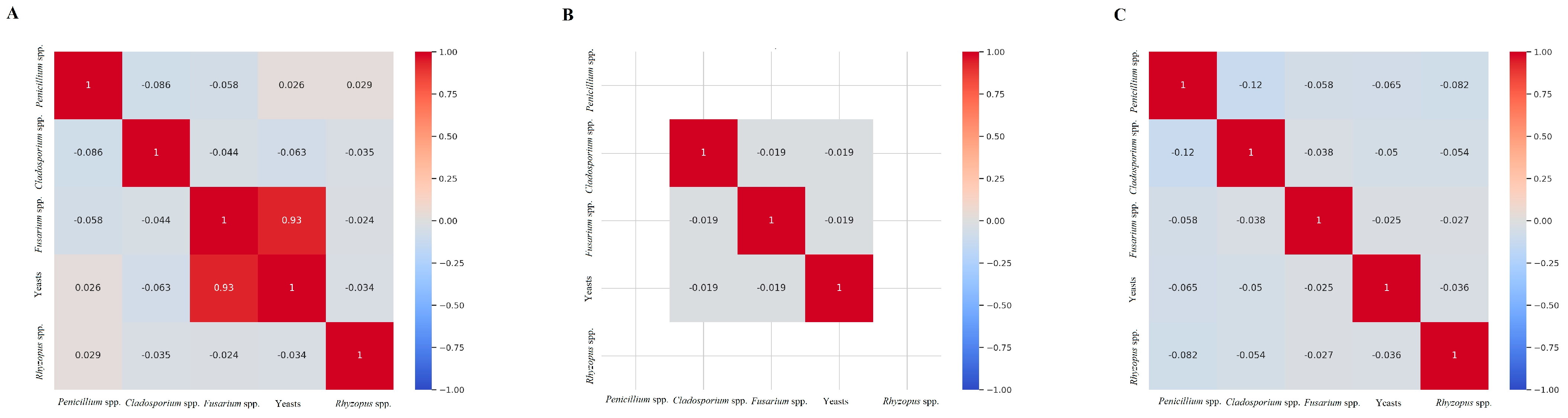
| Treatment | Cladosporium spp. | ||||||
| Without dilution | 10−1 | 10−2 | 10−3 | 10−4 | 10−5 | Total | |
| MB-PRE | 1 | 1 | 356 | 28 | 4 | 1 | 391 |
| MB-POST | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| MB + AA-POST | 0 | 0 | 28 | 3 | 3 | 1 | 35 |
| Fusarium spp. | |||||||
| Without dilution | 10−1 | 10−2 | 10−3 | 10−4 | 10−5 | Total | |
| MB-PRE | 0 | 1 | 8 | 130 | 7 | 2 | 148 |
| MB-POST | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| MB + AA-POST | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Penicillium spp. | |||||||
| Without dilution | 10−1 | 10−2 | 10−3 | 10−4 | 10−5 | Total | |
| MB-PRE | 38 | 23 | 2 | 1 | 1 | 2 | 67 |
| MB-POST | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MB + AA-POST | 5 | 3 | 1 | 1 | 1 | 0 | 11 |
| Rhizopus spp. | |||||||
| Without dilution | 10−1 | 10−2 | 10−3 | 10−4 | 10−5 | Total | |
| MB-PRE | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| MB-POST | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MB + AA-POST | 0 | 0 | 1 | 0 | 0 | 1 | 2 |
| Yeasts | |||||||
| Without dilution | 10−1 | 10−2 | 10−3 | 10−4 | 10−5 | Total | |
| MB-PRE | 24 | 2 | 0 | 126 | 0 | 0 | 152 |
| MB-POST | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| MB + AA-POST | 30 | 1 | 0 | 0 | 0 | 0 | 31 |
| Source of Variation | LR Chisq | Df | Pr (>Chisq) |
|---|---|---|---|
| Treatment (T) | 31.949 | 2 | 1.15 × 10−7 * |
| Fungal genus (F) | 30.069 | 4 | 4.73 × 10−6 * |
| T × F | 12.842 | 8 | 0.1174 |
| Adjusted mean (log-transformed) | |||
| Treatments | Fungal genus | ||
| MB-PRE | 0.1391 ab | Cladosporium spp. | 0.4440 a |
| Yeasts | 0.3170 a | ||
| MB-POST | >0.0001 b | Fusarium spp. | 0.0980 b |
| Penicillium spp. | 0.0007 c | ||
| MB + AA-POST | 1.0678 a | Rhizopus spp. | 0.0001 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valicenti, T.; Manno, C.; Poo, J.I.; Dinolfo, M.I.; Martínez, M.; Enriquez, A. Impact of Malt Bagasse Silage on Fungal Diversity, Fusarium Species, and Mycotoxin Contamination Under a Circular Economy Approach to Climate Change Mitigation. J. Fungi 2025, 11, 505. https://doi.org/10.3390/jof11070505
Valicenti T, Manno C, Poo JI, Dinolfo MI, Martínez M, Enriquez A. Impact of Malt Bagasse Silage on Fungal Diversity, Fusarium Species, and Mycotoxin Contamination Under a Circular Economy Approach to Climate Change Mitigation. Journal of Fungi. 2025; 11(7):505. https://doi.org/10.3390/jof11070505
Chicago/Turabian StyleValicenti, Tania, Carolina Manno, Juan Ignacio Poo, María Inés Dinolfo, Mauro Martínez, and Andrea Enriquez. 2025. "Impact of Malt Bagasse Silage on Fungal Diversity, Fusarium Species, and Mycotoxin Contamination Under a Circular Economy Approach to Climate Change Mitigation" Journal of Fungi 11, no. 7: 505. https://doi.org/10.3390/jof11070505
APA StyleValicenti, T., Manno, C., Poo, J. I., Dinolfo, M. I., Martínez, M., & Enriquez, A. (2025). Impact of Malt Bagasse Silage on Fungal Diversity, Fusarium Species, and Mycotoxin Contamination Under a Circular Economy Approach to Climate Change Mitigation. Journal of Fungi, 11(7), 505. https://doi.org/10.3390/jof11070505








