Exploring the Characteristics of Atoxigenic Aspergillus flavus Isolates and Their Biocontrol Impact on Soil Fungal Communities
Abstract
1. Introduction
2. Materials and Methods
2.1. A. flavus Screening and Culture Conditions
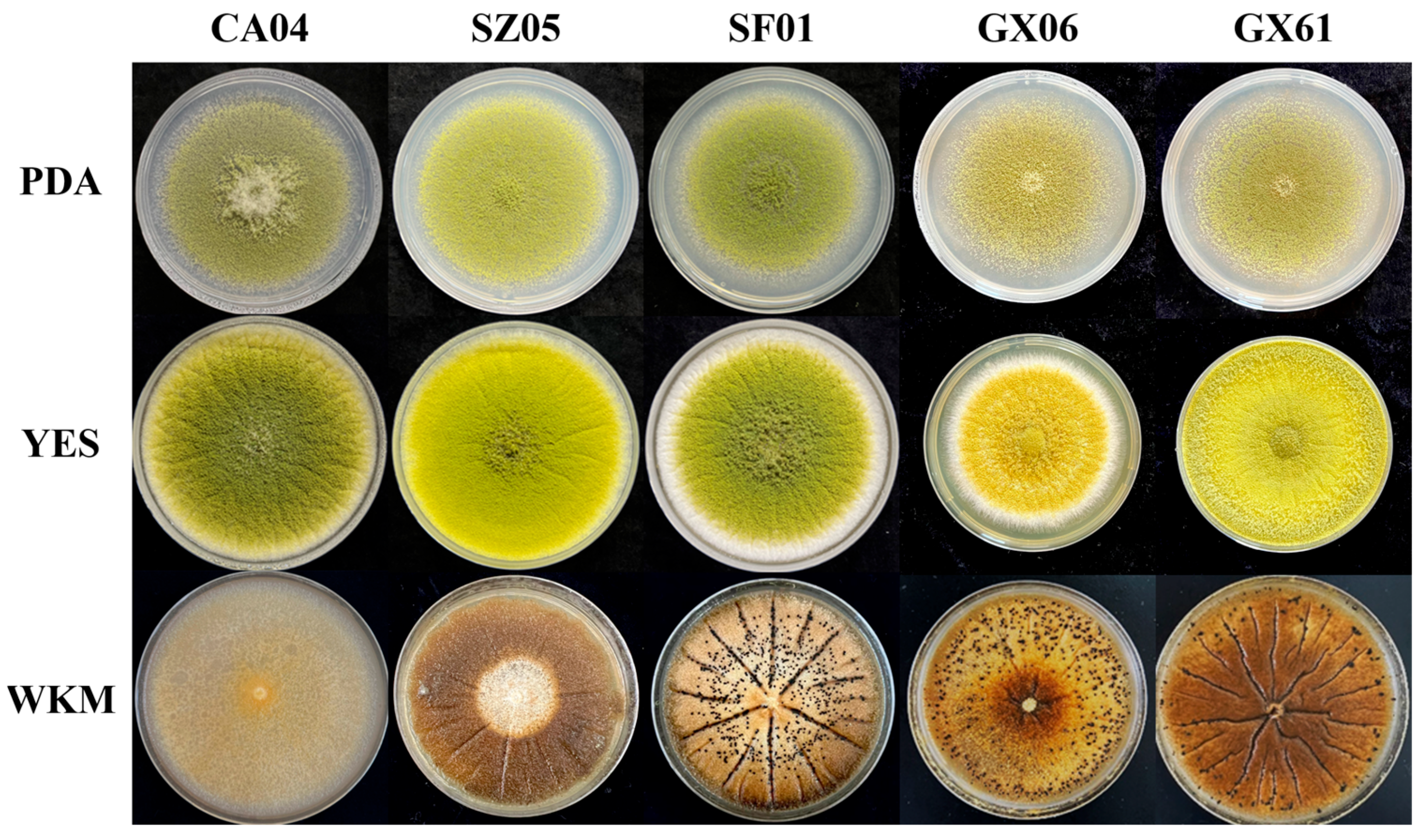
2.2. Morphological Characteristics of Atoxigenic A. flavus
2.3. Production of Aflatoxin and Cyclopiazonic Acid by Atoxigenic A. flavus
2.4. Vegetative Compatibility Group Analyses
2.5. Cluster Amplification Pattern and Microsatellite Loci Analysis of Atoxigenic A. flavus
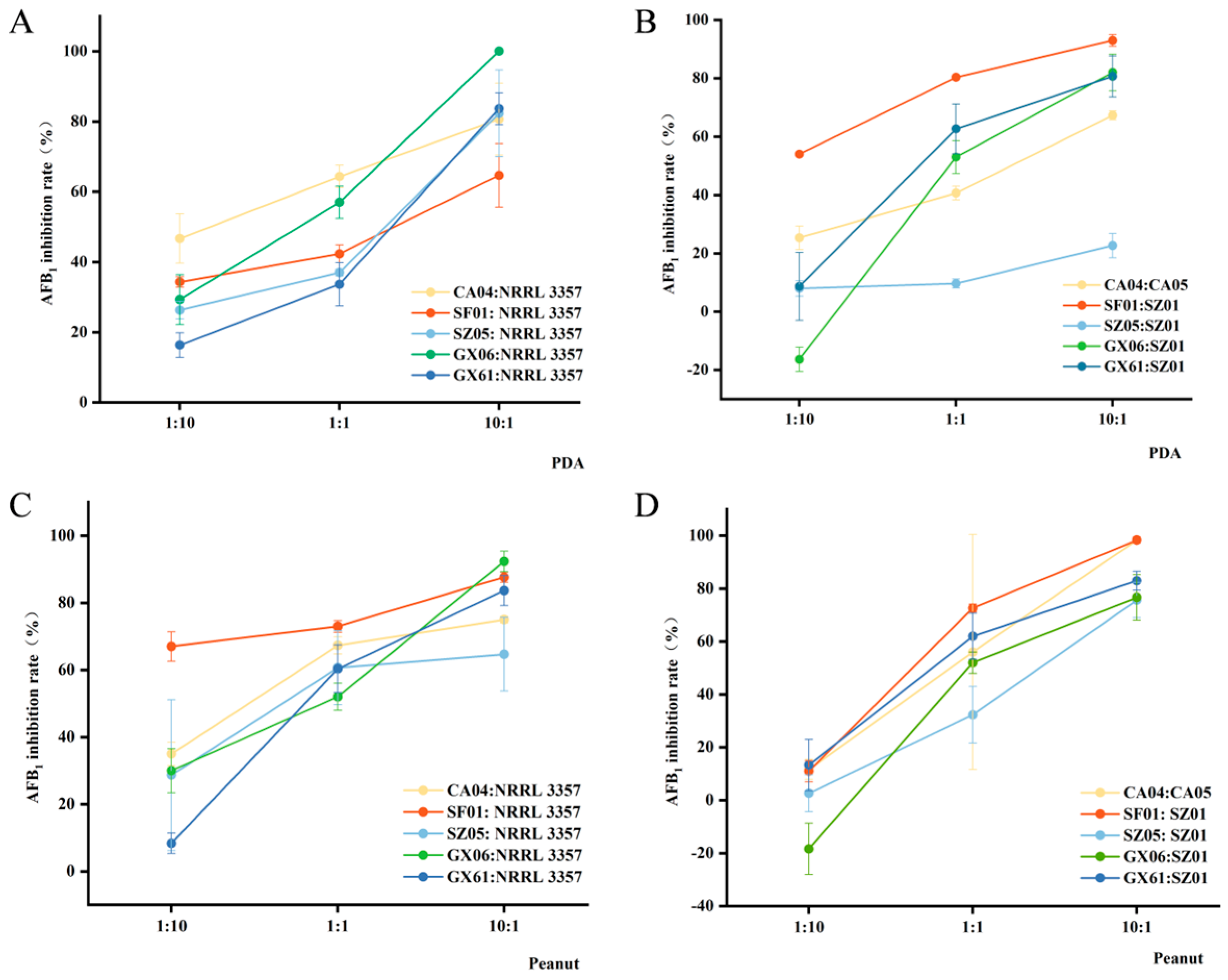
2.6. Competitive Analysis Between Atoxigenic A. flavus and A. flavus
2.6.1. Competitive Analysis on Peanuts
2.6.2. Competitive Analysis on PDA Medium
2.7. Field Trials
2.8. The Processing of Soil Samples
2.9. 97% OTU Clustering Biological Classification
2.10. Data Analysis
3. Results
3.1. Sample Fungus Composition and Characteristics of Atoxigenic Isolates
3.2. Detection of Aflatoxins and CPA Production Capability in A. flavus
3.3. Cluster Amplification Pattern Analysis and Microsatellite Analysis
3.4. Atoxigenic A. flavus Significantly Inhibits the Production of Aflatoxins
3.5. Field Experiment
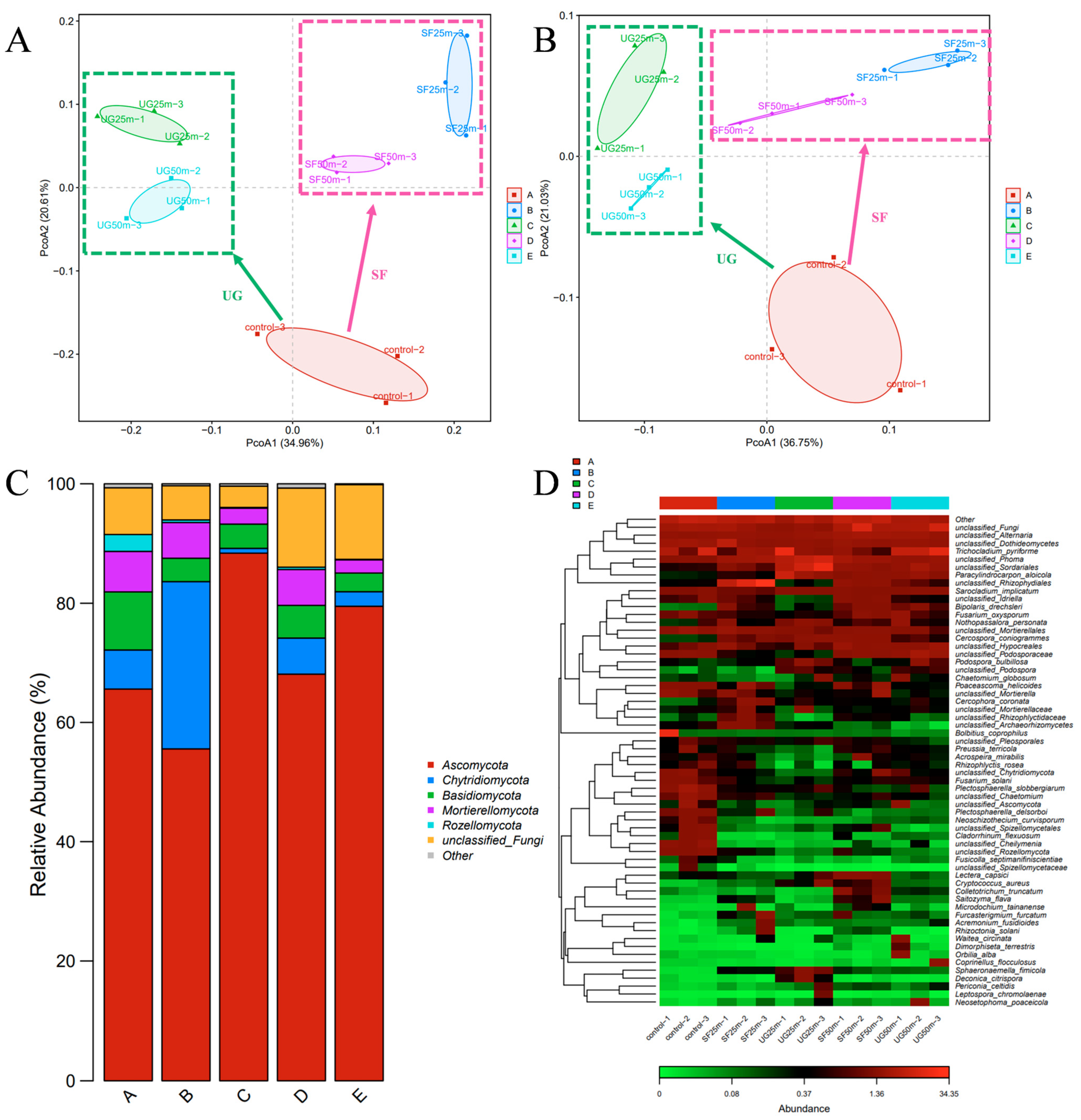
3.6. Analysis of Soil Fungal Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seerat, W.; Akram, A.; Qureshi, R.; Yaseen, G.; Mukhtar, T.; Hanif, N.Q. Light and scanning electron microscopic characterization of aflatoxins producing Aspergillus flavus in the maize crop. Microsc. Res. Tech. 2022, 85, 2894–2903. [Google Scholar] [CrossRef]
- Dessalegn, Y.; Dejene, M.; Mohammed, A.; Chala, A.; Seid, A. Spatiotemporal population dynamics of aflatoxigenic Aspergillus species and their ecological requirements across groundnut-growing areas of Ethiopia. J. Gen. Plant Pathol. 2023, 89, 137–152. [Google Scholar] [CrossRef]
- Xiang, F.; Li, Z.; Zheng, Y.; Ding, C.; Benu, A.; Ma, X.; Xu, X.; Zhu, J.; Abubakar, B.Z.; Shi, A.; et al. Characterization and correlation of engineering properties with microstructure in peanuts: A microscopic to macroscopic analysis. J. Integr. Agric. 2024, 24, 339–352. [Google Scholar] [CrossRef]
- Li, F.; Zhao, X.; Jiao, Y.; Duan, X.; Yu, L.; Zheng, F.; Wang, X.; Wang, L.; Wang, J.-S.; Zhao, X.; et al. Exposure assessment of aflatoxins and zearalenone in edible vegetable oils in Shandong, China: Health risks posed by mycotoxin immunotoxicity and reproductive toxicity in children. Environ. Sci. Pollut. Res. 2022, 30, 3743–3758. [Google Scholar] [CrossRef]
- Aminou, M.M.; Falalou, H.; Abdou, H.; Mendu, V. Aflatoxin B1 contamination association with the seed coat biochemical marker polyphenol in peanuts under intermittent drought. J. Fungi 2024, 10, 850. [Google Scholar] [CrossRef]
- Jallow, A.; Xie, H.; Tang, X.; Qi, Z.; Li, P. Worldwide aflatoxin contamination of agricultural products and foods: From occurrence to control. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2332–2381. [Google Scholar] [CrossRef] [PubMed]
- Amaike, S.; Keller, N.P. Aspergillus flavus. Annu. Rev. Phytopathol. 2011, 49, 107–133. [Google Scholar] [CrossRef]
- Chang, P.-K.; Scharfenstein, L.L.; Li, R.W.; Arroyo-Manzanares, N.; De Saeger, S.; Di Mavungu, J.D. Aspergillus flavus aswA, a gene homolog of Aspergillus nidulans oefC, regulates sclerotial development and biosynthesis of sclerotium-associated secondary metabolites. Fungal Genet. Biol. 2017, 104, 29–37. [Google Scholar] [CrossRef]
- Abe, F.; Karaki, H.; Endoh, M. Effects of cyclopiazonic acid and ryanodine on cytosolic calcium and contraction in vascular smooth muscle. Br. J. Pharmacol. 1996, 118, 1711–1716. [Google Scholar] [CrossRef]
- Zuppini, A.; Navazio, L.; Mariani, P. Endoplasmic reticulum stress-induced programmed cell death in soybean cells. J. Cell Sci. 2004, 117, 2591–2598. [Google Scholar] [CrossRef]
- Moon, Y.-S.; Kim, H.-M.; Chun, H.S.; Lee, S.-E. Organic acids suppress aflatoxin production via lowering expression of aflatoxin biosynthesis-related genes in Aspergillus flavus. Food Control 2018, 88, 207–216. [Google Scholar] [CrossRef]
- Lorán, S.; Carramiñana, J.J.; Juan, T.; Ariño, A.; Herrera, M. Inhibition of Aspergillus parasiticus growth and aflatoxins production by natural essential oils and phenolic acids. Toxins 2022, 14, 384. [Google Scholar] [CrossRef] [PubMed]
- Moral, J.; Garcia-Lopez, M.T.; Camiletti, B.X.; Jaime, R.; Michailides, T.J.; Bandyopadhyay, R.; Ortega-Beltran, A. Present status and perspective on the future use of aflatoxin biocontrol products. Agronomy 2020, 10, 491. [Google Scholar] [CrossRef]
- Rahman, M.A.H.; Selamat, J.; Shaari, K.; Ahmad, S.; Samsudin, N.I.P. Extrolites from non-aflatoxigenic Aspergillus flavus: Potentials and challenges as emerging control strategy against Aspergillus flavus infection and aflatoxin contamination. Curr. Opin. Food Sci. 2024, 59, 101214. [Google Scholar] [CrossRef]
- Einloft, T.C.; de Oliveira, P.B.; Radünz, L.L.; Dionello, R.G. Biocontrol capabilities of three Bacillus isolates towards aflatoxin B1 producer A. flavus in vitro and on maize grains. Food Control 2021, 125, 107978. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Algboory, H.L.; Kadum, H.; Mohammed, N.K.; Saari, N.; Hassan, Z.; Hussin, A.S.M. Antifungal activity determination for the peptides generated by Lactobacillus plantarum TE10 against Aspergillus flavus in maize seeds. Food Control 2020, 109, 106898. [Google Scholar] [CrossRef]
- Atehnkeng, J. Distribution and toxigenicity of Aspergillus species isolated from maize kernels from three agro-ecological zones in Nigeria. Int. J. Food Microbiol. 2008, 122, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Stoev, S.D. Food security, underestimated hazard of joint mycotoxin exposure and management of the risk of mycotoxin contamination. Food Control 2024, 159, 110235. [Google Scholar] [CrossRef]
- Wokorach, G.; Landschoot, S.; Lakot, A.; Karyeija, S.A.; Audenaert, K.; Echodu, R.; Haesaert, G. Characterization of Ugandan endemic Aspergillus species and identification of non-aflatoxigenic isolates for potential biocontrol of aflatoxins. Toxins 2022, 14, 304. [Google Scholar] [CrossRef]
- Ortega-Beltran, A.; Grubisha, L.C.; Callicott, K.A.; Cotty, P.J. The vegetative compatibility group to which the US biocontrol agent Aspergillus flavus AF36 belongs is also endemic to Mexico. J. Appl. Microbiol. 2016, 120, 986–998. [Google Scholar] [CrossRef]
- Perrone, G.; Gallo, A.; Logrieco, A.F. Biodiversity of Aspergillus section Flavi in Europe in relation to the management of aflatoxin risk. Front. Microbiol. 2014, 5, 377. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.G. Practical considerations will ensure the continued success of pre-harvest biocontrol using non-aflatoxigenic Aspergillus flavus strains. Crit. Rev. Food Sci. Nutr. 2021, 62, 4208–4225. [Google Scholar] [CrossRef] [PubMed]
- Hamad, G.M.; Amer, A.; El-Nogoumy, B.; Ibrahim, M.; Hassan, S.; Siddiqui, S.A.; El-Gazzar, A.M.; Khalifa, E.; Omar, S.A.; Abd-Elmohsen Abou-Alella, S.; et al. Evaluation of the effectiveness of charcoal, Lactobacillus rhamnosus, and Saccharomyces cerevisiae as aflatoxin adsorbents in chocolate. Toxins 2022, 15, 21. [Google Scholar] [CrossRef]
- Ehrlich, K.C. Non-aflatoxigenic Aspergillus flavus to prevent aflatoxin contamination in crops: Advantages and limitations. Front. Microbiol. 2014, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Mamo, F.T.; Shang, B.; Selvaraj, J.N.; Zheng, Y.; Liu, Y. Biocontrol efficacy of atoxigenic Aspergillus flavus strains against aflatoxin contamination in peanut field in Guangdong province, South China. Mycology 2021, 13, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.K.; Zablotowicz, R.M.; Bruns, H.A.; Abel, C.A. Biocontrol of aflatoxin in corn by inoculation with non-aflatoxigenic Aspergillus flavus isolates. Biocontrol Sci. Technol. 2007, 16, 437–449. [Google Scholar] [CrossRef]
- Garcia-Lopez, M.T.; Meca, E.; Jaime, R.; Puckett, R.D.; Michailides, T.J.; Moral, J. Sporulation and dispersal of the biological control agent Aspergillus flavus AF36 under field conditions. Phytopathology 2024, 114, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.N.; Bandyopadhyay, R.; Cotty, P.J. Degeneration of aflatoxin gene clusters in Aspergillus flavus from Africa and North America. AMB Express 2016, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zanon, M.S.A.; Chiotta, M.L.; Giaj-Merlera, G.; Barros, G.; Chulze, S. Evaluation of potential biocontrol agent for aflatoxin in Argentinean peanuts. Int. J. Food Microbiol. 2013, 162, 220–225. [Google Scholar] [CrossRef]
- Wang, X.; Wadl, P.A.; Wood-Jones, A.; Windham, G.; Trigiano, R.N.; Scruggs, M.; Pilgrim, C.; Baird, R. Characterization of expressed sequence tag–derived simple sequence repeat markers for Aspergillus flavus: Emphasis on variability of isolates from the southern united states. Mycopathologia 2012, 174, 371–382. [Google Scholar] [CrossRef]
- Ajilogba, C.F.; Babalola, O.O. Bambara groundnut soil metagenomics data. Data Brief 2020, 30, 105542. [Google Scholar] [CrossRef] [PubMed]
- Cotty, P.J. Comparison of four media for the isolation of Aspergillus flavus group fungi. Mycopathologia 1994, 125, 157–162. [Google Scholar] [CrossRef]
- Habibi, A.; Afzali, D. Aspergillus section Flavi from four agricultural products and association of mycotoxin and sclerotia production with isolation source. Curr. Microbiol. 2021, 78, 3674–3685. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, M.; Wang, P.; Kong, Q. The gene vepN regulated by global regulatory factor veA that affects aflatoxin production, morphological development and pathogenicity in Aspergillus flavus. Toxins 2024, 16, 174. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-K.; Horn, B.W.; Dorner, J.W. Clustered genes involved in cyclopiazonic acid production are next to the aflatoxin biosynthesis gene cluster in Aspergillus flavus. Fungal Genet. Biol. 2009, 46, 176–182. [Google Scholar] [CrossRef]
- Picot, A.; Doster, M.; Islam, M.-S.; Callicott, K.; Ortega-Beltran, A.; Cotty, P.; Michailides, T. Distribution and incidence of atoxigenic Aspergillus flavus VCG in tree crop orchards in California: A strategy for identifying potential antagonists, the example of almonds. Int. J. Food Microbiol. 2018, 265, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Callicott, K.A.; Mutegi, C.; Bandyopadhyay, R.; Cotty, P.J. Distribution of active ingredients of a commercial aflatoxin biocontrol product in naturally occurring fungal communities across Kenya. Microb. Biotechnol. 2021, 14, 1331–1342. [Google Scholar] [CrossRef]
- Senghor, L.A.; Ortega-Beltran, A.; Atehnkeng, J.; Callicott, K.A.; Cotty, P.J.; Bandyopadhyay, R. The atoxigenic biocontrol product Aflasafe SN01 is a valuable tool to mitigate aflatoxin contamination of both maize and groundnut cultivated in Senegal. Plant Dis. 2020, 104, 510–520. [Google Scholar] [CrossRef]
- Ajmal, M.; Alshannaq, A.F.; Moon, H.; Choi, D.; Akram, A.; Nayyar, B.G.; Gibbons, J.G.; Yu, J.-H. Characterization of 260 isolates of Aspergillus Section Flavi obtained from sesame seeds in Punjab, Pakistan. Toxins 2022, 14, 117. [Google Scholar] [CrossRef]
- Atayde, D.D.; Reis, T.A.; Godoy, I.J.; Zorzete, P.; Reis, G.M.; Corrêa, B. Mycobiota and aflatoxins in a peanut variety grown in different regions in the state of São Paulo, Brazil. Crop Prot. 2012, 33, 7–12. [Google Scholar] [CrossRef]
- Tran-Dinh, N.; Pitt, J.I.; Markwell, P.J. Use of microsatellite markers to assess the competitive ability of nontoxigenic Aspergillus flavus strains in studies on biocontrol of aflatoxins in maize in Thailand. Biocontrol Sci. Technol. 2018, 28, 215–225. [Google Scholar] [CrossRef]
- Mallikarjunaiah, N.H.; Jayapala, N.; Puttaswamy, H.; Ramachandrappa, N.S. Characterization of non-aflatoxigenic strains of Aspergillus flavus as potential biocontrol agent for the management of aflatoxin contamination in groundnut. Microb. Pathog. 2017, 102, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, M.L.; Carlucci, A. Characterization and pathogenicity assessment of Plectosphaerella species associated with stunting disease on tomato and pepper crops in Italy. Plant Pathol. 2017, 67, 626–641. [Google Scholar] [CrossRef]
- Anco, D.J.; Thomas, J.S.; Jordan, D.L.; Shew, B.B.; Monfort, W.S.; Mehl, H.L.; Small, I.M.; Wright, D.L.; Tillman, B.L.; Dufault, N.S.; et al. Peanut yield loss in the presence of defoliation caused by late or early leaf spot. Plant Dis. 2020, 104, 1390–1399. [Google Scholar] [CrossRef]




| Year | Area | District | Sample | Isolate | VCG | AFB1 (μg/L) | AFB2 (μg/L) | CPA | Sclerotia |
|---|---|---|---|---|---|---|---|---|---|
| 2022 | Shandong | Qingdao | Corn | CA04 | QC001 | ND | ND | + | L |
| 2022 | Shandong | Qingdao | Corn | CA05 | QC002 | 22.11 ± 0.09 | 575.23 ± 8.30 | + | L |
| 2022 | Fujian | Zhangzhou | Soil | SZ01 | ZS001 | 184.23 ± 12.65 | 6937.53 ± 31.67 | + | L |
| 2022 | Fujian | Zhangzhou | Soil | SZ02 | ZS001 | 94.37 ± 3.19 | 1049.32 ± 5.38 | + | L |
| 2022 | Fujian | Zhangzhou | Soil | SZ03 | ZS002 | 287.59 ± 2.67 | 969.24 ± 10.76 | + | L |
| 2022 | Fujian | Zhangzhou | Soil | SZ04 | ZS001 | 145.58 ± 3.87 | 1797.91 ± 32.89 | + | L |
| 2022 | Fujian | Zhangzhou | Soil | SZ05 | ZS003 | ND | ND | + | L |
| 2022 | Fujian | Fuzhou | Soil | SF01 | FS001 | ND | ND | + | L |
| 2022 | Fujian | Fuzhou | Soil | SF02 | FS002 | 162.8 ± 11.77 | 2564.1 ± 37.67 | + | L |
| 2022 | Fujian | Fuzhou | Soil | SF03 | FS003 | 1371.19 ± 40.23 | 20,767.48 ± 98.47 | + | S |
| 2023 | Fujian | Fuzhou | Soil | SF04 | FS004 | 168.45 ± 9.46 | 1069.79 ± 34.78 | + | L |
| 2022 | Fujian | Qvanzhou | Soil | SQ01 | QS001 | 168.4 ± 7.08 | 535.37 ± 18.75 | + | L |
| 2023 | Fujian | Sanming | Soil | SM01 | MS001 | 3231.64 ± 27.65 | 1592.78 ± 45.18 | + | L |
| 2023 | Guangxi | - | Soil | GX06 | GS001 | ND | ND | − | L |
| 2023 | Guangxi | - | Soil | GX61 | GS001 | ND | ND | − | L |
| 2023 | Shandong | Tai’an | Soil | TA03 | TS001 | 320.47 ± 5.62 | 6982.31 ± 65.39 | + | L |
| 2023 | Shandong | Tai’an | Soil | TA04 | TS001 | 468.54 ± 6.59 | 1889.73 ± 13.62 | + | L |
| 2023 | Shandong | Tai’an | Soil | TA08 | TS002 | 267.43 ± 3.55 | 4285.67 ± 54.87 | + | L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, W.; Piao, C.; Li, W.; Cotty, P.J.; Shan, S.; Rasheed, U.; Migheli, Q.; Kong, Q. Exploring the Characteristics of Atoxigenic Aspergillus flavus Isolates and Their Biocontrol Impact on Soil Fungal Communities. J. Fungi 2025, 11, 491. https://doi.org/10.3390/jof11070491
Zhang Y, Wang W, Piao C, Li W, Cotty PJ, Shan S, Rasheed U, Migheli Q, Kong Q. Exploring the Characteristics of Atoxigenic Aspergillus flavus Isolates and Their Biocontrol Impact on Soil Fungal Communities. Journal of Fungi. 2025; 11(7):491. https://doi.org/10.3390/jof11070491
Chicago/Turabian StyleZhang, Yanyan, Wanning Wang, Chenggui Piao, Wenjin Li, Peter J. Cotty, Shihua Shan, Usman Rasheed, Quirico Migheli, and Qing Kong. 2025. "Exploring the Characteristics of Atoxigenic Aspergillus flavus Isolates and Their Biocontrol Impact on Soil Fungal Communities" Journal of Fungi 11, no. 7: 491. https://doi.org/10.3390/jof11070491
APA StyleZhang, Y., Wang, W., Piao, C., Li, W., Cotty, P. J., Shan, S., Rasheed, U., Migheli, Q., & Kong, Q. (2025). Exploring the Characteristics of Atoxigenic Aspergillus flavus Isolates and Their Biocontrol Impact on Soil Fungal Communities. Journal of Fungi, 11(7), 491. https://doi.org/10.3390/jof11070491








