Silent Allies: Endophytic Entomopathogenic Fungi Promote Biological Control and Reduce Spittlebug Mahanarva spectabilis Distant, 1909 (Hemiptera: Cercopidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining Fungi and Urochloa ruziziensis Seeds
2.2. Seed Treatment for Greenhouse and Field Bioassays
2.3. Inoculation of Fungi in Seeds
2.4. Cultivation of Urochloa ruziziensis Containing Entomopathogenic Fungi in the Greenhouse
2.5. Obtaining the Insect Pest Mahanarva spectabilis
2.6. Bioassays in the Greenhouse
2.6.1. Control of Mahanarva spectabilis Nymphs and Adults with Endophytic Fungi
2.6.2. Evaluation of Persistence of Fungi in Nymph and Adult Corpses in the Greenhouse
2.6.3. Damage Score of the Plants
2.6.4. Confirming Persistence of Fungi in Plant Tissues
2.7. Bioassay in the Field: Control of Mahanarva spectabilis Adults with Endophytic Fungi
2.7.1. Cultivation of Urochloa ruziziensis Containing Entomopathogenic Fungi in the Field
2.7.2. Artificial Infestation of Plants by Spittlebugs
2.7.3. Natural Occurrence of Adults
2.7.4. Evaluation of Persistence of Fungi in Nymph and Adult Corpses in the Field
2.7.5. Damage Score
2.7.6. Confirming the Persistence of Fungi in the Plant Tissue of Urochloa ruziziensis
2.8. Statistical Analysis
3. Results
3.1. Mortality of Mahanarva spectabilis Nymphs and Adults Caused by the Fungi Fusarium multiceps and Metarhizium anisopliae in a Greenhouse
3.2. Mortality and Population Fluctuations of Mahanarva spectabilis Nymphs and Adults During Artificial and Natural Infestations in the Field
3.3. Confirmation of the Presence of Fungi in Nymphs and Adults of Mahanarva spectabilis Killed While Feeding on Different Plants in the Greenhouse and Field
3.4. Confirmation of the Presence of Fungi in Plant Tissues Under Greenhouse and Field Conditions
3.5. Leaf Damage Caused by Mahanarva spectabilis Nymphs and Adults in the Greenhouse and Field
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Resende, T.T.; Auad, A.M.; Fonseca, M.G. How many adults of Mahanarva Spectabilis (Hemiptera: Cercopidae) should be used for screening; Brachiaria Ruziziensis; (Poales: Poaceae) resistance? J. Econ. Entomol. 2014, 107, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Congio, G.F.S.; de Almeida, P.C.; Barreto, T.R.; Tinazo, V.A.; da Silva, T.A.C.C.; Costa, D.F.A.; Corsi, M. Spittlebug damage on tropical grass and its impact in pasture-based beef production systems. Sci. Rep. 2020, 10, 10758. [Google Scholar] [CrossRef]
- Resende, T.T.; Sobrinho, F.S.; Campagnani, M.O.; Veríssimo, B.A.; Calsavara, L.A.; Gonçalves, F.M.A.; Nunes, J.A.R.; Auad, A.M. Sixteen years of recurrent selection of ruzi grass for resistance to spittlebugs (Hemiptera: Cercopidae). Agronomy 2024, 14, 1516. [Google Scholar] [CrossRef]
- IBGE Censo Agropecuário. Available online: https://www.fao.org/fileadmin/templates/ess/ess_test_folder/World_Census_Agriculture/WCA_2020/WCA_2020_new_doc/BRA_REP1_POR_2017.pdf (accessed on 24 June 2025).
- Valério, J.R.; Nakano, O. Danos causados pelo adulto da cigarrinha Zulia Entreriana na produção e qualidade de Brachiaria Decumbens. Pesqui. Agropecu. Bras. 1988, 23, 447–453. [Google Scholar]
- Holmann, F.; Peck, D.C. Economic damage caused by spittlebugs (Homoptera: Cercopidae) in Colombia: A First approximation of impact on animal production in Brachiaria Decumbens pastures. Neotrop. Entomol. 2002, 31, 275–284. [Google Scholar] [CrossRef]
- Valerio, J.R. Cigarrinhas-Das-Pastagens, 1st ed.; EMBRAPA: Campo Grande, Brazil, 2009; Volume 1, pp. 1–51. [Google Scholar]
- (ONU). Objetivos de Desenvolvimento Sustentável: Relatório de Acompanhamento Global; ONU Brasil: Brasília, Brazil, 2024; 300p, Available online: https://brasil.un.org/pt-br/sdgs (accessed on 24 June 2025).
- Auad, A.M.; Simões, A.D.; Pereira, A.V.; Braga, A.L.F.; Souza Sobrinho, F.; Lédo, F.J. da S.; Paula-Moraes, S.V.; Oliveira, S.A.; Ferreira, R.B. Seleção de genótipos de capim-elefante quanto à resistência à cigarrinha-das-pastagens. Pesqui. Agropecu. Bras. 2007, 42, 1077–1081. [Google Scholar] [CrossRef]
- Auad, A.M.; Calsavara, L.A.; Souza Sobrinho, F.; Lédo, F.J.d.S.; Machado, J.C.; Pereira, A. Vander Lack of antibiosis against Mahanarva Spectabilis (Distant) (Hemiptera: Cercopidae) in Cenchrus Purpureus (Schumach.) Morrone germplasm. Crop Breed. Appl. Biotechnol. 2024, 24. [Google Scholar] [CrossRef]
- Souza Sobrinho, F.; Borges, V.; Lédo, F.J.d.S.; Kopp, M.M. Repetibilidade de características agronômicas e número de cortes necessários para seleção de Urochloa ruziziensis. Pesqui. Agropecu. Bras. 2010, 45, 579–584. [Google Scholar] [CrossRef]
- Alvarenga, R.; Auad, A.M.; Moraes, J.C.; Silva, S.E. Do silicon and nitric oxide induce resistance to Mahanarva spectabilis (Hemiptera: Cercopidae) in forage grasses? Pest. Manag. Sci. 2019, 75, 3282–3292. [Google Scholar] [CrossRef]
- Alvarenga, R.; Auad, A.M.; Moraes, J.C.; Silva, S.E.B.; Rodrigues, B.S.; Silva, G.B. Spittlebugs (Hemiptera: Cercopidae) and their host plants: A strategy for pasture diversification. Appl. Entomol. Zool. 2017, 52, 653–660. [Google Scholar] [CrossRef]
- Leandro Dias, M.; Machado Auad, A.; de Resende, T.T. Insecticidal effects of thymol on Mahanarva spectabilis (Hemiptera: Cercopidae) in two evaluation methodologies. J. Agric. Crop Res. 2019, 7, 181–185. [Google Scholar] [CrossRef]
- Nascimento, V.F.; Auad, A.M.; de Resende, T.T. Olfactory response of Mahanarva spectabilis (Distant, 1909) (Hemiptera: Cercopidae) to volatile aqueous extracts of plant origin applied to elephant grass plants (Pennisetum purpureum Schum). Agronomy 2021, 11, 856. [Google Scholar] [CrossRef]
- Alvarenga, R.; Auad, A.M.; Moraes, J.C.; da Silva, S.E.B.; Rodrigues, B.S. Tolerance to nymphs and adults of Mahanarva spectabilis (Hemiptera: Cercopidae) by forage plants in fertilized soils. Pest. Manag. Sci. 2019, 75, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, D.d.M.; Auad, A.M.; Fonseca, M.d.G.; Leite, M.V. Brachiaria ruziziensis responses to different fertilization doses and to the attack of Mahanarva spectabilis (Hemiptera: Cercopidae) nymphs and adults. Sci. World J. 2014, 2014, 1–8. [Google Scholar] [CrossRef][Green Version]
- Campagnani, M.O.; Campos, W.G.; Amorim, S.S.; Rosa, L.H.; Auad, A.M.; Cangussú, M.A.; Maurício, R.M. Prospection and fungal virulence associated with Mahanarva spectabilis (Hemiptera: Cercopidae) in an amazon silvopastoral system. Fla. Entomol. 2017, 100, 426–432. [Google Scholar] [CrossRef]
- de Oliveira Netto, P.M.; Auad, A.M.; Campagnani de Mendonça, M.O.; Resende, T.T.; Duarte, M.; Veríssimo, B.A.; Calsavara, L.A.; Oliveira, C.M. Endophytic potential of entomopathogenic fungi associated with Urochloa ruziziensis (Poaceae) for spittlebug (Hemiptera: Cercopidae) control. Fla. Entomol. 2024, 112. [Google Scholar] [CrossRef]
- Campagnani, M.O.; Auad, A.M.; Maurício, R.M.; Madureira, A.P.; Cangussú, M.A.; Rosa, L.H.; Pereira, M.F.A.; Muniz, M.; Souza, S.R.O.; Silva, N.B.M.; et al. Endophytic capacity of entomopathogenic fungi in a pasture grass and their potential to control the spittlebug Mahanarva spectabilis (Hemiptera: Cercopidae). Agronomy 2024, 14, 943. [Google Scholar] [CrossRef]
- Auad, A.M.; Resende, T.T.; Oliveira, C.M. A decade of sampling reveals spittlebug population dynamics in different cultivation system. Glob. Ecol. Conserv. 2025, 59, e03534. [Google Scholar] [CrossRef]
- Duarte, M.; Calsavara, L.A.; Auad, A.M. Thermal stress as a critical factor in the viability and duration of spittlebug eggs. Stresses 2024, 4, 676–684. [Google Scholar] [CrossRef]
- Quesada Moraga, E. Entomopathogenic fungi as endophytes: Their broader contribution to ipm and crop production. Biocontrol Sci. Technol. 2020, 30, 864–877. [Google Scholar] [CrossRef]
- Yousef-Yousef, M.; Morente, M.; González-Mas, N.; Fereres, A.; Quesada-Moraga, E.; Moreno, A. Direct and indirect effects of two endophytic entomopathogenic fungi on survival and feeding behaviour of meadow spittlebug Philaenus spumarius. Biol. Control 2023, 186, 105348. [Google Scholar] [CrossRef]
- Karthi, S.; Vasantha-Srinivasan, P.; Senthil-Nathan, S.; Han, Y.S.; Shivakumar, M.S.; Murali-Baskaran, R.K.; Kalaivani, K.; Radhakrishnan, N.; Park, K.B.; Malafaia, G. Entomopathogenic fungi promising biocontrol agents for managing lepidopteran pests: Review of current knowledge. Biocatal. Agric. Biotechnol. 2024, 58, 103146. [Google Scholar] [CrossRef]
- Alves, S.B. Controle Microbiano de Insetos; Biblioteca de Ciências Agrárias Luiz de Queiroz: São Paulo, Brazil, 1998; 1163p. [Google Scholar]
- Barta, M.; Lalík, M.; Rell, S.; Kunca, A.; Horáková, M.K.; Mudrončeková, S.; Galko, J. Hypocrealean fungi associated with Hylobius abietis in Slovakia, their virulence against weevil adults and effect on feeding damage in laboratory. Forests 2019, 10, 634. [Google Scholar] [CrossRef]
- Rajula, J.; Rahman, A.; Krutmuang, P. Entomopathogenic fungi in southeast asia and africa and their possible adoption in biological control. Biol. Control 2020, 151, 104399. [Google Scholar] [CrossRef]
- Carvalho, C.R.; Gonçalves, V.N.; Pereira, C.B.; Johann, S.; Galliza, I.V.; Alves, T.M.A.; Rabello, A.; Sobral, M.E.G.; Zani, C.L.; Rosa, C.A.; et al. The diversity, antimicrobial and anticancer activity of endophytic fungi associated with the medicinal plant Stryphnodendron adstringens (Mart.) Coville (Fabaceae) from the brazilian savannah. Symbiosis 2012, 57, 95–107. [Google Scholar] [CrossRef]
- Ferreira, M.C.; Cantrell, C.L.; Wedge, D.E.; Gonçalves, V.N.; Jacob, M.R.; Khan, S.; Rosa, C.A.; Rosa, L.H. Diversity of the endophytic fungi associated with the ancient and narrowly endemic neotropical plant Vellozia gigantea from the endangered brazilian rupestrian grasslands. Biochem. Syst. Ecol. 2017, 71, 163–169. [Google Scholar] [CrossRef]
- Keyser, C.A.; Thorup-Kristensen, K.; Meyling, N.V. Metarhizium seed treatment mediates fungal dispersal via roots and induces infections in insects. Fungal Ecol. 2014, 11, 122–131. [Google Scholar] [CrossRef]
- Lopes, R.B.; Martins, I.; Souza, D.A.; Faria, M. Influence of some parameters on the germination assessment of mycopesticides. J. Invertebr. Pathol. 2013, 112, 236–242. [Google Scholar] [CrossRef]
- Klieber, J.; Reineke, A. The entomopathogen Beauveria bassiana has epiphytic and endophytic activity against the tomato leaf miner Tuta absoluta. J. Appl. Entomol. 2016, 140, 580–589. [Google Scholar] [CrossRef]
- Ahmad, I.; Zaib, S.; Alves, P.C.M.S.; Luthe, D.S.; Bano, A.; Shakeel, S.N. Molecular and physiological analysis of drought stress responses in Zea mays treated with plant growth promoting Rhizobacteria. Biol. Plant 2019, 63, 536–547. [Google Scholar] [CrossRef]
- Cardona, C.; Miles, J.W.; Sotelo, G. An Improved methodology for massive screening of Brachiaria Spp. genotypes for resistance to Aeneolamia varia (Homoptera: Cercopidae). J. Econ. Entomol. 1999, 92, 490–496. [Google Scholar] [CrossRef]
- Pabón, A.; Cardona, C.; Miles, J.W.; Sotelo, G. Response of resistant and susceptible Brachiaria Spp. genotypes to simultaneous infestation with multiple species of spittlebugs (Hemiptera: Cercopidae). J. Econ. Entomol. 2007, 100, 1896–1903. [Google Scholar] [CrossRef]
- Ahmad, I.; Jiménez-Gasco, M.d.M.; Luthe, D.S.; Barbercheck, M.E. Systemic colonization by Metarhizium robertsii enhances cover crop growth. J. Fungi 2020, 6, 64. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.10.7. 2021. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 24 June 2025).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biometr. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Quesada-Moraga, E.; Garrido-Jurado, I.; Yousef-Yousef, M.; González-Mas, N. Multitrophic interactions of entomopathogenic fungi in biocontrol. BioControl 2022, 67, 457–472. [Google Scholar] [CrossRef]
- Jaber, L.R.; Ownley, B.H. Can we use entomopathogenic fungi as endophytes for dual biological control of insect pests and plant pathogens? Biol. Control 2018, 116, 36–45. [Google Scholar] [CrossRef]
- Yapa, A.T.; Thambugala, K.M.; Samarakoon, M.C.; de Silva, N. Metarhizium species as bioinsecticides: Potential, progress, applications andfuture perspectives. N. Z. J. Bot. 2025, 63, 439–461. [Google Scholar] [CrossRef]
- García-Espinoza, F.; Quesada-Moraga, E.; García del Rosal, M.J.; Yousef-Yousef, M. Entomopathogenic fungi-mediated solubilization and induction of fe related genes in melon and cucumber plants. J. Fungi 2023, 9, 258. [Google Scholar] [CrossRef]
- Ambele, C.F.; Ekesi, S.; Bisseleua, H.D.B.; Babalola, O.O.; Khamis, F.M.; Djuideu, C.T.L.; Akutse, K.S. Entomopathogenic fungi as endophytes for biological control of subterranean termite pests attacking cocoa seedlings. J. Fungi 2020, 6, 126. [Google Scholar] [CrossRef]
- Macedo, D.; Alves, S.B.; Vieira, S.A. Seleção de isolados de Metarhizium anisopliae (Metsch.) Sorok. a Mahanarva fimbriolata (Stal, 1854) (Hemiptera: Cercopidae). Semin. Cienc. Agrar. 2006, 27, 47. [Google Scholar] [CrossRef]
- Iwanicki, N.S.; Pereira, A.A.; Botelho, A.B.R.Z.; Rezende, J.M.; Moral, R.d.A.; Zucchi, M.I.; Delalibera Júnior, I. Monitoring of the field application of Metarhizium anisopliae in Brazil revealed high molecular diversity of Metarhizium spp. in insects, soil and sugarcane roots. Sci. Rep. 2019, 9, 4443. [Google Scholar] [CrossRef]
- Loureiro, E.S.; Batista Filho, A.; Almeida, J.E.M.; Pessoa, L.G.A. Screening of Metarhizium anisopliae (Metsch.) Sorok. strains against the sugarcane root spittlebug Mahanarva fimbriolata (Stål) (Hemiptera: Cercopidae) in laboratory. Neotrop. Entomol. 2005, 34, 791–798. [Google Scholar] [CrossRef]
- Mateus, M.P.d.B.; Loureiro, E. de S.; Pessoa, L.G.A.; Adão, D.V. Estudo comparativo de bioinseticidas a base de Metarhizium anisopliae (Ascomycota: Clavicipitaceae) no controle de Mahanarva fimbriolata (Hemiptera: Cercopidae). Res. Soc. Dev. 2020, 9, e1579108322. [Google Scholar] [CrossRef]
- Garrido-Jurado, I.; Resquín-Romero, G.; Amarilla, S.P.; Ríos-Moreno, A.; Carrasco, L.; Quesada-Moraga, E. Transient endophytic colonization of melon plants by entomopathogenic fungi after foliar application for the control of Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae). J. Pest Sci. 2017, 90, 319–330. [Google Scholar] [CrossRef]
- Kershaw, M.J.; Moorhouse, E.R.; Bateman, R.; Reynolds, S.E.; Charnley, A.K. The role of destruxins in the pathogenicity of Metarhizium anisopliae for three species of insect. J. Invertebr. Pathol. 1999, 74, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Quesada-Moraga, E.; Muñoz-Ledesma, F.J.; Santiago-Álvarez, C. Systemic protection of Papaver somniferum L. against Iraella luteipes (Hymenoptera: Cynipidae) by an endophytic strain of Beauveria bassiana (Ascomycota: Hypocreales). Environ. Entomol. 2009, 38, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Vega, F.E.; Posada, F.; Catherine Aime, M.; Pava-Ripoll, M.; Infante, F.; Rehner, S.A. Entomopathogenic fungal endophytes. Biol. Control 2008, 46, 72–82. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Vey, A. Bassiacridin, a protein toxic for locusts secreted by the entomopathogenic fungus Beauveria bassiana. Mycol. Res. 2004, 108, 441–452. [Google Scholar] [CrossRef]
- Gurulingappa, P.; Sword, G.A.; Murdoch, G.; McGee, P.A. Colonization of crop plants by fungal entomopathogens and their effects on two insect pests when in planta. Biol. Control 2010, 55, 34–41. [Google Scholar] [CrossRef]
- Akello, J.; Sikora, R. Systemic Acropedal Influence of endophyte seed treatment on Acyrthosiphon pisum and Aphis fabae offspring development and reproductive fitness. Biol. Control 2012, 61, 215–221. [Google Scholar] [CrossRef]
- Amandio, D.T.T.; Nesi, C.N.; Poltronieri, A.S.; Ribeiro, L. do P. Endophytic entomopathogenic fungi isolates as growth promoters of the grass Urochloa Brizantha. Fungal Ecol. 2024, 70, 101355. [Google Scholar] [CrossRef]
- Bamisile, B.S.; Akutse, K.S.; Siddiqui, J.A.; Xu, Y. Model application of entomopathogenic fungi as alternatives to chemical pesticides: Prospects, challenges, and insights for next-generation sustainable agriculture. Front. Plant Sci. 2021, 12, 741804. [Google Scholar] [CrossRef] [PubMed]
- Rondot, Y.; Reineke, A. endophytic Beauveria bassiana in Grapevine vitis Vinifera (L.) reduces infestation with piercing-sucking insects. Biol. Control 2018, 116, 82–89. [Google Scholar] [CrossRef]

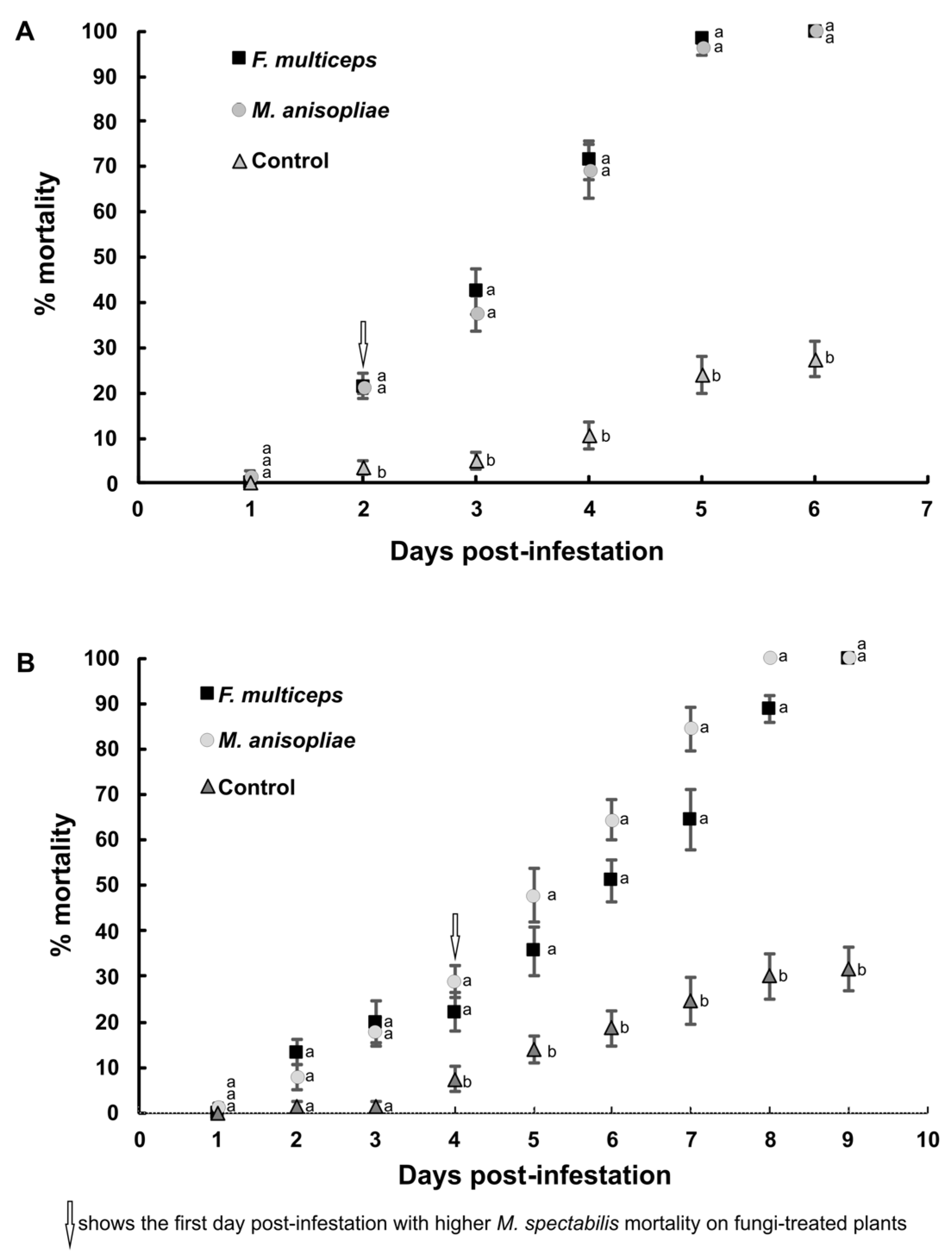
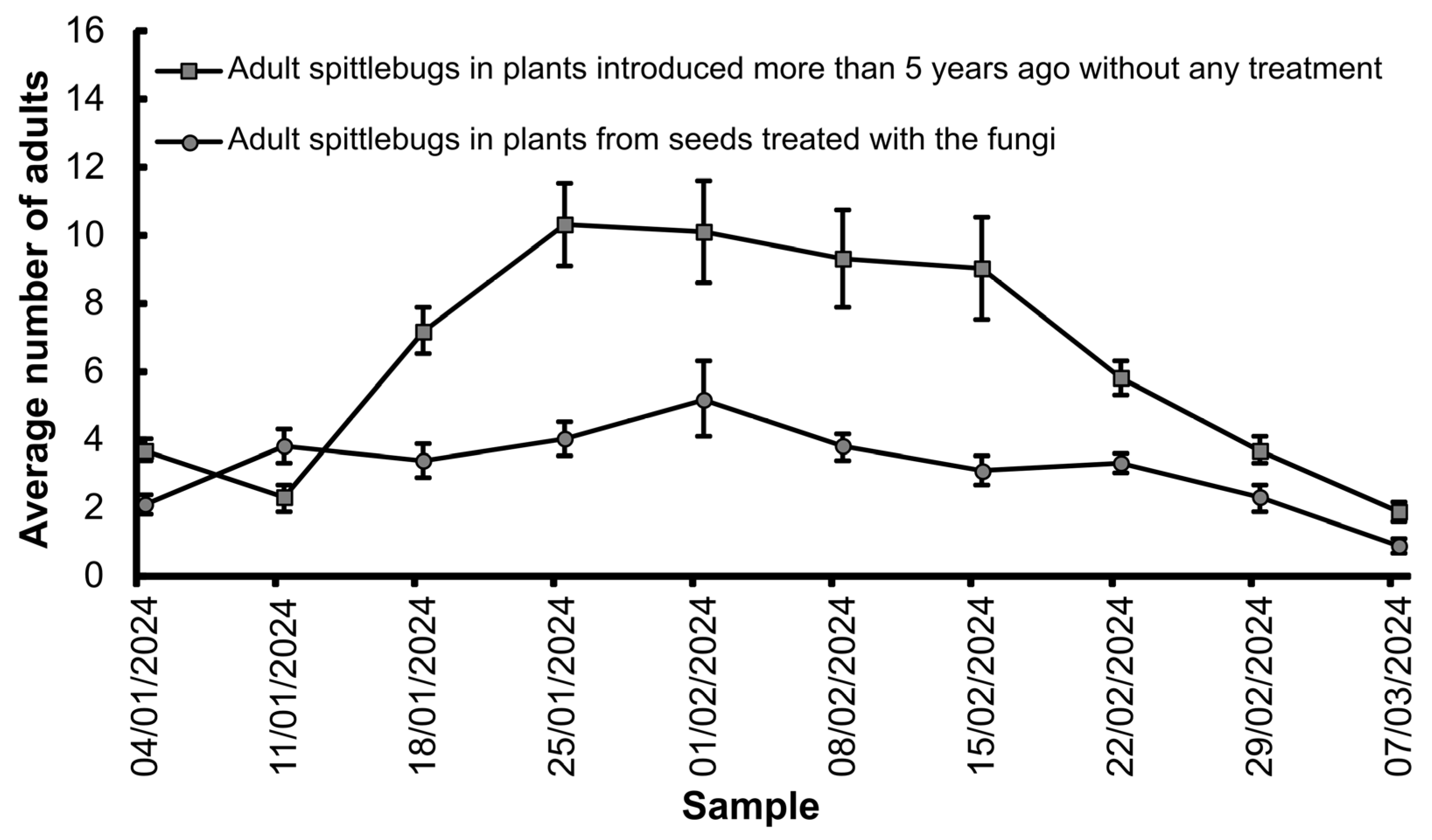
| Location | Insect Stage | Treatments | Average (%) | |
|---|---|---|---|---|
| Greenhouse | Nymphs | Fusarium multiceps | 53.3 ± 11.2 a | χ2 = 227.19; p = 0.008 |
| Metarhizium anisopliae | 52.9 ± 11.1 a | |||
| Control | 0 b | |||
| Adults | Fusarium multiceps | 77.7 ± 6.0 a | χ2 = 183.75; p = 0.020 | |
| Metarhizium anisopliae | 58.9 ± 11.7 a | |||
| Control | 0 b | |||
| Field: Artificial Infestation (*) | Nymphs | Fusarium multiceps | 90.5 ± 6.1 a | X22;11 = 65.70; p < 0.0001 |
| Metarhizium anisopliae | 100 ± 0.0 a | |||
| Control | 0 b | |||
| Adults | Fusarium multiceps | 48.8 ± 5.2 a | X22;131 = 10.18; p = 0.0062 | |
| Metarhizium anisopliae | 44.6 ± 4.9 a | |||
| Control | 0 b | |||
| Field: Natural Infestation (**) | Nymphs | Fusarium multiceps | 51.9 ± 7.4 a | X22;59 = 9.71; p = 0.008 |
| Metarhizium anisopliae | 47.7 ± 6.8 a | |||
| Control | 0 b | |||
| Adults | Fusarium multiceps | 57.5 ± 5.3 a | X22;111 = 21.63; p < 0.0001 | |
| Metarhizium anisopliae | 75.3 ± 4.6 a | |||
| Control | 0 b |
| Location | Treatment | Days After Treatment | ||||||
|---|---|---|---|---|---|---|---|---|
| 30 | 60 | 90 | 120 | 150 | 180 | 210 | ||
| F. multiceps | 77.8 ± 4.7 a | 77.8 ± 14.7 a | 66.7 ± 16.7 a | 44.4 ± 17.6 a | 33 ± 16.7 a | 22 ± 14.7 a | 11 ± 11.1 a | |
| Greenhouse | M. anisopliae | 88.9 ± 11.1 a | 88.9 ± 11.1 a | 88.9 ± 11.1 a | 77.8 ±14.7 b | 33 ± 16.7 a | 11 ± 11.1 a | 11 ±11.1 a |
| Control | 0 b | 0 b | 0 b | 0 a | 0 a | 0 a | 0 a | |
| F. multiceps | 85.7 ± 14.3 a | 71.4 ± 18.4 a | 85.7 ± 14.3 a | 85.7 ± 14.3 a | 28.6 ± 18.4 a | 28.6 ± 18.4 a | 14.3 ± 14.3 a | |
| Field | M. anisopliae | 78.5 ± 14.3 a | 78.4 ± 18.4 a | 78.4 ± 14.3 a | 64.3 ± 18.4 a | 43.8 ± 20.2 a | 28.6 ± 18.4 a | 28.6 ± 18.4 a |
| Control | 0 b | 0 b | 0 b | 0 b | 0 a | 0 a | 0 a | |
| Location | Insect Stage | Treatment | Damage Score (%) | |
|---|---|---|---|---|
| Greenhouse | F. multiceps | 46.8 ± 4.5 a | ||
| Nymphs | M. anisopliae | 19.5 ± 4.4 b | X22;24 = 23.24; p < 0.0001 | |
| Control | 76.3 ± 2.1 c | |||
| F. multiceps | 48.3 ± 3.1 a | |||
| Adults | M. anisopliae | 46.3 ± 1.3 a | X22;24 = 13.65; p < 0.0001 | |
| Control | 71.1 ± 3.1 b | |||
| F. multiceps | 46.7 ± 4.6 a | |||
| Field | M. anisopliae | 62.5 ± 3.2 b | X22;54 = 22.09; p < 0.0001 | |
| Control | 71.9 ± 3.7 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campagnani, M.O.; Calsavara, L.A.; de Oliveira, C.M.; Auad, A.M. Silent Allies: Endophytic Entomopathogenic Fungi Promote Biological Control and Reduce Spittlebug Mahanarva spectabilis Distant, 1909 (Hemiptera: Cercopidae). J. Fungi 2025, 11, 492. https://doi.org/10.3390/jof11070492
Campagnani MO, Calsavara LA, de Oliveira CM, Auad AM. Silent Allies: Endophytic Entomopathogenic Fungi Promote Biological Control and Reduce Spittlebug Mahanarva spectabilis Distant, 1909 (Hemiptera: Cercopidae). Journal of Fungi. 2025; 11(7):492. https://doi.org/10.3390/jof11070492
Chicago/Turabian StyleCampagnani, Michelle O., Luís Augusto Calsavara, Charles Martins de Oliveira, and Alexander Machado Auad. 2025. "Silent Allies: Endophytic Entomopathogenic Fungi Promote Biological Control and Reduce Spittlebug Mahanarva spectabilis Distant, 1909 (Hemiptera: Cercopidae)" Journal of Fungi 11, no. 7: 492. https://doi.org/10.3390/jof11070492
APA StyleCampagnani, M. O., Calsavara, L. A., de Oliveira, C. M., & Auad, A. M. (2025). Silent Allies: Endophytic Entomopathogenic Fungi Promote Biological Control and Reduce Spittlebug Mahanarva spectabilis Distant, 1909 (Hemiptera: Cercopidae). Journal of Fungi, 11(7), 492. https://doi.org/10.3390/jof11070492







