Assessing Venturia inaequalis Response to Common Fungicides in Morocco
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Strategy
2.2. Pathogen Isolation
2.3. Identification of the Fungal Species: Morphological Observations and Molecular Analysis
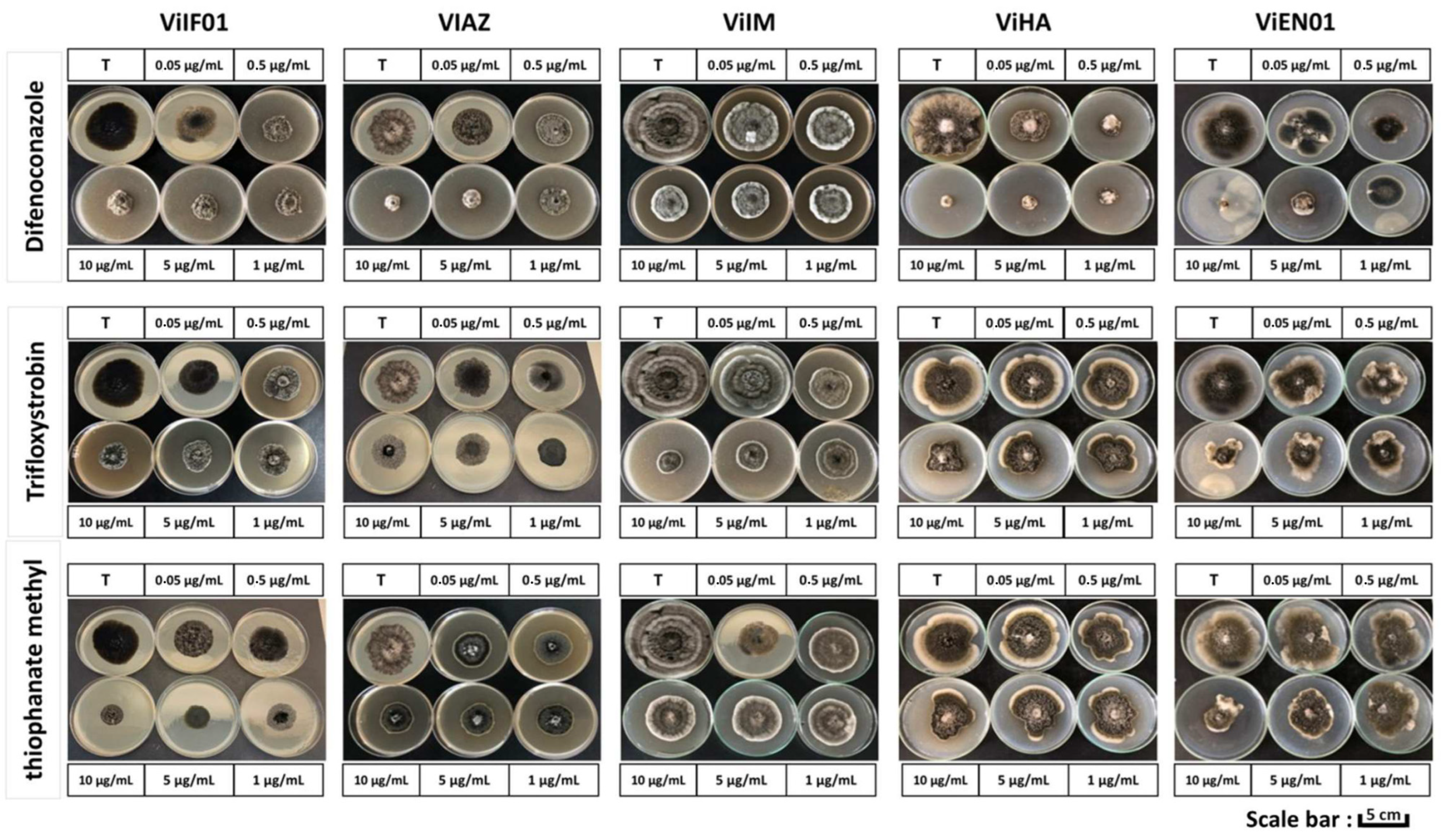
2.4. In Vitro Fungicide Sensitivity Tests
2.5. In Vivo Test of Fungicide Efficacy Against V. inaequalis on Detached Leaves
2.6. Data Analysis
3. Results
3.1. Isolate Identification
3.2. In Vitro Evaluation of Fungicide Effectiveness and EC50 Determination
3.3. In Vivo Evaluation of Fungicide Efficacy on Detached Apple Leaves
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bowen, J.K.; Mesarich, C.H.; Bus, V.G.M.; Beresford, R.M.; Plummer, K.M.; Templeton, M.D. Venturia inaequalis: The causal agent of apple scab. Mol. Plant Pathol. 2011, 12, 105–122. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, E.; Armengol, J.; Rossi, V. Biology and epidemiology of Venturia species affecting fruit crops: A review. Front. Plant Sci. 2017, 8, 1496. [Google Scholar] [CrossRef] [PubMed]
- Jamar, L.; Lefrancq, B.; Lateur, M. Control of apple scab (Venturia inaequalis) with bicarbonate salts under controlled environment bekämpfung Des apfelschorfs (Venturia inaequalis) Mit bicarbonatsalzen unter kontrollierten bedingungen. J. Plant Dis. Prot. 2007, 114, 221–227. [Google Scholar] [CrossRef]
- Polat, Z.; Bayraktar, H. Resistance of Venturia inaequalis to multiple fungicides in turkish apple orchards. J. Phytopathol. 2021, 169, 360–368. [Google Scholar] [CrossRef]
- Tibebu, B.; Boyraz, N. Critical review on apple scab (Venturia inaequalis) biology, epidemiology, economic importance, management and defense mechanisms to the causal agent. J. Plant Physiol. Pathol. 2017, 5. [Google Scholar] [CrossRef]
- Stević, M.; Tamaš, N.; Miletić, N.; Vukša, P. Different toxicity of the strobilurin fungicides kresoxim-methyl and trifloxistrobin to Venturia inaequalis isolates from serbia. J. Environ. Sci. Health B 2015, 50, 633–637. [Google Scholar]
- Lahlali, R.; Moinina, A.; Ezrari, S.; Maclean, D.; Boulif, M. Apple Scab disease severity in the Sais region of Morocco and Its sensitivity to three commercial fungicides. Not. Sci. Biol. 2019, 11, 249–257. [Google Scholar] [CrossRef][Green Version]
- Ma, Z.; Michailides, T.J. Advances in understanding molecular mechanisms of fungicide resistance and molecular detection of resistant genotypes in phytopathogenic fungi. Crop Prot. 2005, 24, 853–863. [Google Scholar] [CrossRef]
- Cordero-Limon, L.; Shaw, M.W.; Passey, T.A.J.; Robinson, J.D.; Xu, X. Cross-resistance between myclobutanil and tebuconazole and the genetic basis of tebuconazole resistance in Venturia inaequalis. Pest. Manag. Sci. 2021, 77, 844–850. [Google Scholar] [CrossRef]
- Lesemann, S.S.; Schimpke, S.; Dunemann, F.; Deising, H.B. Mitochondrial heteroplasmy for the cytochrome b gene controls the level of strobilurin resistance in the Apple powdery mildew fungus podosphaera leucotricha (Ell. & Ev.) E.S. Salmon. J. Plant Dis. Prot. 2006, 113, 259–266. [Google Scholar] [CrossRef]
- FRAC. Pathogen Risk List. September 2019. Available online: https://www.frac.info/media/qluf3unn/frac-pathogen-list-2019.pdf (accessed on 6 February 2024).
- Chapman, K.S.; Sundin, G.W.; Beckerman, J.L. Identification of resistance to multiple fungicides in field populations of Venturia inaequalis. Plant Dis. 2011, 95, 921–926. [Google Scholar] [CrossRef]
- Braun, P.G. Development and decline of a population of Venturia inaequalis resistant to sterol-inhibiting fungicides. Norw. J. Agric. Sci. 1994, 17, 173–184. [Google Scholar]
- Fiaccadori, R. Researches on methodologies to verify reduced sensitivities of Venturia inaequalis in Field to difenoconazole and first indications of a survey in Italy. Am. J. Plant Sci. 2017, 8, 2056–2068. [Google Scholar] [CrossRef]
- Fontaine, S.; Remuson, F.; Fraissinet-Tachet, L.; Micoud, A.; Marmeisse, R.; Melayah, D. Monitoring of Venturia inaequalis harbouring the QoI resistance G143A mutation in French orchards as revealed by PCR assays. Pest. Manag. Sci. 2009, 65, 74–81. [Google Scholar] [CrossRef]
- Jobin, T.; Carisse, O. Incidence of myclobutanil- and kresoxim-methyl-insensitive isolates of Venturia inaequalis in Quebec orchards. Plant Dis. 2007, 91, 1351–1358. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koenraadt, H.; Jones, A.L. The use of allele-specific oligonucleotide probes to characterize resistance to benomyl in field strains of Venturia inaequalis. Am. Phytopathol. Soc. 1992, 82, 1354–1358. [Google Scholar] [CrossRef]
- Marine, S.C.; Schmale, D.G.; Yoder, K.S. Resistance to myclobutanil in populations of Venturia inaequalis in Winchester, Virginia. Plant Health Prog. 2007, 8. [Google Scholar] [CrossRef]
- Mondino, P.; Casanova, L.; Celio, A.; Bentancur, O.; Leoni, C.; Alaniz, S. Sensitivity of Venturia inaequalis to trifloxystrobin and difenoconazole in Uruguay. J. Phytopathol. 2015, 163, 1–10. [Google Scholar] [CrossRef]
- Quello, K.L.; Chapman, K.S.; Beckerman, J.L. In situ detection of benzimidazole resistance in field isolates of Venturia inaequalis in indiana. Plant Dis. 2010, 94, 744–750. [Google Scholar] [CrossRef]
- Köller, W.; Parker, D.M.; Turechek, W.W.; Avila-Adame, C. A Two-phase resistance response of Venturia inaequalis populations to the QoI fungicides kresoxim-methyl and trifloxystrobin. Plant Dis. 2004, 88, 537–544. [Google Scholar] [CrossRef]
- Gouit, S.; Chair, I.; Belabess, Z.; Legrifi, I.; Goura, K.; Tahiri, A.; Lazraq, A.; Lahlali, R. Harnessing Trichoderma spp.: A promising approach to control apple Scab disease. Pathogens 2024, 13, 752. [Google Scholar] [CrossRef] [PubMed]
- Oukabli, A. Le Pommier: Une culture de terroir En zones d’altitude. Transf. Techmol. 2018, 523, 77–80. [Google Scholar]
- Moinina, A.; Lahlali, R.; Maclean, D.; Boulif, M. Farmers’ Knowledge, Perception and practices in apple pest management and climate change in the Fes-Meknes region, Morocco. Horticulturae 2018, 4, 42. [Google Scholar] [CrossRef]
- Office National de Sécurité Sanitaire des Produits Alimentaires (ONSSA). Phytosanitary Index; ONSSA: Rabat, Morocco, 2024. Available online: https://eservice.onssa.gov.ma/IndPesticide.aspx (accessed on 6 February 2024).
- Bora, T.; Karaca, I. Measurement of Disease and Injury in Cultivated Plants; Assistant Textbook; Ege University: Bornova, Türkiye, 1970; No. 167. [Google Scholar]
- Kavak, H.; Celik, A. Comparison of some morphological and physiological characters of Apple Scab pathogen (Venturia inaequalis) in two different agricultural ecology of turkey. Erwerbs-Obstbau 2021, 63, 47–52. [Google Scholar] [CrossRef]
- Nicholson, R.; Scoyoc, S.; Kuc, J.; Williams, E. Response of Detached Apple leaves to Venturia inaequalis. Phytopathology 1973, 63, 649–650. [Google Scholar] [CrossRef]
- Olivier, J.; Lespinasse, Y. Study of the efficacy of fungicides against scab after contamination of apple in the glasshouse. I. Method. of study on young seedlings. Phytiatr.-Phytopharm. 1980, 29, 13–22. [Google Scholar] [CrossRef]
- Doyle, J. DNA protocols for plants. In Molecular Techniques in Taxonomy; Springer: Berlin/Heidelberg, Germany, 1991; pp. 283–293. [Google Scholar] [CrossRef]
- White, J.F., Jr.; Morrow, A.C.; Morgan-Jones, G. Endophyte-host associations in forage grasses. XII. A fungal endophyte of Trichachne Insularis belonging to Pseudocercosporella. Mycologia 1990, 82, 218–226. [Google Scholar] [CrossRef]
- Parisi, L.; Guillaumes, J.; Olivier, J.; Gaudin, J. Variabilité de l’efficacité curative d’inhibiteurs de La biosynthèse des stérols Vis-à-Vis de Venturia inaequalis. Agronomie 1990, 10, 573–579. [Google Scholar] [CrossRef]
- Chevalier, M.; Lespinasse, Y.; Renaudin, S. A Microscopic study of the different classes of symptoms coded by the Vf gene in Apple for resistance to Scab (Venturia inaequalis). Plant Pathol. 1991, 40, 249–256. [Google Scholar] [CrossRef]
- Yepes, L.M.; Aldwinckle, H.S. Selection of resistance to Venturia Inaequalis using detached leaves from in vitro-grown Apple shoots. Plant Sci. 1993, 93, 211–216. [Google Scholar] [CrossRef]
- Calenge, F.; Faure, A.; Goerre, M.; Gebhardt, C.; Van de Weg, W.E.; Parisi, L.; Durel, C.-E. Genetics and resistance Quantitative Trait Loci (QTL) analysis reveals both broad-spectrum and isolate-specific QTL for Scab resistance in an Apple progeny challenged with eight isolates of Venturia inaequalis. Phytopathology 2004, 94, 370–379. [Google Scholar] [CrossRef]
- Tehon, L.R.; Stout, G.L. Epidemic diseases of fruit trees in Illinois 1922–1928. In Illinois Natural History Survey Bulletin; IOPN: London, UK, 1930. [Google Scholar]
- Russell, P.E. Sensitivity Baselines in Fungicide Resistance Research and Management, FRAC Monograph No. 3; Fungicide Resistance Action Committee (FRAC): Brussels, Belgium, 2004; Available online: https://www.frac.info/media/2djnn1f1/monograph-3.pdf (accessed on 27 May 2025).
- Roig, E.; Neumann, P.; Simon, J. Growth and isoenzyme comparison of five isolates of Venturia inaequalis. Phytoprotection 1990, 71, 65. [Google Scholar] [CrossRef]
- Kollar, A. Present research on the most important pathogen of apple, the Apple Scab fungus Venturia inaequalis. Nachrichtenbl. Deut. Pflanzenschutzd. 1997, 49, 131–136. [Google Scholar] [CrossRef]
- Yaegashi, H.; Hirayama, K.; Akahira, T.; Ito, T. Point mutation in CYP51A1 of Venturia inaequalis is associated with low sensitivity to sterol demethylation Inhibitors. J. Gen. Plant Pathol. 2020, 86, 245–249. [Google Scholar] [CrossRef]
- Mubashir, S.S.; Bhat, Z.A.; Bhat, M.A.; Masoodi, K.Z.; Shafi, F.; Mukhtar, M.; Nargis, S.; Farhana, W. Profiling Difenoconazole and Flusilazole Resistance, Fitness Penalty and Phenotypic Stability in Venturia inaequalis. Sci. Rep. 2025, 15, 4855. [Google Scholar] [CrossRef] [PubMed]
- Nabi, A.; Ahmad, M.; Shah, M.D.; Padder, B.A.; Dar, M.S.; Banday, S. First report of myclobutanil resistance and shift in sensitivity to difenoconazole and flusilazole in north-western himalyan Venturia inaequalis populations. Australas. Plant Pathol. 2023, 52, 13–22. [Google Scholar] [CrossRef]
- Stević, M.; Vukša, P.; Elezović, I. Resistance of Venturia inaequalis to demethylation inhibiting (DMI) fungicides. Zemdirbyste 2010, 97, 65–72. [Google Scholar]
- Villani, S.M.; Hulvey, J.; Hily, J.M.; Cox, K.D. Overexpression of the CYP51A1 gene and repeated elements Are associated with differential sensitivity to DMI fungicides in Venturia inaequalis. Am. Phytopath Soc. 2016, 106, 562–571. [Google Scholar] [CrossRef]
- Tziros, G.T.; Ainalidou, A.; Samaras, A.; Kollaros, M.; Karamanoli, K.; Menkissoglu-Spiroudi, U.; Karaoglanidis, G.S. Differences in defence-related gene expression and metabolite accumulation reveal insights into the resistance of greek grape wine cultivars to Botrytis Bunch Rot. OENO One 2022, 56, 111–123. [Google Scholar] [CrossRef]
- Friederike, M.-J.; Thekla, T.; Andreas, M. Molecular Genetic Detection of Mutations Conferring QoI Resistance in Venturia inaequalis via Pyrosequencing; Fungicide Resistance Action Committee and Bayer AG: Monheim, Germany, 2016; Available online: https://www.frac.info/media/w5ant4aa/ventin-pyro-monitoring-method-bayer-20162bf42b2c512362eb9a1eff00004acf5d.pdf (accessed on 28 May 2025).
- Theodorides, V.; Scott, G.; Laderman, M. Strains of haemonchus contortus resistant against benzimidazole anthelmintics. Am. J. Vet. Res. 1970, 31, 859–864. [Google Scholar] [CrossRef]
- Young, J.R.; Tomaso-Peterson, M.; De La Cerda, K.; Wong, F.P. Two mutations in β-Tubulin 2 gene associated with thiophanate-methyl resistance in Colletotrichum cereale isolates from creeping bentgrass in mississippi and alabama. Plant Dis. 2010, 94, 207–212. [Google Scholar] [CrossRef] [PubMed][Green Version]


| Isolate Code | Province | GPS Coordinates of the Orchard | Apple Production Systems |
|---|---|---|---|
| ViIF01 | Ifrane | 33°36′35″ N 5°9′45″ W | Commercial orchard |
| ViAZ | Azrou | 33°22′11″ N 5°23′25″ W | Commercial orchard |
| ViIM | Immouzer | 33°46′4″ N 5°0′58″ W | Commercial orchard |
| ViHA | EL Hajeb | 33°49′49″ N 5°9′14″ W | Commercial orchard |
| ViEN01 | Meknes | 33°50′34.70″ N, 5°28′35.22″ W | An untreated experimental orchard |
| Fungicide | Concentration (µg/mL) | ViIF01 | ViAZ | ViIM | ViHA | ViEN01 |
|---|---|---|---|---|---|---|
| Difenoconazole | 0.05 | 38.96 ± 2.60 b | 20.19 ± 2.79 c | 20.25 ± 3.08 c | 50.33 ± 1.67 a | 24.32 ± 3.46 c |
| 0.5 | 49.80 ± 2.93 b | 45.67 ± 2.38 b | 35.70 ± 2.56 c | 67.56 ± 1.54 a | 54.05 ± 3.10 b | |
| 1.0 | 53.14 ± 2.71 b | 56.46 ± 3.28 b | 40.25 ± 2.64 c | 74.75 ± 3.17 a | 64.19 ± 2.42 b | |
| 5.0 | 62.51 ± 2.59 b | 80.40 ± 3.43 a | 62.66 ± 3.35 b | 81.44 ± 3.06 a | 77.03 ± 3.06 a | |
| 10.0 | 71.45 ± 2.35 b | 93.69 ± 2.27 a | 74.37 ± 1.64 b | 91.47 ± 3.24 a | 94.59 ± 1.74 a | |
| Trifloxystrobin | 0.05 | 6.18 ± 2.78 c | 19.68 ± 2.03 b | 13.24 ± 2.74 bc | 11.39 ± 2.22 bc | 18.92 ± 2.84 b |
| 0.5 | 28.37 ± 1.79 b | 38.77 ± 3.05 a | 27.30 ± 2.72 b | 14.56 ± 2.37 c | 22.30 ± 1.92 bc | |
| 1.0 | 41.14 ± 3.39 a | 48.82 ± 2.41 a | 31.32 ± 2.73 b | 28.48 ± 2.90 b | 34.46 ± 1.76 b | |
| 5.0 | 56.56 ± 2.54 a | 50.43 ± 2.64 a | 38.17 ± 3.39 b | 43.99 ± 1.62 b | 36.49 ± 2.13 b | |
| 10.0 | 62.79 ± 2.33 a | 53.39 ± 1.54 a | 42.26 ± 2.86 b | 50.32 ± 2.83 a | 54.73 ± 2.23 a | |
| Thiophanate-methyl | 0.05 | 8.61 ± 2.64 c | 9.92 ± 1.82 c | 6.01 ± 1.82 c | 16.46 ± 2.24 b | 8.11 ± 3.45 c |
| 0.5 | 11.77 ± 2.38 bc | 17.49 ± 2.81 b | 9.28 ± 1.72 c | 24.68 ± 3.14 a | 18.92 ± 2.44 b | |
| 1.0 | 24.93 ± 3.48 a | 18.86 ± 2.01 b | 14.77 ± 2.81 bc | 27.22 ± 1.69 a | 22.30 ± 3.45 b | |
| 5.0 | 35.61 ± 1.70 a | 21.61 ± 2.43 b | 26.90 ± 1.78 ab | 34.81 ± 3.18 a | 28.38 ± 2.71 ab | |
| 10.0 | 54.30 ± 1.92 a | 25.74 ± 1.99 c | 35.13 ± 1.89 b | 38.61 ± 1.69 b | 32.43 ± 2.98 b |
| Isolate | Difenoconazole EC50 ± SD (Group) | Status | Trifloxystrobin EC50 ± SD (Group) | Status | Thiophanate-Methyl EC50 ± SD (Group) | Status |
|---|---|---|---|---|---|---|
| ViIF01 | 0.43 ± 0.04 (bc) | R | 2.94 ± 0.29 (a) | R | 14.84 ± 1.48 (a) | MR |
| ViAZ | 0.51 ± 0.05 (c) | R | 3.60 ± 0.36 (b) | R | 1237.20 ± 12.37 (e) | HR |
| ViIM | 1.46 ± 0.15 (d) | R | 29.62 ± 2.96 (e) | R | 132.22 ± 13.22 (c) | HR |
| ViHA | 0.05 ± 0.01 (a) | S | 12.56 ± 1.26 (c) | R | 126.66 ± 12.67 (b) | HR |
| ViEN01 | 0.36 ± 0.04 (b) | R | 15.17 ± 1.52 (d) | R | 193.51 ± 19.35 (d) | HR |
| Isolate | Fungicide | Concentration (µg/mL) | Preventive Severity (%) (Mean ± SD, Group) | Preventive Efficacy (%) (Mean ± SD, Group) | Curative Severity (%) (Mean ± SD, Group) | Curative Efficacy (%) (Mean ± SD, Group) |
|---|---|---|---|---|---|---|
| ViEN01 | Difenoconazole | 0.00 (Control) | 99.75 ± 0.5 (d) | 0.0 ± 0.0 (d) | 98.13 ± 0.5 (d) | 0.0 ± 0.0 (d) |
| 0.05 | 57.40 ± 2.1 (c) | 41.5 ± 2.1 (c) | 81.76 ± 3.0 (c) | 16.7 ± 3.1 (c) | ||
| 0.5 | 48.98 ± 1.8 (bc) | 50.1 ± 1.8 (bc) | 74.54 ± 2.5 (bc) | 24.0 ± 2.5 (bc) | ||
| 1 | 32.56 ± 1.5 (ab) | 66.8 ± 1.5 (ab) | 64.18 ± 2.8 (ab) | 34.6 ± 2.9 (ab) | ||
| 5 | 21.67 ± 1.2 (a) | 77.9 ± 1.2 (a) | 43.49 ± 1.9 (a) | 55.7 ± 1.9 (a) | ||
| 10 | 14.20 ± 0.9 (a) | 85.5 ± 0.9 (a) | 27.16 ± 1.4 (a) | 72.3 ± 1.4 (a) | ||
| Trifloxystrobin | 0.00 (Control) | 98.13 ± SD (d) | 0.0 ± 0.0 (d) | 98.13 ± SD (d) | 0.0 ± 0.0 (d) | |
| 0.05 | 86.64 ± SD (c) | 11.7 ± SD (c) | 90.86 ± SD (c) | 7.4 ± SD (c) | ||
| 0.5 | 77.87 ± SD (bc) | 20.6 ± SD (bc) | 83.56 ± SD (bc) | 14.8 ± SD (bc) | ||
| 1 | 76.09 ± SD (bc) | 22.5 ± SD (bc) | 78.21 ± SD (bc) | 20.3 ± SD (bc) | ||
| 5 | 66.57 ± SD (b) | 32.1 ± SD (b) | 75.42 ± SD (b) | 23.1 ± SD (b) | ||
| 10 | 48.90 ± SD (a) | 50.2 ± SD (a) | 59.55 ± SD (a) | 39.3 ± SD (a) | ||
| Thiophanate-methyl | 0.00 (Control) | 98.13 ± 2.0 (d) | 0.0 ± 2.0 (d) | 98.13 ± 2.0 (d) | 0.0 ± 2.0 (d) | |
| 0.05 | 85.45 ± 2.0 (c) | 12.9 ± 2.0 (c) | 87.24 ± 2.0 (c) | 11.1 ± 2.0 (c) | ||
| 0.5 | 80.60 ± 2.0 (bc) | 17.9 ± 2.0 (bc) | 84.11 ± 2.0 (bc) | 14.3 ± 2.0 (bc) | ||
| 1 | 74.61 ± 2.0 (b) | 23.9 ± 2.0 (b) | 80.91 ± 2.0 (b) | 17.5 ± 2.0 (b) | ||
| 5 | 55.54 ± 2.0 (a) | 43.4 ± 2.0 (a) | 79.95 ± 2.0 (a) | 18.5 ± 2.0 (a) | ||
| 10 | 57.41 ± 2.0 (a) | 41.5 ± 2.0 (a) | 75.78 ± 2.0 (a) | 22.7 ± 2.0 (a) | ||
| ViIF01 | Difenoconazole | 0.00 (Control) | 98.81 ± 0.5 (d) | 0.0 ± 0.0 (d) | 98.81 ± 0.5 (d) | 0.0 ± 0.0 (d) |
| 0.05 | 85.19 ± 2.1 (c) | 13.8 ± 2.1 (c) | 90.26 ± 3.0 (c) | 8.6 ± 3.1 (c) | ||
| 0.5 | 71.30 ± 1.8 (bc) | 27.8 ± 1.8 (bc) | 83.15 ± 2.5 (bc) | 15.8 ± 2.5 (bc) | ||
| 1 | 61.08 ± 1.5 (ab) | 38.2 ± 1.5 (ab) | 70.72 ± 2.8 (ab) | 28.4 ± 2.9 (ab) | ||
| 5 | 59.10 ± 1.2 (a) | 40.2 ± 1.2 (a) | 63.01 ± 1.9 (a) | 36.2 ± 1.9 (a) | ||
| 10 | 46.88 ± 0.9 (a) | 52.5 ± 0.9 (a) | 51.42 ± 1.4 (a) | 47.9 ± 1.4 (a) | ||
| Trifloxystrobin | 0.00 (Control) | 98.81 ± 0.5 (d) | 0.0 ± 0.0 (d) | 98.81 ± 0.5 (d) | 0.0 ± 0.0 (d) | |
| 0.05 | 87.67 ± 2.1 (c) | 11.3 ± 2.1 (c) | 96.21 ± 3.0 (c) | 2.6 ± 3.1 (c) | ||
| 0.5 | 80.88 ± 1.8 (bc) | 18.1 ± 1.8 (bc) | 88.69 ± 2.5 (bc) | 10.2 ± 2.5 (bc) | ||
| 1 | 71.96 ± 1.5 (ab) | 27.2 ± 1.5 (ab) | 74.58 ± 2.8 (ab) | 24.5 ± 2.9 (ab) | ||
| 5 | 65.87 ± 1.2 (a) | 33.3 ± 1.2 (a) | 69.00 ± 1.9 (a) | 30.2 ± 1.9 (a) | ||
| 10 | 44.94 ± 0.9 (a) | 54.5 ± 0.9 (a) | 56.72 ± 1.4 (a) | 42.6 ± 1.4 (a) | ||
| Thiophanate-methyl | 0.00 (Control) | 98.81 ± 0.5 (d) | 0.0 ± 0.0 (d) | 98.81 ± 0.5 (d) | 0.0 ± 0.0 (d) | |
| 0.05 | 88.86 ± 2.1 (c) | 10.1 ± 2.1 (c) | 97.75 ± 3.0 (c) | 1.1 ± 3.1 (c) | ||
| 0.5 | 84.47 ± 1.8 (bc) | 14.5 ± 1.8 (bc) | 89.90 ± 2.5 (bc) | 9.0 ± 2.5 (bc) | ||
| 1 | 76.06 ± 1.5 (ab) | 22.9 ± 1.5 (ab) | 80.54 ± 2.8 (ab) | 18.5 ± 2.9 (ab) | ||
| 5 | 61.70 ± 1.2 (a) | 37.6 ± 1.2 (a) | 71.36 ± 1.9 (a) | 27.7 ± 1.9 (a) | ||
| 10 | 55.44 ± 0.9 (a) | 43.9 ± 0.9 (a) | 62.70 ± 1.4 (a) | 36.5 ± 1.4 (a) | ||
| ViAZ | Difenoconazole | 0.00 (Control) | 99.75 ± 0.5 (d) | 0.0 ± 0.0 (d) | 99.75 ± 0.5 (d) | 0.0 ± 0.0 (d) |
| 0.05 | 87.87 ± 1.8 (c) | 11.9 ± 1.8 (c) | 88.67 ± 2.0 (c) | 11.1 ± 2.0 (c) | ||
| 0.5 | 66.10 ± 1.5 (bc) | 33.7 ± 1.5 (bc) | 76.20 ± 1.9 (bc) | 23.6 ± 1.9 (bc) | ||
| 1 | 45.89 ± 1.2 (ab) | 54.0 ± 1.2 (ab) | 54.09 ± 1.5 (ab) | 45.7 ± 1.5 (ab) | ||
| 5 | 40.01 ± 0.9 (a) | 59.9 ± 0.9 (a) | 40.31 ± 1.0 (a) | 59.6 ± 1.0 (a) | ||
| 10 | 27.97 ± 0.7 (a) | 71.9 ± 0.7 (a) | 39.04 ± 0.8 (a) | 60.9 ± 0.8 (a) | ||
| Trifloxystrobin | 0.00 (Control) | 99.75 ± 0.5 (d) | 0.0 ± 0.0 (d) | 99.75 ± 0.5 (d) | 0.0 ± 0.0 (d) | |
| 0.05 | 94.86 ± 2.0 (c) | 4.9 ± 2.0 (c) | 92.58 ± 2.2 (c) | 7.2 ± 2.2 (c) | ||
| 0.5 | 86.76 ± 1.8 (bc) | 13.0 ± 1.8 (bc) | 83.86 ± 1.9 (bc) | 15.9 ± 1.9 (bc) | ||
| 1 | 78.03 ± 1.5 (ab) | 21.7 ± 1.5 (ab) | 69.07 ± 1.6 (ab) | 30.7 ± 1.6 (ab) | ||
| 5 | 75.00 ± 1.2 (a) | 24.8 ± 1.2 (a) | 55.93 ± 1.3 (a) | 43.9 ± 1.3 (a) | ||
| 10 | 68.76 ± 0.9 (a) | 31.1 ± 0.9 (a) | 50.18 ± 1.0 (a) | 49.7 ± 1.0 (a) | ||
| Thiophanate-methyl | 0.00 (Control) | 99.75 ± 0.5 (d) | 0.0 ± 0.0 (d) | 99.75 ± 0.5 (d) | 0.0 ± 0.0 (d) | |
| 0.05 | 90.00 ± 2.0 (c) | 9.8 ± 2.0 (c) | 95.49 ± 2.2 (c) | 4.3 ± 2.2 (c) | ||
| 0.5 | 89.84 ± 1.8 (bc) | 10.0 ± 1.8 (bc) | 92.83 ± 1.9 (bc) | 6.9 ± 1.9 (bc) | ||
| 1 | 85.87 ± 1.5 (ab) | 13.9 ± 1.5 (ab) | 87.22 ± 1.6 (ab) | 12.5 ± 1.6 (ab) | ||
| 5 | 79.67 ± 1.2 (a) | 20.1 ± 1.2 (a) | 80.09 ± 1.3 (a) | 19.7 ± 1.3 (a) | ||
| 10 | 77.33 ± 0.9 (a) | 22.5 ± 0.9 (a) | 79.86 ± 1.0 (a) | 19.9 ± 1.0 (a) | ||
| ViIM | Difenoconazole | 0.00 (Control) | 98.88 ± 0.5 (d) | 0.0 ± 0.0 (d) | 98.88 ± 0.5 (d) | 0.0 ± 0.0 (d) |
| 0.05 | 85.07 ± 2.0 (c) | 13.9 ± 2.0 (c) | 90.00 ± 3.0 (c) | 8.9 ± 3.1 (c) | ||
| 0.5 | 79.10 ± 1.8 (bc) | 20.0 ± 1.8 (bc) | 82.73 ± 2.5 (bc) | 16.3 ± 2.5 (bc) | ||
| 1 | 67.08 ± 1.5 (ab) | 32.1 ± 1.5 (ab) | 74.54 ± 2.8 (ab) | 24.6 ± 2.9 (ab) | ||
| 5 | 52.13 ± 1.2 (a) | 47.3 ± 1.2 (a) | 61.08 ± 1.9 (a) | 38.2 ± 1.9 (a) | ||
| 10 | 38.87 ± 0.9 (a) | 60.7 ± 0.9 (a) | 48.66 ± 1.4 (a) | 50.8 ± 1.4 (a) | ||
| Trifloxystrobin | 0.00 (Control) | 98.88 ± 4.94 (d) | 0.0 ± 0.0 (d) | 98.88 ± 4.94 (d) | 0.0 ± 0.0 (d) | |
| 0.05 | 90.00 ± 4.50 (c) | 9.0 ± 4.5 (c) | 84.77 ± 4.24 (c) | 14.2 ± 4.2 (c) | ||
| 0.5 | 85.89 ± 4.29 (bc) | 13.1 ± 4.3 (bc) | 75.00 ± 3.75 (bc) | 24.1 ± 3.8 (bc) | ||
| 1 | 79.65 ± 3.98 (ab) | 19.5 ± 4.0 (ab) | 70.01 ± 3.50 (ab) | 29.2 ± 3.5 (ab) | ||
| 5 | 73.43 ± 3.67 (a) | 25.7 ± 3.7 (a) | 65.40 ± 3.27 (a) | 33.8 ± 3.3 (a) | ||
| 10 | 69.90 ± 3.50 (a) | 29.3 ± 3.5 (a) | 52.65 ± 2.63 (a) | 46.7 ± 2.6 (a) | ||
| Thiophanate-methyl | 0.00 (Control) | 98.88 ± 4.94 (d) | 0.0 ± 0.0 (d) | 98.88 ± 4.94 (d) | 0.0 ± 0.0 (d) | |
| 0.05 | 97.65 ± 4.88 (c) | 1.2 ± 4.9 (c) | 98.27 ± 4.91 (c) | 0.6 ± 4.9 (c) | ||
| 0.5 | 90.87 ± 4.54 (bc) | 8.1 ± 4.5 (bc) | 92.79 ± 4.64 (bc) | 6.1 ± 4.6 (bc) | ||
| 1 | 83.31 ± 4.17 (ab) | 15.7 ± 4.2 (ab) | 76.79 ± 3.84 (ab) | 22.3 ± 3.8 (ab) | ||
| 5 | 78.17 ± 3.91 (a) | 20.9 ± 3.9 (a) | 74.25 ± 3.71 (a) | 24.9 ± 3.7 (a) | ||
| 10 | 70.90 ± 3.55 (a) | 28.3 ± 3.5 (a) | 73.65 ± 3.68 (a) | 25.5 ± 3.7 (a) | ||
| ViHA | Difenoconazole | 0.00 (Control) | 99.20 ± 0.5 (d) | 0.0 ± 0.0 (d) | 99.20 ± 0.5 (d) | 0.0 ± 0.0 (d) |
| 0.05 | 81.00 ± 2.0 (c) | 18.3 ± 2.0 (c) | 82.32 ± 3.0 (c) | 17.0 ± 3.1 (c) | ||
| 0.5 | 68.34 ± 1.8 (bc) | 31.1 ± 1.8 (bc) | 72.27 ± 2.5 (bc) | 27.1 ± 2.5 (bc) | ||
| 1 | 57.47 ± 1.5 (ab) | 42.0 ± 1.5 (ab) | 70.32 ± 2.8 (ab) | 29.1 ± 2.9 (ab) | ||
| 5 | 50.25 ± 1.2 (a) | 49.3 ± 1.2 (a) | 50.24 ± 1.9 (a) | 49.4 ± 1.9 (a) | ||
| 10 | 30.90 ± 0.9 (a) | 68.8 ± 0.9 (a) | 40.87 ± 1.4 (a) | 58.8 ± 1.4 (a) | ||
| Trifloxystrobin | 0.00 (Control) | 99.20 ± 4.96 (d) | 0.0 ± 0.0 (d) | 99.20 ± 4.96 (d) | 0.0 ± 0.0 (d) | |
| 0.05 | 85.09 ± 4.25 (c) | 14.2 ± 4.3 (c) | 98.87 ± 4.94 (c) | 0.3 ± 4.9 (c) | ||
| 0.5 | 80.65 ± 4.03 (bc) | 18.7 ± 4.0 (bc) | 91.67 ± 4.58 (bc) | 7.6 ± 4.6 (bc) | ||
| 1 | 68.87 ± 3.44 (ab) | 30.6 ± 3.4 (ab) | 87.98 ± 4.40 (ab) | 11.2 ± 4.4 (ab) | ||
| 5 | 62.60 ± 3.13 (a) | 36.9 ± 3.1 (a) | 79.89 ± 3.99 (a) | 19.5 ± 4.0 (a) | ||
| 10 | 50.16 ± 2.51 (a) | 49.4 ± 2.5 (a) | 68.50 ± 3.43 (a) | 30.9 ± 3.4 (a) | ||
| Thiophanate-methyl | 0.00 (Control) | 99.20 ± 4.96 (d) | 0.0 ± 0.0 (d) | 99.20 ± 4.96 (d) | 0.0 ± 0.0 (d) | |
| 0.05 | 93.33 ± 4.67 (c) | 5.9 ± 4.7 (c) | 87.73 ± 4.39 (c) | 11.6 ± 4.4 (c) | ||
| 0.5 | 91.70 ± 4.59 (bc) | 7.6 ± 4.6 (bc) | 76.68 ± 3.83 (bc) | 22.7 ± 3.8 (bc) | ||
| 1 | 80.22 ± 4.01 (ab) | 19.1 ± 4.0 (ab) | 68.44 ± 3.42 (ab) | 30.9 ± 3.4 (ab) | ||
| 5 | 78.31 ± 3.92 (a) | 21.0 ± 3.9 (a) | 66.68 ± 3.33 (a) | 32.8 ± 3.3 (a) | ||
| 10 | 73.50 ± 3.68 (a) | 25.9 ± 3.7 (a) | 50.16 ± 2.51 (a) | 49.4 ± 2.5 (a) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouit, S.; Chiadmi, S.; Goura, K.; Legrifi, I.; El Jarroudi, M.; Belabess, Z.; Tahiri, A.; Lazraq, A.; Baala, M.; Lahlali, R. Assessing Venturia inaequalis Response to Common Fungicides in Morocco. J. Fungi 2025, 11, 493. https://doi.org/10.3390/jof11070493
Gouit S, Chiadmi S, Goura K, Legrifi I, El Jarroudi M, Belabess Z, Tahiri A, Lazraq A, Baala M, Lahlali R. Assessing Venturia inaequalis Response to Common Fungicides in Morocco. Journal of Fungi. 2025; 11(7):493. https://doi.org/10.3390/jof11070493
Chicago/Turabian StyleGouit, Safae, Safae Chiadmi, Khadija Goura, Ikram Legrifi, Moussa El Jarroudi, Zineb Belabess, Abdessalem Tahiri, Abderrahim Lazraq, Mohammed Baala, and Rachid Lahlali. 2025. "Assessing Venturia inaequalis Response to Common Fungicides in Morocco" Journal of Fungi 11, no. 7: 493. https://doi.org/10.3390/jof11070493
APA StyleGouit, S., Chiadmi, S., Goura, K., Legrifi, I., El Jarroudi, M., Belabess, Z., Tahiri, A., Lazraq, A., Baala, M., & Lahlali, R. (2025). Assessing Venturia inaequalis Response to Common Fungicides in Morocco. Journal of Fungi, 11(7), 493. https://doi.org/10.3390/jof11070493







