Abstract
The name Clavaria zollingeri Lév. is currently applied to striking violet species producing branched basidiomata and lacking clamp connections, two typical characteristics of the genus Clavaria Pers. Interestingly, as currently interpreted, C. zollingeri has been globally assessed as Vulnerable by the IUCN and is red-listed in several European countries. However, the type material of C. zollingeri, examined here, possesses clamp connections and should be referred to the genus Clavulinopsis Van Overeem. Thus, the name C. zollingeri is being misapplied. Based on the taxonomic revision of the specimens, along with morphological and molecular studies of the nrDNA ITS-LSU regions, three species differing in spore characters, basidial size and distribution are recognized. After our nomenclatural revision we conclude that one of the species should be named Clavaria amethystina (Holmsk.) Bull., characterized by ellipsoid spores and distribution in the Northern Hemisphere; the second C. lilacina Jungh., with subglobose spores and present in Eastern Asia and Oceania; whereas the third, also with subglobose spores and distributed in Europe and North America, is newly described as C. violaceopulchra. Clavaria orientalis is proposed to be a later synonym of C. lilacina. Nine type specimens were examined, the name C. amethystina is typified and the combination of C. zollingeri in Clavulinopsis is proposed.
1. Introduction
The name Clavaria zollingeri Lév., originally described from Java [1], is applied to one, but in reality several, striking, dichotomously branched and deep violet-colored species (referred to as C. zollingeri s. auct. below), the color of which fades to pink with age. Clavaria zollingeri s. auct. is distributed in North America [2], South America [3], Asia [4], Oceania [5] and Europe, where it is red-listed in the Czech Republic [6], Denmark [7], Norway [8], Poland [9] and Sweden [10]. More recently, Jordal and Kautmanová [11] assessed C. zollingeri s. auct. globally and listed it as Vulnerable. The branched, deeply violet-colored fruitbodies of C. zollingeri s. auct. are unique within Clavaria L. nom. sanct. Fr. [12], a genus characterized by simple clavarioid fruitbodies, hyaline spores, absence of clamp connections on the context hyphae [13], and basidia of chiastic nuclear division [14,15]. Molecular data also support the placement of C. zollingeri s. auct. in Clavaria [16,17].
Clavaria zollingeri s. auct. was known in Europe before Léveillé introduced the name C. zollingeri in 1846 (e.g., [18] (p. 117, sub Clavaria purpurea Schaeff.); [19] (p. 110, sub Ramaria amethystina Holmsk.); [20] (p. 169, sub Clavaria amethystina (Holmsk.) Bull.); [21], (p. 38, sub C. amethystina)). Interestingly, earlier names that appear to refer to the same taxon exist—some even based on European material—such as Clavaria brachycera Pers. [22] (p. 61), C. violacea Vill. [23] (p. 1050), C. lilacina Jungh. [1] (p. 216), C. alcicornis Zoll. & Moritzi [24] (p. 382) or C. amethystina (Holmsk.) Bull. nom. sanct. Fr., but these names are not in current use. Olariaga [25] examined the holotype of C. zollingeri and observed clamp connections on the context hyphae, a characteristic suggesting that C. zollingeri was not a species of Clavaria. Therefore, the name C. zollingeri is currently being misapplied and even used for endangered species without clamp connections.
Evidence from various sources suggests that more than one species is subsumed under the name C. zollingeri s. auct. Even within Europe, the spore size values provided by different authors show significant variation. While some authors describe the spores as subglobose [25], sub Clavaria schaefferi Sacc. [26], others characterize them as ellipsoid [12,27]. Additionally, records assigned to C. zollingeri from Australasia and the Americas have been suggested to belong to a different species from that found in Europe [11] as well. Recently, Yan et al. [4] described two new species closely related to C. zollingeri based on material from China: C. orientalis P. Zhang & Ju. Yan, differing from C. zollingeri in its shorter basidia, and C. tongdaoensis P. Zhang & Ju. Yan., characterized by a very pale lilac color. Although these authors referred to Corner [12] and Franchi & Marchetti [26], they did not consider or discuss earlier names potentially applicable to the newly proposed taxa. Our analyses of sequences deposited in public databases show a high sequence divergence, supporting also the idea that more than one violet, branched species of Clavaria may exist. No global revision including molecular data has been conducted to date.
In this framework, the three main goals of this study are to test species boundaries within the C. zollingeri s. auct. complex based on morphological and molecular data, to propose the correct names for the taxa recognized by examining available type specimens, as well as reference specimens and pertinent typifications for each name to stabilize their interpretations.
2. Materials and Methods
2.1. Herbarium Specimens and Morphological Study
Descriptions were compiled from notes made from fresh material, with further details observed upon dry material. Color codes follow the Royal Horticultural Society [28] for fresh material and Munsell Color Corporation [29] for dry material. Basidiospores were measured in side view from hymenium mountings excluding the apiculus using KOH 5%. Abbreviations referring to basidiospores are the following: Lm = mean length, Wm = mean width, Qm = Lm/Wm; 25 basidiospores were measured per collection. Water and Congo red in ammonia were also used to examine the material. The original literature and nomenclatural types were examined in person. The specimens examined are deposited in ARAN, BCC, BIO, C, BZ, FH, G, H, K, O, PAD, PC, PDD, SALA and UPS herbaria [30]. LAZA refers to the herbarium of the “Sociedad Micológica Salmantina Lazarillo” mycological society. The herbarium specimens examined were filed under the name Clavaria zollingeri Lév. unless otherwise stated.
2.2. Nomenclature
The Articles and Examples cited in this paper have been extracted from the current International Code for Nomenclature of algae, fungi and plants [31].
2.3. DNA Extraction, PCR Amplification, Sequencing and Alignment
DNA was extracted from fresh and dried collections with the DNeasy Plant Mini Kit (QIAGEN, Crawley, West Sussex, UK), following the manufacturer’s protocol. The primer combination ITS5-ITS4 [32] was used to PCR amplify the ITS region, and LR0R [33] and LR5 [33,34] for the LSU region. PCR products were cleaned using ExoSAP-IT® (USB, Cleveland, OH, USA). The PCR amplicons were sequenced in both directions using the same primers. Sequences were edited and assembled using Sequencher v. 4.10 (Gene Codes Corporation Ann Arbor, MI, USA) and have been deposited in GenBank (Table 1). The ITS and LSU sequences were aligned manually in AliView 1.30 [35] using Muscle v5 [36]. Additional sequences were downloaded from the EMBL/GenBank and UNITE databases. Regions that could not be unambiguously aligned were visually detected and removed. Based on Birkebak et al. [37], sequences of Clavaria fumosa Pers. (KP257126), C. cf. fumosa (KP257127) and C. cf. rubicundula Leathers (HQ877697) were set as outgroup.

Table 1.
Sequenced specimens and their respective original names, UNITE and GenBank accession numbers, mean spore length (Lm), width (Wm) and length/width (Wm). Newly generated sequences are marked in bold.
2.4. Phylogenetic Analyses and OTU Delimitation
The ML analysis was conducted in IQ-TREE [38], starting from a random tree and letting IQ-TREE test the best partitioning scheme under default options. To evaluate branch confidence, 1000 ML bootstrap repetitions were performed using standard bootstrapping. Bootstrap values were considered significant when ≥70% [39]. The Bayesian analysis was carried out in MrBayes v. 3.2.7 [40] via the CIPRES Science Gateway [41]. Two parallel runs of eight Metropolis-coupled Markov chain Monte Carlo (MCMCMC) chains were implemented for 30 M generations, starting from a random tree, and sampling one tree every 100th generation from the posterior distribution. The same partition scheme as in the ML analysis was set, with model parameters unlinked across partitions. Substitution models were sampled across the GTR space during the MCMC simulation [40]. Stationarity was assumed when average standard deviation of split frequencies fell below 0.01. Convergence was further visually diagnosed using Tracer v. 1.7.2. [42]. A burn-in sample of the first 50% of the trees was discarded. To assess branch confidence, a 50% majority rule consensus tree was calculated with the remaining trees using the command SUMT of MrBayes. Bayesian posterior probability (PP) values ≥ 0.95 were considered to be significant.
3. Results
3.1. Morphological Study
The Lm and Wm spore values of thirty-eight sequenced specimens of C. zollingeri complex were plotted (Figure 1, Table 1) for comparison, in addition to the spore values of the type material of C. rosolana. A group of specimens had ellipsoid spores with a Qm value ranging between 1.27–1.78, whereas another group showed more rounded, subglobose spores, with a Qm range of 1.13–1.28. The spores from the type of Clavaria rosolana Petch were the longest (Lm = 6.5) and the most ellipsoid ones (Qm = 1.93).
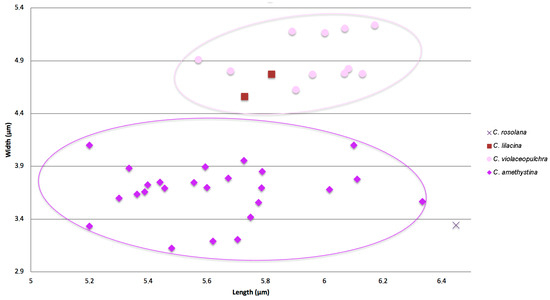
Figure 1.
Diagram showing spore measurements from collections within the Clavaria zollingeri complex. Spore measurements of C. rosolana Petch were obtained from the isotype (K[M]168006).
3.2. Molecular Study
Fifty ITS and nine LSU sequences were generated from fifty specimens (Table 1), from which representative sequences were selected for phylogenetic analyses. The alignment contained 1229 positions and 51 sequences. The ML and Bayesian analyses resulted in consensus trees with highly similar topologies, and congruent, well-supported clades (Figure 2). Specimens assigned to C. zollingeri s. auct. formed a large strongly supported clade (ML-BP 78; PP 0.96) basal to the three sequences of C. tongdaoensis. The large clade is composed of a subclade attributed here to C. amethystina (Holsmk.) Bull. (ML-BP 100; PP 1) and another supported subclade (ML-BP 100; PP 1) that contains two smaller subclades described below as C. violaceopulchra (ML-BP 100; PP 1) and C. lilacina Jungh. (ML-BP 93; PP 1), respectively. The latter two subclades show long branches, indicating a high sequence divergence between both. The three clades show a sequence divergence that is correlated with their geographic origin. The C. amethystina clade contains six supported and one unsupported subclades, as well as a branch composed by a single sequence. Two of the clades contain only North American material, four only European, and two Asian sequences, respectively. The C. lilacina clade contains three supported clades composed of material from China, Australia and New Zealand, respectively. The C. violaceopulchra clade does not contain any supported clade, but a sequence obtained from North American material is in a long branch. The clade named C. amethystina (statistics based on sequenced material alone) is characterized by having very variable, but more ellipsoid (Qm range = [1.27]1.37–1.91) and narrower spores (Wm range= 3.1–4.1), whereas the other two, C. lilacina (Qm range = 1.22–1.26; Wm range = 4.6–4.8) and C. violaceopulchra (Qm range = 1.08–1.28; Wm range = 3.6–5.2), possess broadly ellipsoid to subglobose spores.
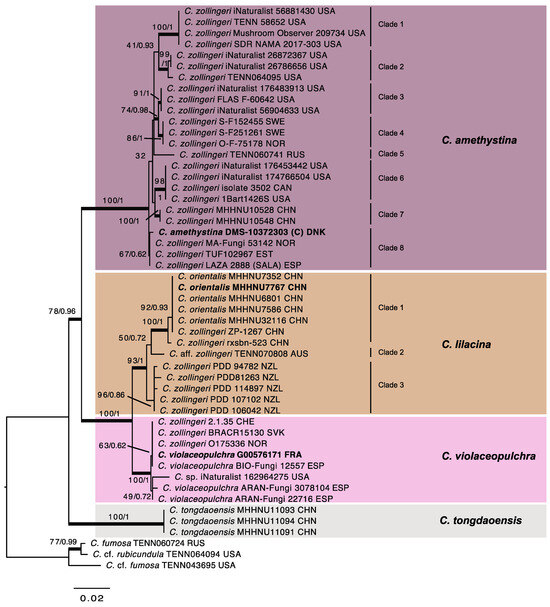
Figure 2.
Most Likely Tree from Maximum Likelihood analysis of selected sequences within the Clavaria zollingeri s. auct. complex. Maximum Likelihood bootstrap values (ML-Boot)/Bayesian Posterior probabilities (PP) are shown by nodes, ordered as ML-BP/PP. Thickened branches received support in both analyses (ML-BP ≥ 70% and/or PP ≥ 0.95). Voucher specimens and ISO 3166-1 alpha-3 codes [43] for countries are provided after the original identifications of the sequences. Sequences in bold refer to type or reference specimens.
3.3. Type Study of Clavaria zollingeri
Léveillé [1] (p. 155) described C. zollingeri based on a specimen collected by Zollinger in Java, which Zollinger [24] (p. 381) himself had earlier published as Clavaria amethystina. Léveillé indicated as collection data of the specimen “Hab. ad truncos, Java. Zollinger, nº 992” (Figure 3A). A type specimen of C. zollingeri is housed in PC herbarium (PC0093981), which is kept in the original paper envelope. A printed label of Plantae Javanicae is glued to it, with the following handwritten text: “Clavaria Zollingeri Lév. No 99.z Clavaria amethystina Bull. Fr.? Zoll! In Natur-en Genesch. Arch. 1844. p. 381. Im Wäldchen auf der Erde beim Kampong(dorf). Tjikoya. Febr. 1843”. A second label with a different handwriting is glued on it, reading: “nº 99. Clavaria Zollingeri Lev. sp. nov. elle differe de l’amethystina de Bull par ses rameaux dichotomes. Scripsit Leveille” (Figure 3B). The paper sheet where the specimen is placed bears the notation “ad truncos” on it (seen by I. Olariaga).

Figure 3.
(A). Original description of Clavaria zollingeri by Léveillé reproduced from Ann. Sci. Nat., Bot., sér. 3, 5: 155. 1846. Source: Biodiversity Heritage Library. (B). Label of the holotype collection of C. zollingeri Lév. (PC0093981). Photograph: I. Olariaga.
The specimen in PC was thus seen by Léveillé. The writing “ad truncos”, and above all, the accompanying Léveillé handwriting demonstrate that it was the single specimen on which Léveillé based his description of C. zollingeri. The discrepancy noted by Van Overeem [44] between the collection number given on the label (“99.z”) and the protologue (“992”), is interpreted here as an oversight by Léveillé, since no. 992 in Plantae Javanicae corresponds to the cyperaceous plant “Fimbristylis albescens Steud.” [45] (p. 61) and the numbers were unique. Moritzi [46], in his account of the material sent by Zollinger to Europe, and Zollinger himself [45], refer to the C. zollingeri material sent to Léveillé as “99.z”. The similarity between “99.z” and “992” may explain the oversight. “Z” refers to specimens containing little material and not duplicated [46] (p. iv). Zollinger [45] (p. 12) himself also marked collection 99.z as “HZ 99” (“Herbarium proprium Zollingeri”), meaning that the specimen was kept in his herbarium [45] (p. ix) and was not distributed. The location of this material is unknown to us (see below). A second discrepancy exists between the habitat information on the label and the protologue. The label reads “Im Wäldchen auf der Erde”, which is translated as “in the small forest on soil”. This conforms to what Zollinger ([24,45], p. 12) and Moritzi [46] (p. 124) cited for the material sent to Léveillé. The inscription “ad truncos” appears to have been added by Léveillé to the paper sheet on which the specimen was kept. The fact that Zollinger and Moritzi consistently cited the specimen sent to Léveillé suggests that “ad truncos” was erroneously written or translated by Léveillé. Regardless of whether this was an error, it is clear that this specimen was seen by Léveillé and that he coined the name Clavaria zollingeri based on it.
Another type specimen numbered as Plantae Javanicae 99.z was located at FH within the Patouillard herbarium (FH 00290380). The label bears the same information as the specimen kept at PC, but it is typed. We regard the specimen at PC as the one seen by Léveillé, and hence, the holotype, whereas the specimen at FH is a duplicate, i.e., an isotype. The material kept in Zollinger’s own herbarium could not be found at AMD, B, BO, G, K, L, S or UPS.
As expected, examination of the holotype and isotype revealed identical morphological characteristics. Both consist of small and fragmented pieces of branches, black, 1–2.7 mm in diam. Microscopically, the structures are collapsed, but relatively well observable when rehydrated in KOH 5%. Basidiospores are broadly ellipsoid, smooth, 5–7 × 3.5–5 µm (Lm = 6.1, Wm = 4.5, Qm = 1.35; n = 25). Basidia are claviform, long stalked, sometimes sclerified (wall up to 1 µm thick), 44–58 × 5.5–7 µm. The context is formed by a mixture of wide and narrow hyphae, cylindrical to swollen, hyaline, septate, with clamp connections in many septa, 8–12 µm in diam. (Figure 4).
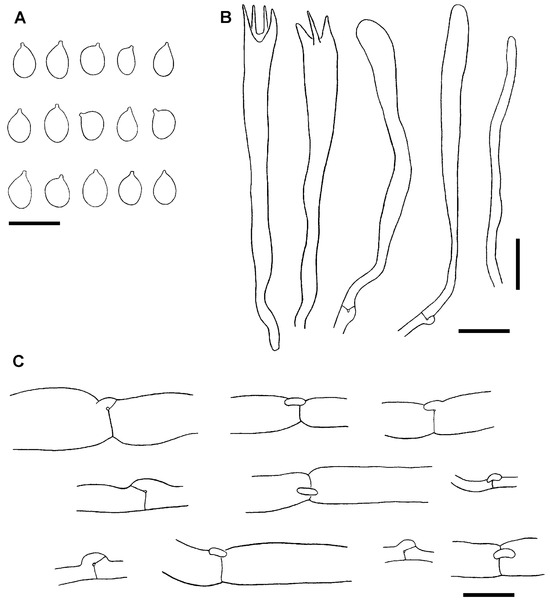
Figure 4.
Microscopic structures of the holotype collection of Clavaria zollingeri Lév. (PC0093981). (A). Basidiospores. (B). Basidia and basidioles. (C). Hyphae from the context with clamp connections. Scale bars = 10 µm. Author I. Olariaga.
Both type specimens differ from C. zollingeri s. auct. in having clamp connections on the context hyphae, a diagnostic character at the generic rank within the Clavariaceae [12,13]. The presence of sclerified basidia, typically found in this family [25,47], suggests that the holotype of C. zollingeri belongs to the Clavariaceae lineage within the clavarioid fungi. Due to the presence of clamp connections, hyaline spores and long basidia, we propose below to combine C. zollingeri in Clavulinopsis.
3.4. Taxonomic Treatment
3.4.1. Clavaria amethystina
Clavaria amethystina (Holmsk.) Bull., Herb. France 11: Table 496, Figure 2. 1791 [“amethystea”] nom. sanct. Fr., Syst. Mycol. 1: 472. 1821. ≡ Ramaria amethystina Holmsk., Beata Ruris 1: 110. 1790 [basionym]. ≡ Clavulina amethystina (Holmsk.) Donk, Meded. Bot. Mus. Herb. Rijks. Univ. Utrecht 9: 23. 1933. ≡ Cladaria amethystina (Holmsk.) Doty, Lloydia 13(1): 14. 1950. Lectotype (designated here): [icon] “Ramaria amethystina” in Holmskjold, Beata ruris 1: t. 11. 1790. MycoBank Typification: MBT 10020236. Reference specimen: Denmark. Midtjylland, Engelsholm, Sønderskov, on earth in broadleaf forest among other clavarioids, Hygrocybe spp., Hodophilus spp., etc., 21 August 2023, leg. T. Læssøe, DMS-10372303 (C!).
= Clavaria lavendula Peck., Bull. New York State Mus. 139: 47. 1910 − Lectotype: USA, Massachusetts, Stow, chestnut grove, 26 July 1909, leg. S. Davis, NYSf1666 (designation achieved by Coker [2] accepting it as the type, according to Art. 7.11).
=? Clavaria schaefferi Sacc., Syll. Fung. 6: 693. 1888. [“schäfferi”, corrected according to Art. 60.7] [nom. nov. based on Clavaria lilacina Fr.]. ≡ Clavaria lilacina Fr., Hymenomyc. Eur.: 667. 1874 [nom. illeg. Art. 53, non Clavaria lilacina Jungh., Ann. Sci. Nat., Bot., sér. 3, 2: 216. 1844]. ≡ Clavaria sublilacina P. Karst., Bidrag Kännedom Finlands Natur Folk 48: 375. 1889. [nom. nov. based on Clavaria lilacina Fr.; nom. illeg. Art. 52, the homotypic name Clavaria schaefferi with priority ought to have been adopted]. ≡ Clavaria amethystina var. purpurea Bourdot & Galzin, Hymenomyc. France: 106. 1928. [“1927”]—Lectotype (here designated): Norway, Hordaland, Bergens stift, Etne praestgaard, paa fuktig mark, paa Rottene, 4 August 1864, leg. C. Sommerfelt, UPS F-127181 (!; in sched. sub Clavaria lilacina). Mycobank MBT 10026998.
=? Clavaria rosolana Petch, Ann. Roy. Bot. Gard. Peradeniya 7(4): 290. 1922—Isotype: Sri Lanka, Waga, on the ground, August 1917, leg. T. Petch 5249 (K(M)168006 (!)).
− Clavaria purpurea Schaeff., Fung. Bavar. Palat. Nasc. 4: 117. 1774 pro parte (description & plate) typus excluded [“Clvaria”]
- Description
Basidiomata gregarious, 25–60 × 13–25 mm, branched, sometimes with an indistinct stipe. Branching dichotomous to irregularly dichotomous or trichotomous, with U-shaped angles, with a few V-shaped, parallel, branching rank 2–4. Branches cylindrical, solid, bright violet (8A, 8B), fading to pale pink (62D) in age, when dried light ochre (7.5YR 7/2, 8/2) or ochre with a violet hue (5YR 7/2, 8/2). Apices obtuse, sometimes subacute, concolorous or slightly darker. Stipe when present 8–18 × 3–5 mm, cylindrical, bright to paler violet lilac (84A). Whitish mycelium present at the base, seldom forming erect tufts. Context white, taste and odor not recorded. Reaction with iron salts not tested. Basidiospores ellipsoid in side view, a few broadly ellipsoid, thin-walled, smooth, non-amyloid, (4–)5–6.5(–8) × 3–4(–4.5) µm (Lm range= 5.2–6.7, Wm range = 3.1–3.9, Qm range = (1.27–)1.37–1.91. Basidia clavate, (2–)4-spored, without clamps, 32–42 × 6–8 µm. Context formed by tightly interwoven hyphae, cylindrical to fusiform, closely septate, thin-walled, hyaline to very pale yellow, smooth, without clamps, 4–15 µm broad. Figure 5.

Figure 5.
Clavaria amethystina (Holmsk.) Bull. (DMS-10372303(C), reference specimen). (A–C). Basidiomata in situ. (D). Basidiospores. Scale bar = 10 µm. Photographs: T. Læssøe (A–C), I. Olariaga (D).
- Distribution and ecology
Widely distributed in the Northern Hemisphere, in temperate areas of continental Europe, confirmed records from Czech Republic, Denmark, Finland, Norway, Slovakia, Sweden, Spain and United Kingdom (following Cotton and Wakefield [48]), North America (Canada, USA) and Asia (China). Records from the Southeast insular Asia to be confirmed. Mainly occurring in non-fertilized pastures in Europe, whereas its prefers habitats are broadleaf and coniferous forests in North America and Asia [4].
- Specimens examined
CZECH REPUBLIC. Czech Republic, Špindlerův Mlýn, Jelení boudy, in mosses and grass in mountain meadow, 27 October 2014, leg. D. Bureš, BRACR21846.
FINLAND. Etelä-Häme Prov., Juupajoki, Hyytiälä Forest Field Station surroundings, in grass, 6 September 2005, leg. S. Jacobsson, BRACR4318. Etelä-Häme Prov., Tammela, Mustiala, 12 August 1866, leg. P.A. Karsten, H6002010, (in sched. sub C. sublilacina); duplicate in UPS F-121023, (in sched. sub C. amethystina). Varsinais-Suomi, Naantali, Merimasku June 1860, leg. P.A. Karsten, H6081401(!).
FRANCE. Aveyron, Evès, sous des châtaigniers, leg. Galzin, 1 October 1914, Galzin 14845 (UPS).
NORWAY. Agder, Lillesand, Skolehusheia, oak forest with hazel and spruce, 30 September 2023, leg. D. Pettersen & I.L. Fonneland, O-F-260485. Akershus, Nesodden Røer, in mixed deciduous forest, 9 September 2009, leg. V. Kautman, BRACR13263; in broadleaved forest with hazel and oak, 30 August 2008, leg. A. Molia & A.O. Folkestad, O-F-069072. Akershus, Vestby, Gjekstad gard, under Picea abies, mossy clearing in the forest, August 2002, leg. P.P. Daniëls, Daniëls 1196 (MA-Fungi 53142). Aust-Agder, Arendal, Rønningheia, in rich deciduous forest, among Plagiochila asplenoides, 19 September 2004, leg. T.H. Dahl, O-F-187574. Aust-Agder, Arendal, Tromøy, on bare ground, hazel, oak, lime, 3 August 1998, leg. T.H. Dahl, O-F-090541. Aust-Agder, Grimstad, Tjore, in deciduous forest, hazel, in mosses, 15 September 1999, leg. I.L. Fonneland, O-F-132134. Aust-Agder, Grimstad, Vatnestrand, under hazel, in ground and mosses, 2 September 2000, leg. I.L. Fonneland, O-F-161801. Bomlo, Vestre Vika, sheep grazed seminatural grassland, 6 October 2023, leg. D. Pettersen, BRACR39246. Hedmark, Os, Dalengvollen, in open forest, 21 August 2010, leg. P. Marstad, O-F-244573. Hordaland, Bømlo, Sønstebømakjo, Melhus Lynghei, pasture, 1 October 2011, leg. P. Fadnes & A. Knutsen, O-F-242537. Hordaland, Bergens stift, Etne praestgaard, 22 July 1865, leg. C. Sommerfelt, UPS F-127180 (syntype of Clavaria schaefferi, in sched. sub C. lilacina). Hordaland, Bømlo, Vestre Vika, in pasture, 25 October 2006, leg. A. Knutsen, O-F-361554. Hordaland, Lindås, Lygra, in pasture, 24 September 2014, leg. J.B. Jordal, O-F-251624. Hordaland, Stord, Kjølsvika, Føyno, shore meadow, 1 October 2011, O-F-242581. Møre og Romsdal, Aure, Husfest, seminatural pasture, 29 September 2020, leg. J.B. Jordal, O-F-312241. Møre og Romsdal, Herøy, Runde, Goksøy, in pasture, 6 October 2016, leg. J.B. Jordal, O-F-254064. Møore og Romsdal, Sande, Sandsøya, Ulandsvika, at the edge of heath, in pasture, 24 September 2009, leg. J.B. Jordal, O-F-291170. Møre og Romsdal, Sunndal, Hagen, in pasture, 8 September 2016, leg. J.B. Jordal, O-F-254066. Møre og Romsdal, Sunndal, Kalvhusvøttu, in pasture, 27 July 1999, leg. J.B.Jordal, O-F-240114. More og Romsdal, Tingvoll, Gyl, in pine forest with scattered deciduous trees, 7 September 2001, leg. G. Gaarder & K. Bang, O-F-176149. Møre og Romsdal, Tingvoll, Liaslettet øst, near road under hazel shrubs, 12 September 2004, leg. G. Gaarder, O-F-360957. Nord Trøndelag, Lierne, Nordli, Kvernvika, in meadow, 12 September 2005, leg. G. Gaarder, O-F-281193. Nord Trøndelag, Steinkjer, Høgmennen, lakeshore, partially outgrown, 7 August 2005, leg. S. Reiso, O-F-285708. Nordland, Hemnes, Solhaug, in pasture, 22 July 2013, leg. G. Gaarder, O-F-2461666. Nordland, Steigen, Hesta sletta ved Laskestad, in rich calcareous pasture, 8 September 2002, leg. G. Gaarder, O-F-223492. Nordland, Vevelstad, Almoselva, in sheep grazed meadow, 1 September 2003, leg. G. Gaarder & T. Hofton, O-F-223396. Oppland, Vestre Toten, Finnstad, in pasture, 17 August 2000, leg. B.H. Larsen & G. Gaarder, O-F-224204. Oslo Fylke, Brannfjell S-V for Svarta, in mosses, in wet shaded forest, 11 July 2002, leg. G. Flatabø, O-F-065543. Rennebu, Hol, Sostuggu, 13 September 2016, leg. S. Vatne, BRACR25781. Rennebu, Rise, 8 September 2016, leg. S. Vatne, BRACR25780. Sogn og Fjordane, Gloppen, Nord for Fella, in cattle pasture, in calcareous soil, in grass, 1 August 2001, leg. G. Gaarder, O-F-176179. Sogn og Fjordane, Selje, Honningsvagen, in pasture, calcareous, 3 October 2000, leg. G. Gaarder & J.B. Jordal, O-F-223561. Sogn og Fjordane, Stryn, Bøasetra, in poor soil, 5 August 2001, leg. G. Gaarder, O-F-176147. Spyssoya, Myra, sheep grazed meadow, 7 October 2023, leg. F. Fuljer, BRACR39145. Steinkjer, Mokk, in grass, 3 September 2009, leg. M. Jeppson & E. Larsson, BRACR13372. Sør-Trøndelag, Oppdal, Gorsetlia, in pasture, 21 August 2008, leg. J.B. Jordal, O-F-287940. Trøndelag, Oppdal, Vinstradalen, in pasture, 21 August 2017, leg. J.B. Jordal, O-F-257161. Vestfold, Hof, Sæteråsen, Lagurtskog, 13 September 2011, leg. T.N. Kristiansen & P. Marstad, O-F-244642. Vestfold, Ramnes, Fossan/Ås, wet ridge, 10 September 1998, leg. K. Geelmuyden, O-F-63441.
SLOVAKIA. Nízke Tatry, Malužiná, Michalovo, in mowed and grazed meadow, 1 September 2010, leg. V. Kautman, BRACR15965. Nízke Tatry, Malužiná village, Michalovo valley, “Nad Gašperíkom”, 22 August 2014, leg. V. Kautman, BRACR36900. Nízke Tatry, Malužiná, Michalovo valley, in pasture, 17 September 2016, leg. I. Kautmanová, BRACR27015. Považský Inovec, Kálnica, Medňanské lúky, pasture, 17 September 2014, leg. J. Herman, BRACR36938. Revúcka vrchovina, Betliar, Straková, 1 July 2013, leg. M. Merva, BRACR24274. Stolicke vrchy, Kokava nad Rimavicou, Háj, in pasture, 10 September 2014, leg. M. Smiková & P. Smik, BRACR28365. Veporské vrchy, Kokava—Háj, pasture, 3 October 2014, leg. V. Kautman, BRACR36929.
SPAIN. Basque Country, Gipuzkoa, Oiartzun, Oieleku, under Pteridium aquilinum, on the ground, 10 July 1990, leg. J.M. Lekuona, ARAN-Fungi A3020304. Castilla y León, Salamanca, El Cabaco, La Dehesa, on humus of Quercus pyrenaica forest, 26 May 2011, leg. E. Rolo, J.I. Gómez & L.A. Fernández, LAZA 2888 (SALA).
SWEDEN. Gästrikland, Sandviken, Sandvikens kyrka, Åsgatan, i gräsmatta mot kyrkogårdsmuren (in grass), 5 September 1997, leg. O. Lennström, UPS F-013096. Medelpad, Torp sn., Finnsjön, äng (open grassland with scattered trees), 25 August 2009, leg. J.-O. Tedebrand & L. Vessberg, S-F251261). Södermanland, Handen, Getporsvägen 5, lawn in a garden, 19 September 2011, leg. L. Poile, S-F198736. Uppland, Upplands-Väsby, Runby hage, bland gräs och mossa i hagmark (among grass and mosses in grassland), 27 September 1943, leg. L.J. Söderström, UPS F-121017. Värmland, Hagfors kommun, Gustav Adolf församling, Malmbackarna. Naturbetesmark (natural grazed grassland), 17 September 2009, leg. F. Turander, S-F152455. Östergötland, Kvarsebo, 9 September 1951, leg. O. Lundell, UPS F-121011.
- Taxonomic comments
Clavaria amethystina appears to be the most common among the violet species of Clavaria, and most records from Europe and North America belong to this species. It differs clearly from C. violaceopulchra and C. lilacina in having more ellipsoid (Qm range = [1.27]1.37–1.91) and narrower (Wm range = 3.1–3.9 µm) spores. Macroscopically, basidiomata of C. amethystina show less tendency to fade to pink in age. Although the Holmskjold plate selected as the lectotype could represent any of the three violet Clavaria species of the complex, the most common species in the Nordic countries—and the only one known from Denmark, also present close to the Holmskjold collection area—is the one with ellipsoid spores, to which we think the epithet amethystina should be attached. Accordingly, we propose a reference specimen collected near the Holmskjold collecting area.
In the sanctioning works, under C. amethystina Fries [49] made reference to several plates, among which the ones by Schaeffer [50], Holmskjold [19] and Nees von Esenbeck [20] belong to Clavaria zollingeri s. auct. in our opinion, while the one by Bulliard [51] clearly shows basidiomata of a species of Clavulina J. Schröt, possibly Cl. coralloides (L.) J. Schröt parasitized by Helminthosphaeria clavariarum (Desm.) Fuckel. Probably due to that, the name C. amethystina was interpreted in both ways by later authors: (i) as a Clavaria species—as done here (e.g., [2,48,52,53,54]) or (ii) in Clavulina J. Schröt. as Cl. amethystina (Holmsk.) Donk (e.g., [12,55,56]). The latter interpretation, proposed by Donk [14], was likely based on the Bulliard plate. This interpretation of C. amethystina as a species of Clavulina is currently accepted (e.g., [26], although the name has been applied to several Clavulina species without a consistent taxonomic interpretation [26,57]). Interestingly, many herbarium specimens and publications attributing the name to Clavulina (as Clavulina amethystina) actually refer to species of Clavaria within the C. zollingeri s. auct. complex (e.g., [58,59]). Considering this, we follow the interpretation of C. amethystina as a Clavaria species to provide a stable name for an endangered species, rather than treating it as a Clavulina where it would likely become a synonym of Cl. coralloides if typified—according to our taxonomic interpretation of the Bulliard plate—or remain a dubious name applied to several species.
Considering that C. amethystina is widespread in North America (Figure 2), following previous authors, we treat here C. lavendula Peck as a later synonym of C. amethystina. Petersen and Olexia [60] examined the type specimen, and the spore measurements provided by them (5.7–6.8 × 2.9–4.0 µm) conform to the material of C. amethystina examined by us. The synonymy between C. lavendula and C. amethystina s. auct. was already proposed ([2,14], synonym under C. zollingeri) [12], and considered probable by Petersen and Olexia [60]. Our decision is supported by the fact that C. amethystina is widespread in North America and that the C. amethystina clade shows a low sequence divergence in North America.
Ellipsoid-spored collections similar to those of C. amethystina have been cited from Southeast insular Asia under the names C. rosolana Petch [61], C. zollingeri [12,44,61,62] and C. alcicornis Zoll. & Moritzi [63]. Regarding C. rosolana, described from Sri Lanka, the isotype we examined lacked clamp connections, and its spores (5.5–7 × 3–3.5 µm) are also similar to those of C. amethystina. We also examined two specimens collected in the Buitenzorg Botanic Garden (25 May 1921, BO 464; 24 May 1921, BO 360) used by Van Overeem [44,62] for his treatments of C. zollingeri, which included a beautiful color painting showing ellipsoid spores. Our measurements from BO 464 (4.8–6.5 × 2.5–3.2 µm) were almost identical to the ones provided by him (4.5–6.5 × 2.5–3 µm) and therefore similar to the ones of C. amethystina. Additionally, Petersen [63] attributed to C. zollingeri s. auct. a specimen labelled as C. alcicornis from L (see also Excluded Names) after observing closely septate hyphae with occasional secondary septa and spores measuring 5.9–6.7 × 3.3–4.1 (Qm = 1.69) that conform to C. amethystina. From all this evidence, we conclude that C. amethystina or a species closely related to it is present in Southeast insular Asia, but further material in better condition and supported by molecular characters would be desirable to confirm this hypothesis. Regrettably, our efforts to loan more recent material from those areas were unfruitful.
- Nomenclatural comments on Clavaria amethystina and its synonyms
The nomenclatural background of the names C. amethystina and C. schaefferi is very intricate with changes in spelling, interpretations of earlier names, invalidly published names, and illegitimate names involved. Therefore, a detailed nomenclatural account of the involved names is deemed necessary.
Some authors (e.g., [2,64]) considered Coralloides amethystina Battarra as the basionym of C. amethystina. As Donk stated repeatedly [65,66,67,68], with whom we concur, Battarra [69] did not adhere to the binomial system, and according to Art. 23.7(b), even those names that are composed of two words—including Coralloides amethystina—must be regarded as not validly published in predominantly polynomial works. Therefore, the basionym of C. amethystina is Ramaria amethystina Holmsk. [19], as it is the oldest validly published name following a binomial nomenclature system.
Clavaria amethystina (Holmsk.) Bull. is a correct combination based on R. amethystina Holmsk., even though Bulliard [51] (Table 496, Figure 2) misspelled the original epithet as “amethystea” and misinterpreted the name because his plate shows basidiomata of Clavulina coralloides (L.) J. Schröt (=Clavulina cristata (Holmsk.) J. Schröt.), parasitized by Helminthosphaeria clavariarum (Desm.) Fuckel (see Art. 7.3). Bulliard did not cite any basionym or description in his Herbier de France [51], but he made two references to “Coralloides amethystina BATT. Fung. 22. Tab. I.” and “Clavaria purpurea. SCHOEFF. Fung. Tom.II. Table 172” in his Histoire des Champignons de France [70] (p. 200). Those two references were also included by Holmskjold [19] under Ramaria amethystina, because both authors considered “Coralloides amethystina” the basionym. Thus, in accordance with Art. 41.4, since Bulliard presumably intended to make a new combination and a potential basionym (Ramaria amethystina Holmsk.) applying to the same taxon exists, Bulliard published a valid combination even though he misspelled the original epithet as “amethystea” [51] (p. 496). Fries sanctioned Clavaria amethystina in the Systema Mycologicum [49] (p. 472, sub “C. amethystina. Bull. t. 496. f. 2”) and also in the Index of the third volume [71]; (p. 71, sub “Clavaria amethystina Bull. I. 472”), referring only to Bulliard. According to Art. F.3.2 last sentence, the spelling used in the sanctioned name is to be maintained and Fries used consistently “amethystina”, the same epithet used in the basionym by Holmskjold.
Clavaria purpurea Schaeff. [18] (p. 117) is an illegitimate name (Art. 52) since Schaeffer included the type of Clavaria palmata Scop. as synonym, a name that ought to have been adopted. Therefore, nomenclaturally, the name C. purpurea becomes a homotypic synonym of C. palmata, currently Thelephora palmata (Scop.) Fr. nom. sanct., despite the fact that Schaeffer’s plate [50] and description [18] correspond to a Clavaria species and not to a Thelephora Willd.
Fries was aware that Schaeffer’s plate and description of C. purpurea did not match with T. palmata and created the name C. lilacina [54] (p. 667), based on Schaeffer’s plate and description of C. purpurea, excluding the type of C. palmata by citing this name under T. palmata [54] (p. 634). However, the name C. lilacina Fr. is also illegitimate since it is a later homonym of C. lilacina Jungh. [1] (p. 216), whereas the replacement names C. schaefferi and C. amethystina var. purpurea, based on C. lilacina, are legitimate.
The name C. sublilacina P. Karst. is also illegitimate because the homotypic name C. schaefferi with priority ought to have been adopted (Art. 52).
Clavaria schaefferi is considered here a synonym of C. amethystina after examining two syntypes (UPS F-127180, UPS F-127181). Both syntypes belong to C. amethystina as treated here. The spores of the syntype in better state (UPS F-127181) measure 5–6 × 3–4 µm and thus conform to our measurements of C. amethystina. Thus, this specimen is selected above as lectotype.
3.4.2. Clavaria lilacina
Clavaria lilacina Jungh., Ann. Sci. Nat., Bot., sér. 3, 2: 216. Oct. 1844. Lectotype (designated here): Indonesia, Java, ad truncos, without date, PC (!). Mycobank typification: MBT 10025179.
= Clavaria orientalis P. Zhang & Jun Yan, Mycokeys 115: 144. 2025. Holotype: China, Hunan Province, Shimen County, Hupingshan Nature Reserve, 11 September 2012, P. Zhang, MHHNU7767.
=? Clavaria bicolor Massee, Bull. Misc. Inform. Kew: 154. 1901 [nom. illeg. Art. 53.1] non Clavaria bicolor Raf., Med. Repos., ser. [“hexade”] 2, 5: 363. 1808. Holotype: Malaysia, Penang, ad truncos, without date, Ridley, K(M)168004 (!).
- Description
Basidiomata gregarious, up to 60 × 35 mm, branched, sometimes with a distinct stipe. Branching more or less dichotomous, sometimes trichotomous, with U-shaped angles, parallel or divergent near the apices, branching rank 2–4. Branches cylindrical, sometimes flattened of longitudinally furrowed, solid, bright violet (8A, 8B) to brownish purple (5YR 5/4, 5/3, 7.5YR 5/4, 5/3), fading to pink (9D) in age, when dried light ochre (10YR 8/2, 2.5Y 7/3, 7/4) or very pale lilac (5YR 8/1). Apices obtuse, rounded, concolorous. Stipe when present cylindrical, bright violet (8A, 8B) to brownish purple (5YR 5/4, 5/3, 7.5YR 5/4, 5/3). Whitish mycelium sometimes present at the base. Context white, taste and odor not recorded. Reaction with iron salts not tested. Basidiospores ovoid to subglobose in side view, thin-walled, smooth, non-amyloid, (5–) 5.5–6.5(–7) × (4–) 4.5–5 (–5.5) µm (Lm range = 5.7–5.8, Wm range = 4.6–4.8, Qm range = 1.22–1.26). Basidia clavate, (2–)4-spored, without clamps, 40–45 × 6–8 µm. Context formed by tightly interwoven hyphae, cylindrical to fusiform, closely septate, thin-walled, hyaline, smooth, without clamps, 7–18 µm wide. Figure 6.

Figure 6.
Clavaria lilacina Jungh. (A) Envelope of the lectotype specimen (PC). (B) Single basidioma of the lectotype specimen (PC). (C) Basidioma in situ (PDD 94782). (D) Basidioma in situ (PDD 81263). (E) Aged basidiomata with brown tones (PDD 81263). (F) Basidiospores (PDD 81263). Scale bar = 10 µm. Photographs: I. Olariaga (A,B), Clive Shirley (C–E), I. Olariaga (F).
- Distribution and ecology
Eastern Hemisphere, with records confirmed by molecular data from New Zealand, Australia and tropical China, whereas the lectotype specimen is from Indonesia (Java). Records from Malaysia and Salomon Islands [72] are to be referred to this complex and are likely to belong to C. lilacina as well. Occurs on soil in forests, rarely (?) also on very decayed wood.
- Specimens examined
NEW ZEALAND. Auckland, Manukau, Murphy’s Bushleg, leg. C. Shirley, 17 June 2004, PDD 81263. Waitakere, Jonkers Road, Forest & Bird, Leptospermum & mixed indigenous scrub, leg. C. Shirley, 13 July 2007, CS AK330 (PDD 94782).
- Taxonomic comments
Clavaria lilacina is characterized by its branched violet basidiomata, which fade to pink in age, and its subglobose spores. The material examined suggests that C. lilacina is morphologically very similar to C. violaceopulchra. However, some specimens of C. lilacina ([5]; Figure 6E) exhibit a brownish purple color not observed in C. violaceopulchra, and the basidia in C. lilacina are shorter than in C. violaceopulchra (own measurements and [4]). Clavaria lilacina appears to be an Eastern hemisphere vicariant of C. violaceopulchra. The significant sequence divergence in the ITS region between the C. lilacina and C. violaceopulchra clades suggests a long-term isolation and supports their separation as well. Sequences within the C. lilacina clade show a high sequence divergence correlated with its geographic origin, suggesting that island populations remain isolated. Although further studies including a richer set of material from Australasia and tropical Asia are still needed, our revision revealed that the name C. zollingeri should no longer be used to refer to specimens from these areas. Clavaria tongdaoensis is a further species of the complex that may be mistaken for C. lilacina, from which it differs in a much paler, paler purple to pale purplish pink color [4].
The original specimen of C. lilacina kept at PC, examined by us (Figure 6A,B), shows a dichotomously branched basidioma with simple obtuse apices that conforms to the more recent specimens attributed to C. lilacina here. The color is reddish brown, as typical in weathered specimens of the species complex treated in this paper. Microscopically, the structures are very collapsed, but no clamp connections were observed by us and the few spores observed measured 6 × 5 µm. Therefore, we propose that this name should be applied to the specimens of Australasia and tropical Asia until a more thorough revision is carried out.
Clavaria orientalis P. Zhang & Ju. Yan is considered here a later synonym of C. lilacina. Yan et al. [4] described C. orientalis primarily based on shorter basidia than noted by Corner [12] and Franchi & Marchetti [26] for C. zollingeri, but these authors included also C. violaceopulchra in their descriptions, which likely accounts for the observed differences. The protologue of C. orientalis did not discuss earlier potentially available names based on Australasian and Asian material, nor did it include sequences originated from material collected in Australia and New Zealand. Although the material from China shows some divergence in the ITS region compared to the material from Australasian collections, we interpret that variation as intraspecific, as morphological differences are absent.
Clavaria bicolor Massee is here regarded as a possible synonym of C. lilacina, but a full synonymy cannot be proposed here due to the poor condition of the holotype, sent in spirit and now kept in dry state. The basidioma studied was branched above, violaceous grey, lacked clamp connections and had very collapsed subglobose spores (5–5.5 × 3.5–4 µm).
- Nomenclatural comments
The correct authorship of this name should be C. lilacina Jungh., and not Jungh ex Lév., following Art. 46.2 (see Ex. 13), since Léveillé is author of the article but not of the whole work—a journal—in which the name was published. Clavaria lilacina Jungh. was published in October 1844 according to the footnote found on the bottom left of page 209. From Léveillé’s statement (“des échantillons”) it can be inferred that the original description is based on several specimens deposited at L (“herb. Lugd. Batav.”). The only material labelled as C. lilacina at L is a specimen from the Persoon herbarium (L-0713518) which lacks information about its collector and geographic origin and thus cannot be considered original material. This collection is very unlikely to have been made in Java, as all the specimens from the Persoon herbarium referenced by Léveillé’s extensive account in [1] originate from the New World. Conversely, a specimen kept at PC bears a label in Léveillé’s handwriting with the notation “Clavaria lilacina Junghn. Lév. Ann. Sc. nat. 3 ser. vol. 2. p. 216. Ad truncos. Java. Lév.”. This specimen is considered original material and matches our description of C. lilacina. Therefore, we designate it as the lectotype.
3.4.3. Clavaria violaceopulchra
Clavaria violaceopulchra Olariaga, L.A. Parra, Læssøe, Velasco, Kautman., Kruys & Salcedo, sp. nov. MB 859599.
Holotype: France, Haute-Savoie, Faverges, Englannaz, grassland, 29 June 2006, leg. L. Francini, G00576171 (!).
Etymology: The epithet violaceopulchra is a compound coming from the Latin words violaceus (violaceous) and pulchra (beautiful).
- Description
Basidiomata gregarious, 42–65 × 14–20 mm, branched, usually without a distinct stipe. Branching irregularly dichotomous, with U-shaped angles, sometimes partly V-shaped, branches parallel or convergent, branching rank 3–4. Branches cylindrical, sometimes flattened, longitudinally furrowed, solid, bright violet (84A), fading to pink (62C, 62D) in age, when dried light ochre (10YR 8/2, 2.5Y 7/3, 7/4) or very pale lilac (5YR 8/1). Apices obtuse, sometimes subacute or truncate, concolorous. Stipe when present 10–15 × 3–5 mm, cylindrical, pinkish lilac (75B, 75C). Whitish mycelium present at the base, seldom forming erect tufts. Context white, taste mild, odor none. No reaction with iron salts. Basidiospores ovoid to subglobose in side view, thin-walled, smooth, non-amyloid, 5–6.5(–7) × 4–6 (–6.5) µm (Lm range = 5.4–6.1, Wm range = 3.9–5.2, Qm range = 1.08–1.28). Basidia clavate, (2–)4-spored, without clamps, 44–58 × 8–9.5 µm. Context formed by tightly interwoven hyphae, cylindrical to fusiform, closely septate, thin-walled, hyaline to very pale yellow, smooth, without clamps, 4–24 µm wide. Figure 7.

Figure 7.
Clavaria violaceopulchra sp. nov. (A) Basidiomata (G00576171, holotype). (B) Basidiomata in situ (BIO-Fungi 12557). (C) Basidiomata in situ (ARAN-Fungi 16434). (D) Basidiospores (G00576171, holotype). Scale bar = 10 µm. Photographs: L. Francini (A), I. Olariaga (B–D).
- Distribution and ecology
Mainly temperate areas of central and south Europe (France, Spain, Switzerland), also present in scattered localities of the Nordic countries (Norway) and North America (California), occurring in non-fertilized pastures, periodically cut Pteridium aquilinum communities and broadleaf forests, often on acidic ground.
- Distribution and ecology
CZECH REPUBLIC. Rychlebská vrchovina, Jeseník, Smetanovy sady, city park, in grass, 21 October 2017, leg. P. Skopal, BRACR29005.
NORWAY. Aust-Agder, Froland, Syd for Bukkelfjel, Snoøløs, hazel, oak, spruce, on bare ground, leg. 17 December 2001, leg. I. L. Fonneland, O-F-175336.
SLOVAKIA. Biele Karpaty, Veľká Javorina, 28 September 2014, leg. L. Janošík, BRACR23959. Jablunkovské medzihorie, Skalité, sheep grazed meadow, 30 October 2018, leg. M. Zajac, BRACR30724. Javorníky, Veľké Rovné, Solisko, mowed meadow, 2 November 2018, leg. F. Fuljer, BRACR30732. Javorníky, Petrovice, Medvedie, mowed meadow, 3 November 2018, leg. F. Fuljer, BRACR30735. Kysucké Beskydy, Oščadnica-Beskydok, 6 October 2021, leg. V. Kautman, BRACR39564. Kysucké Beskydy, Skalité, 6 October 2021, leg. V. Kautman, BRACR39555. Kysucká vrchovina, Oščadnica, Zadedová, mowed meadow, 15 October 2017, leg. F. Fuljer, BRACR41672. Turzovská vrchovina, Korňa, in meadow, 15 September 2010, leg. L. Mikovčáková, BRACR15130; in shrubs (Corylus avellana) on old abandoned pasture, 19 September 2010, leg. L. Mikovčáková, BRACR15909. Veporské vrchy, Kokava-Háj, in pasture, 15 September 2014, leg. P. Pavol, BRACR37053; 10 September 2016, leg. M. Smiková & P. Smik, BRACR82365.
SPAIN. Andalucía, Cádiz, Los Barrios, finca “Murtas”, under Quercus suber, Q. canariensis and Olea europaea var. sylvestris, 5 February 2019, leg. M. Romera, ARAN-Fungi 22716. Andalucía, Huelva, Sierra de Aracena, mixed forest with Pinus sp., Quercus suber, etc., 25 October 2006, leg. S. Silvestre, BIO-Fungi 12538. Basque Country, Gipuzkoa, Berastegi, Artaleku, under Pteridium aquilinum, among mosses and gramineous plants, on acid soil, 12 October 2007, leg. J.A. Albizu & I. Olariaga, BIO-Fungi 12557; 7 October 2005, leg. P.M. Pasaban, ARAN-Fungi A3006065. Gipuzkoa, Getaria, San Anton mendia, 12 December 2021, leg. J. Teres, ARAN-Fungi 16434. Gipuzkoa, Zarautz, Santa Barbara, poor-nutrient pasture, 10 December 2010, leg. J. Teres, ARAN-Fungi A3078104. Catalonia, Barcelona, St. Hilari Sacalm, Joanetes, herbes (herbs), 15 November 1998, SCM 3471 (BCC). Navarre, Larraun, Atako bailara, poor grassland among Pteridium, 6 September 2002, leg. P.M. Pasaban, J.I. López Amiano, J.M. Lekuona & I. Olariaga, BIO-Fungi 9948; BIO-Fungi 9610.
- Taxonomic comments
Clavaria violaceopulchra differs from C. amethystina, the other species of this complex present in temperate areas of the Northern Hemisphere, by its subglobose spores. The analyses of the ITS-LSU regions also support its distinctiveness. Both species have a deep violet basidioma color, but the basidiomata of C. violaceopulchra are usually larger, less branched and a greater tendency to fade to pale pink in age. According to our measurements, basidia are also longer in C. violaceopulchra (44–58 × 8–9.5 µm) than those of C. amethystina (32–42 × 6–8 µm) and Clavaria lilacina (40–45 × 6–8 µm), and this character could be diagnostic. The latter differs further from C. violaceopulchra in sometimes becoming purplish brown in age and an Australiasian distribution.
Some European authors have treated C. violaceopulchra under the name C. amethystina [48] or as C. zollingeri [12,26]. Our nomenclatural revision did not yield any previously published name that can be applied to this taxon, and it is therefore described here as new.
3.4.4. New Combination
Clavulinopsis zollingeri (Lév.) Olariaga comb. nov. MycoBank: MB 853972. ≡ Clavaria zollingeri Lév., Ann. Sci. Nat., Bot., sér. 3, 5: 155. 1846 [basionym]. Holotype: Indonesia, Java, Kampong (dorf) Tjikoya, ad truncos, February 1843, leg. Zollinger, Plantae Javanicae 99.z (PC0093981 [!]). Isotype: FH 00290380 (!).
3.4.5. Excluded Material
The material studied during this project and found not to belong to C. zollingeri s. auct. is provided below.
Specimens examined and excluded. Clavaria sp.—SRI LANKA: Henaratgoda, on the ground, 26 July 1916, leg. T. Petch 4840, K(M)168007 [original material of Clavaria violacea Petch, nom. illeg.]. Scytinopogon echinosporus—INDONESIA: Java, prope Tjikoya, ad terram, leg. Zollinger, Plantae Javanicae 1311, L0836107 [in sched. sub C. zollingeri].
3.4.6. Excluded Names
Names that do not belong to C. amethystina, C. violaceopulchra or C. lilacina, but have been referred to these names within the complex.
- Clavaria alcicornis Zoll. & Moritzi in Zollinger, Natuur-Geneesk. Arch. Ned.-Indië 1: 382. 1844. Lectotype: Indonesia, Java, Tjikoya, March 1843, Plantae Javanicae 1125 (FH) (designation achieved by Petersen [73] accepting it as the holotype, according to Arts. 7.11 and 9.10).
An original specimen of C. alcicornis, deposited at L, was referred to C. zollingeri s. auct. by Petersen [63], as it lacked clamp connections, had typically closely septate context hyphae and ovoid basidiospores (5.8–6.7 × 3.3–4.1 µm) matching C. lilacina as interpreted here. However, Petersen [73] had previously examined another original specimen kept at FH. Lectotype designation was achieved by Petersen accepting this specimen at FH as the holotype (Art. 7.11), which is correctable to lectotype (Art. 9.10). Thus, C. alcicornis must be interpreted according to its lectotypification. Petersen [73] did not find spores in the lectotype at FH, but he reported 4-spored basidia and clamp connections and attributed it to a species of the genus Clavulinopsis. Therefore, the name C. alcicornis should be applied to a species of Clavulinopsis with pink tones, rather than to the group of Clavaria species treated here.
- Clavaria amethystina subsp. coerulescens P. Karst., Meddeland. Soc. Fauna Fl. Fenn. 16: 2. 1888. Type specimen: not kept at H (checked in person by I. Olariaga), probably lost.
The brief protologue by Karsten described this taxon as similar in shape and size with Clavaria flava Schaeff. nom. sanct. Fr. (currently placed in Ramaria as R. flava [Schaeff.] Quél.). The protologue stated that its shape was similar to Clavaria flava Schaeff. (≡Ramaria flava (Schaeff.) Quél., and thus the name is likely to be referred to Ramaria Holmsk.).
- Clavaria bizzozeriana Sacc., Syll. Fung. 6: 693. 1888. [“Bizzozeriana”][nom. nov. based on Clavaria tenuissima Sacc., Michelia 1(4): 436. 1878, nom. illeg. Art. 53, non Clavaria tenuissima Lév., Ann. Sci. Nat., Bot., sér. 3, 5: 156. 1846]. ≡ Ramariopsis bizzozeriana (Sacc.) Schild, Z. Pilzk. 38: 26. 1972. ≡ Clavulinopsis bizzozeriana (Sacc.) Jülich, Int. J. Mycol. Lichenol. 2(1): 120. 1985. Syntypes: Italy, Padova, in uliginosis, October 1878, leg. G. Bizzozero, Mycotheca Veneta 1309 (B, BM, BUCM, DBN, E, FH, GE, HBG, K, L, M, PRE, S, SIENA, STR, TLA, TO, W, WRSL).
Saccardo [74] compared Clavaria bizzozeriana to C. lilacina Fr. in the protologue, noting that C. bizzozeriana differed from the latter by having thinner branches, dichotomous branching and smaller spherical spores. Coker [2] examined the syntype deposited at K, describing its spores as 2.5–3.6 µm thick, and considered C. bizzozeriana a synonym of Ramariopsis pulchella (Boud.) Corner (as Clavaria pulchella Boud.), as subsequent authors (e.g., [12,26]) did. The dichotomously branched, slender basidiomata with violet tones and small spherical spores can only represent R. pulchella according to our current knowledge, but a modern type examination would be desirable to confirm this view.
- Clavaria brachycera Pers., Comm. Fung. Clav.: 61. 1797. Type specimen: not kept at L, probably lost.
Persoon described a clavarioid, branched fungus with a stout stipe, branched apices and a violaceous color. He further referred to a plate by Barrelier [75] (Figure 1261) that shows in our opinion a young basidioma of a species of Ramaria. The robust stipe and the branching pattern of the apices do not conform to C. zollingeri s. auct.
- Clavaria nymaniana Henn. in Warburg, Monsunia 1: 9. 1900. Type: lost (see below).
Clavaria nymaniana Henn. is a further name based on material from Java [76] that has been considered a synonym of C. zollingeri [12]. Clavaria nymaniana was described in the protologue as having branched violet basidiomata, subglobose to ovoid spores measuring 4.5–5 µm and basidia 25–30 µm long. The basidium size is shorter than the one observed in the C. zollingeri s. auct. complex. Important information, such as the presence of clamp connections, is missing in the protologue. Regrettably, no type specimen appears to exist [77], and we are uncertain whether C. nymaniana is a later synonym of C. lilacina or belongs to another genus in the Clavariaceae (Clavulinopsis or Ramariopsis Corner).
- Clavaria violacea Vill., Hist. Pl. Dauphiné 3(2): 1050. 1789. non Clavaria violacea Petch, Ann. Roy. Bot. Gard. Peradeniya 7(4): 290. 1922 [nom. illeg. Art. 53.1].
The protologue of C. violacea [23] describes a clavarioid species with simple branches and a violaceous color. Villars [23] based the description of C. violacea on a handwritten unpublished manuscript by Jullien [78] and included C. violacea among the species with unbranched or sparingly branched basidiomata, whereas he included the branches species among the species with divided branches “a tiges divisées”. Jullien [78] himself also characterized C. violacea as “Cl. ramis simplicibus acutis violaceis”, denoting a species with simple basidiomata. The term “ramis”, usually translated as “branch” and referring to branched structures, was used by Villars [23] and Jullien [78] in a less common sense—club—to refer to simple basidiomata. Although Fries [79] referred C. violacea to C. amethystina (p. 287, sub C. amethystea), the simple basidiomata described in the protologue of C. violacea can hardly refer to the C. amethystina s. auct. complex. Rather, we believe that this name could refer to Alloclavaria purpurea (O.F. Müll.) Dentinger & D.J. McLaughlin, Clavaria fumosa Pers. or even a species of Clavulina J. Schröt. Nevertheless, no type specimen of C. violacea is preserved in the Villars herbarium (GRM), nor does the protologue refer to any original material or illustration. We therefore regard C. violacea as a nomen ambiguum.
Villars [23] did not ascribe the name C. violacea to Jullien, who had earlier accounted C. violacea in a handwritten unpublished manuscript [78] that did not fulfill the requirements for an effective publication (Art. 29.1). Thus, even though Villars acknowledged that Jullien had characterized the species, the name C. violacea must be ascribed to Villars [23] alone.
4. Discussion
The name C. zollingeri has been widely used for several taxa (without clamp connections) not including its type (with clamp connections). The ITS-LSU analyses show that the C. zollingeri s. auct. complex contains at least four clades, assigned here to four species. Sequence divergence, particularly in the ITS region, is high in the C. amethystina and C. lilacina clades, raising the question of whether each encompasses more than one species —with 8 and 3 putative species, respectively. Sequence divergence is strongly correlated with the geographical origin of the specimens, while no consistent morphological differences have been detected within the specimens of each of those three clades. The internal transcribed spacer (ITS) is known to show a high variability across Clavaria, even within morphologically rather homogeneous groups, such as the C. falcata Pers. and C. fragilis Holmsk. clades (see [17]). In our view, the signal of the ITS region in the C. amethystina and C. lilacina clades should be assessed in the light of analyses of multiple protein-coding markers that will allow for a better interpretation of the signal of the ITS region. In this framework, splitting further C. amethystina, C. lilacina and C. violaceopulchra, considering that no morphological characters support this view, appears premature and unsuitable. Rather, we regard the existing divergence in the ITS region as intraspecific variation, recognizing four species within the C. zollingeri s. auct. complex, namely C. amethystina, C. lilacina, C. tongdaoensis and C. violaceopulchra.
At least three of those species—C. amethystina, C. lilacina, and C. violaceopulchra— produce branched deep violet basidiomata and have been subsumed under the misapplied name, C. zollingeri. Therefore, the name C. zollingeri cannot be used for any of those, for which correct names must be adopted. Since C. zollingeri is considered globally endangered [11] and the name is often used by non-taxonomists, a first option considered here is to maintain its usage by proposing to conserve Clavaria zollingeri with a conserved type that represents one of three species. This choice would, however, require coining a new name for the clamped violet C. zollingeri from Java.
As earlier names are available for two of the species treated under the misapplied name C. zollingeri s. auct., the choice made by us is to adopt the oldest names available for those two clades, to describe a third clade as new and to combine C. zollingeri under Clavulinopsis. The name C. amethystina as here typified remains attached to the most common, ellipsoid-spored species present in Europe and North America, while C. violaceopulchra is described as new for the species so far known from Europe and North America, and C. lilacina is proposed to be adopted for the remaining clade present in Australia, New Zealand and China. This decision prevents the name Clavaria amethystina (as Clavulina amethystina [Holmsk.] Donk) from being used in Clavulina, where it is often placed, but it lacks a consistent taxonomic interpretation [57]; see comments under C. amethystina).
Author Contributions
Conceptualization, I.O. and I.S.; methodology, I.O.; nomenclatural study, I.O. and L.A.P.; material, I.O., T.L., J.M.V., I.K. and Å.K.; software, I.O.; writing—original draft preparation, I.O.; writing—review and editing, I.O., L.A.P., T.L., J.M.V., I.K., Å.K. and I.S.; supervision, I.S.; funding acquisition, I.O. and I.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was made possible by a grant for the training of Researchers (2002/2003)” by the Government of the Basque Country. The research of I. Kautmanova was supported by the Operational Program of Research and Innovations and co-financed with the European Fund for Regional Development (EFRD) ITMS2014 + 313021 W683: “DNA barcoding of Slovakia (SK-BOL), as a part of international initiative International Barcode of Life (iBOL)”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be found here: https://www.ncbi.nlm.nih.gov (accessed on 9 April 2025).
Acknowledgments
We extend our thanks to the curators who provided specimens from their respective herbaria available for us and kindly answered our questions: Ferry Bouman (AMD), Harrie J.M. Sipman and René Jarling (B), Atik Retnowati (BO), Philippe Clerc (G), Joelle Chiche (GRM), Begoña Aguirre-Hudson (K), Bart Buyck (PC), Genevieve Lewis-Gentry (FH), Katriina Bendiksen and Mika Bendiksby (O), and Gerard Thijse (L). Mercedes Herrera kindly handled a loan request from BIO herbarium. We also thank Pierre-Arthur Moreau and Laurent Deparis for making available the holotype specimen of Clavaria violaceopulchra. Our appreciation extends to Mariano Romera and Joxepo Teres for supplying valuable material for this study, as well as to Hans-Otto Baral for transcribing the old German label of the holotype of Clavaria zollingeri. Clive Shirley kindly provided us with the photographs of the material of Clavaria lilacina examined here. Mathieu Lefevbre (Museum of Natural History of Grenoble) provided his insightful comments on Villars’ work and for sending to us a scan of Jullien’s unpublished manuscript. We are grateful to the reviewers who anonymously reviewed our manuscript and improved it significantly.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Léveillé, J.H. Descriptions des Champignons de l’herbier du Muséum de Paris. Ann. Sci. Nat. Sér. 3 1846, 5, 111–167. [Google Scholar]
- Coker, W.C. The Club and Coral Mushrooms (Clavarias) of the United States and Canada; University of North Carolina Press: Chapel Hill, NC, USA, 1923. [Google Scholar]
- Vasco-Palacios, A.M.; Franco-Molano, A.E. Diversity of Colombian Macrofungi (Ascomycota-Basidiomycota). Mycotaxon 2013, 121, 1–58. [Google Scholar]
- Yan, J.; Xiong, L.; Yang, L.-X.; He, Z.-M.; Zhang, P.; Liao, K. Morphological and Multi-Locus Phylogenetic Analyses Reveal Three New Branched Species of Clavaria (Clavariaceae, Agaricales) from China. MycoKeys 2025, 115, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.H. The Clavarioid Fungi of New Zealand; Information Series; Department of Scientific and Industrial Research: Auckland, New Zealand, 1988; Volume 236, pp. 1–170.
- Holec, J.; Beran, M. Červený Seznam Hub (makromycetů) České Republiky [Red List of Fungi (macromycetes) of the Czech Republic]. Příroda, Praha 2006, 24, 1–282. [Google Scholar]
- Moeslund, J.E.; Nygaard, B.; Ejrnæs, R.; Alstrup, V.; Baagøe, H.J.; Bell, N.; Bruun, L.D.; Bygebjerg, R.; Carl, H.; Christensen, M.; et al. Den Danske Rødliste; Aarhus Universitet, DCE—Nationalt Center for Miljø og Energi: Aarhus, Denmark, 2023. [Google Scholar]
- Brandrud, T.E.; Bendiksen, E.; Blaalid, R.; Hofton, T.H.; Jordal, J.B.; Nordén, J.; Nordén, B.; Wollan, A.K. Sopper: Vurdering av Fiolett Greinkøllesopp Clavaria Zollingeri for Norge. Rødlista for Arter 2021; Artsdatabanken: Trondheim, Norway, 2021. [Google Scholar]
- Wojewoda, W.; Ławrynowicz, M. Red List of the Macrofungi in Poland. In Red List of Plants and Fungi in Poland; Mirek, Z., Zakrzycki, K., Wojewoda, W., Szeląg, Z., Eds.; Szafer Institute of Botany, Polish Academy of Science: W. Kraków, Poland, 2006; pp. 53–70. [Google Scholar]
- Knutsson, T.; Krikorev, M.; Ottosson, E.; Dahlberg, A.; Edman, M.; Hansen, K.; Jeppson, M.; Karström, M.; Larsson, E.; Nitare, J.; et al. 2020. Rödlista 2020—Expertkommittén För Svampar; SLU Artdatabanken: Uppsala, Sweden, 2020. [Google Scholar]
- Jordal, J.; Kautmanová, I. Clavaria zollingeri. The IUCN Red List of Threatened Species 2019: E.T70402563A70402575. Available online: https://doi.org/10.2305/IUCN.UK.2019-2.RLTS.T70402563A70402575.en (accessed on 5 April 2024).
- Corner, E.J.H. A Monograph of Clavaria and Allied Genera. Ann. Bot. Mem. 1950, 1, 1–740. [Google Scholar]
- Knudsen, H.; Vesterholt, J. Agaricoid, Boletoid, Clavarioid, Cyphelloid and Gastroid Genera. In Funga Nordica, 2nd ed.; Nordsvamp: Copenhagen, Denmark, 2012. [Google Scholar]
- Donk, M.A. Revision Der Niederländischen Homobasidiomycetae-Aphyllophoraceae II. Meded. Ned. Mycol. Ver. 1933, 22, 1–278. [Google Scholar]
- Petersen, R.H. Notes on Clavarioid Fungi. XV. Reorganization of Clavaria, Clavulinopsis and Ramariopsis. Mycologia 1978, 70, 660–671. [Google Scholar] [CrossRef]
- Dentinger, B.T.M.; McLaughlin, D.J. Reconstructing the Clavariaceae Using Nuclear Large Subunit rDNA Sequences and a New Genus Segregated from Clavaria. Mycologia 2006, 98, 746–762. [Google Scholar] [CrossRef]
- Kautmanová, I.; Tomšovský, M.; Dueñas, M.; Martín, M.P. European Species of Clavaria (Agaricales, Agaricomycetes) with Dark Basidiomata—A Morphological and Molecular Study. Persoonia 2012, 29, 133–145. [Google Scholar] [CrossRef]
- Schaeffer, J.C. Fungorum qui in Bavaria et Palatinatu Circa Ratisbonam Nascuntur Icons Natives Coloribus Expressae; typis Zunkelianis: Regensburg, Germany, 1774; Volume 4. [Google Scholar]
- Holmskjold, T. Beata Ruris Otia Fungis Danicis; Friderico Brummer: Havniae [Kjøbenhavn], Denmark, 1790; Volume 1. [Google Scholar]
- Nees von Esenbeck, C.G. Das System der Pilze und Schwamme. Ein Versuch; Stahelschen Buchhandlung: Würzburg, Germany, 1816. [Google Scholar]
- Venturi, A. Studi Micologici; Francisco Cavalieri: Brescia, Italy, 1842. [Google Scholar]
- Persoon, C.H. Commentatio de Fungis Clavaeformibus; Petrum Philippum Wolf: Lipsiae, Germany, 1797. [Google Scholar]
- Villars, M. Histoire des Plantes de Dauphiné. Tome Troisieme. Contenant la Deuxieme Partie & Les Planches des Deux Volumes; Grande Chartreuse: Briançon, France; Gap & de Montelimar: Briançon, France; chez les frères Perisse: Lyon, France; chez Prevost: Paris, France; Grenoble, France, 1789. [Google Scholar]
- Zollinger, H. Observationes Phytographicae Praecipur Genera et Species Nova Nonnula Respicientes. Natuur Geneesk. Arch. Ned. Indië 1844, 1, 372–405. [Google Scholar]
- Olariaga, I. The Order Cantharellales in the Iberian Peninsula and the Balearic Islands; University of the Basque Country (UPV/EHU): Bilbao, Spain, 2009. [Google Scholar]
- Franchi, P.; Marchetti, M. I Funghi Clavarioidi in Italia. Bresadola, A.M., Ed.; Graffica Sette: Trento, Italy, 2021; Volume 1. [Google Scholar]
- Kautmanová, I. Taxonomy of Central-European Representatives of the Genus Clavaria (Basidiomycetes, Clavariaceae); University of Bratislava: Bratislava, Slovakia, 2012. [Google Scholar]
- The Royal Horticultural Society. R.H.S. Colour Charts; RHS: London, UK, 1995. [Google Scholar]
- Munsell Color Corporation Soil Color Charts; Macbeth Division of Kollmorgen Instruments Corporation: New York, NY, USA, 1990.
- Thiers, B. Updated Continuously. Index Herbariorum. A Global Directory of Public Herbaria and Associated Staff; New York Botanical Garden’s Virtual Herbarium: New York, NY, USA, 2019; Available online: http://sweetgum.nybg.org/ih/ (accessed on 9 April 2025).
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Gandhi, K.N.; Gravendyck, J.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Klopper, R.R.; Knapp, S.; et al. International Code of Nomenclature for Algae, Fungi, and Plants (Madrid Code) Adopted by the Twentieth International Botanical Congress Madrid, Spain, July 2024; Regnum Vegetabile; The University of Chicago Press: Chicago, IL, USA, 2025. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols; Elsevier: Amsterdam, The Netherlands, 1990; pp. 315–322. ISBN 9780123721808. [Google Scholar]
- Cubeta, M.A.; Echandi, E.; Abernethy, T.; Vilgalis, R. Characterization of Anastomosis Groups of Binucleate Rhizoctonia species Using Restriction Analysis of an Amplified Ribosomal RNA Gene. Phytopathology 1991, 81, 1395–1400. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid Genetic Identification and Mapping of Enzymatically Amplified Ribosomal DNA from Several Cryptococcus Species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A. AliView: A Fast and Lightweight Alignment Viewer and Editor for Large Datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: A Multiple Sequence Alignment Method with Reduced Time and Space Complexity. BMC Bioinform. 2004, 5, 113. [Google Scholar] [CrossRef]
- Birkebak, J.M.; Adamčík, S.; Looney, B.P.; Matheny, P.B. Multilocus Phylogenetic Reconstruction of the Clavariaceae (Agaricales) Reveals Polyphyly of Agaricoid Members. Mycologia 2016, 108, 860–868. [Google Scholar] [CrossRef] [PubMed]
- Trifinopoulos, J.; Nguyen, L.-T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A Fast Online Phylogenetic Tool for Maximum Likelihood Analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Hillis, D.M.; Bull, J.J. An Empirical Test of Bootstrapping as a Method for Assessing Confidence in Phylogenetic Analysis. Syst. Biol. 1993, 42, 182–192. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for Inference of Large Phylogenetic Trees. In Proceedings of the 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010. [Google Scholar]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- ISO 3166-1; Codes for the Representation of Names of Countries and Their Subdivisions – Part 1: Country Code. International Organization for Standardization: Geneve, Switzerland, 2013.
- Van Overeem, C. Beiträge Zur Pilzflora von Niederländisch Indien. Bull. Jard. Bot. Buitenzorg 1923, 5, 247–293. [Google Scholar]
- Zollinger, H. Systematisches Verzeichniss der im Indischen Archipel in Den Jahren 1842–1848 Gesammelten Sowie der aus Japan Empfangenen Pflanzen; Verlag von E. Kiesling: Zürich, Switzerland, 1854. [Google Scholar]
- Moritzi, A. Systematisches Verzeichniss der von H. Zollinger; Verlag des Verfassers: Solothurn, Switzerland, 1846. [Google Scholar]
- Petersen, R.H. Notes on Clavarioid Fungi. Persoonia 1971, 6, 219–229. [Google Scholar]
- Cotton, A.D.; Wakefield, E.M. A Revision of the British Clavariae. Trans. Br. Mycol. Soc. 1919, 6, 164–198. [Google Scholar] [CrossRef]
- Fries, E.M. Systema Mycologicum; Ernesti Mauritii: Greifswaldiae, Germany, 1821; Volume 1. [Google Scholar]
- Schaeffer, J.C. Fungorum qui in Bavaria et Palatinatu Circa Ratisbonam Nascuntur Icons Natives Coloribus Expressae; typis Zunkelianis: Regensburg, Germany, 1763; Volume 2. [Google Scholar]
- Bulliard, G.B.F. Herbier de la France; Didot J.ne, Debure, Libraires: Paris, France, 1791; Volume 11. [Google Scholar]
- Berkeley, M.J. Outlines of British Fungology; Lovell Reeve: London, UK, 1860. [Google Scholar]
- Cooke, M.C. A Plain and Easy Account of British Fungi; Robert Hardwicke: London, UK, 1862. [Google Scholar]
- Fries, E.M. Hymenomycetes Europaei; Ed. Berling: Uppsala, Sweden, 1874. [Google Scholar]
- Pilát, A. Přehled Hub Kyjankovitych-Clavariaceae Se Zvláštním Zřetelem K československým Druhûm. Sborn. Nár. Mus. Praze Řada B Přir. Vědy 1958, 14, 129–255. [Google Scholar]
- Jülich, W. Die Nichtblätterpilze, Gallerpilze und Bauchpilze Kleine Kryptogamenflora Band II; Gustav Fischer Verlag: Sttutgart, Germany, 1984. [Google Scholar]
- Olariaga, I.; Jugo, B.M.; García-Etxebarria, K.; Salcedo, I. Species Delimitation in the European Species of Clavulina (Cantharellales, Basidiomycota) Inferred from Phylogenetic Analyses of ITS Region and Morphological Data. Mycol. Res. 2009, 113, 1261–1270. [Google Scholar] [CrossRef]
- Calonge, F.D.; de Sequeira, M.M.; Rocha, E.; Hernández, C.J.C. Algunos Hongos Interesantes de Madeira (Portugal). Bull. Sem. Féd. Assoc. Mycol. Médit. 2010, 37, 29–34. [Google Scholar]
- Castro-Marcote, J.L. Clavulina amethystina (Bull.) Donk. In Bolets de Catalunya 29; Impressors de Barcelona S.L.: Barcelona, Spain, 2010; p. 1420. [Google Scholar]
- Petersen, R.H.; Olexia, P.D. Type Studies in the Clavarioid Fungi. I. The Taxa Described by Charles Horton Peck. Mycologia 1967, 59, 767–802. [Google Scholar] [CrossRef]
- Petch, B.A. Additions to Ceylon Fungi (II.). Ann. Roy. Bot. Gard. (Peradeniya) 1922, 7, 279–322. [Google Scholar]
- Van Overeem, C. Clavariaceae. In Icones Fungorum Malayensium 4; Van Overeem, C., Weese, J., Eds.; Selbstverlag des Mykologischen Museums in Weesp: Wien, Austria, 1923; pp. 1–2. [Google Scholar]
- Petersen, R.H. Type Studies in the Clavariaceae. VI. Four Pivotal Types from the Pacific Tropics. Mycotaxon 1980, 12, 281–286. [Google Scholar]
- Rea, C. British Basidiomycetidae. A Handbook to the Larger British Fungi; University Press: Cambridge, UK, 1922. [Google Scholar]
- Donk, M.A. Nomenclatural Notes on Generic Names of Agarics (Fungi: Agaricales). Bull. Jard. Bot. Buitenzorg Sér. 3 1949, 18, 271–402. [Google Scholar]
- Donk, M.A. The Generic Names Proposed for the Hymenomycetes— III. “Clavariaceae.” Reinwardtia 1954, 2, 441–493. [Google Scholar]
- Donk, M.A. The Generic Names Proposed for Polyporaceae. Persoonia 1960, 2, 173–302. [Google Scholar]
- Donk, M.A. The Generic Names Proposed for Agaricaceae. Nova Hedwigia (Beih.) 1962, 5, 1–320. [Google Scholar]
- Battarra, A.J. Fungi Agri Ariminensis Historia; Typis Martinianis: Faenza, Italy, 1759. [Google Scholar]
- Bulliard, G.B.F. Histoire des Champignons de La France; Barrois le Jeune, Belin: Paris, France, 1791; Volume 1. [Google Scholar]
- Fries, E.M. Systema Mycologicum, Vol. 3. (Index); Ernesti Mauritii: Greifswaldae, Germany, 1832. [Google Scholar]
- iNaturalist. Naturalist: A World-Wide Database of Biodiversity Observations. Available online: https://www.inaturalist.org/observations?place_id=any&subview=map&taxon_id=126160 (accessed on 10 May 2024).
- Petersen, R.H. Type Studies in the Clavariaceae. Sydowia 1967, 21, 105–122. [Google Scholar]
- Saccardo, P.A. Sylloge Fungorum; Typis Seminarii: Padua, Italy, 1888; Volume 6. [Google Scholar]
- Barrelier, J. Plantae per Galliam, Hispaniam et Italiam Observatae; Stephane Ganeau: Paris, France, 1714. [Google Scholar]
- Hennings, P. Fungi. In Monsunia Beiträge zur Kenntniss der Vegetation süd-und Ostasiatischen Monsungebietes; Warburg, O., Ed.; Verlag von Wilhelm Engelman: Leipzig, Germany, 1900; pp. 9–38. [Google Scholar]
- Hein, B. Liste der Arten und Arten und Infraspecifischen taxa von P. Hennings. Englera 1988, 10, 1–374. [Google Scholar]
- Jullien-[l’Abbe]. Decuria Fungorum Quos Legi in Pago Thejiano. Unpublished manuscript kept in the Museum of Natural History of Grenoble (GRM). 1784.
- Fries, E.M. Observationes Mycologicae; Gehr. Bonnier: Kjøbenhavn, Denmark, 1818; Volume 2. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).