Pezizales in Israel: Molecular Phylogenetic and δ13Cδ15N Stable Isotope Data Reveal New Records and Potential Discrepancies in Their Trophic Ecology
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Processing
2.2. Molecular Methods
2.3. Phylogenetic Analysis and Trophic State Estimation Based on Molecular Data
2.4. Stable Isotope Analysis
2.5. Data Recording
2.6. Data Analysis and Statistics
3. Results
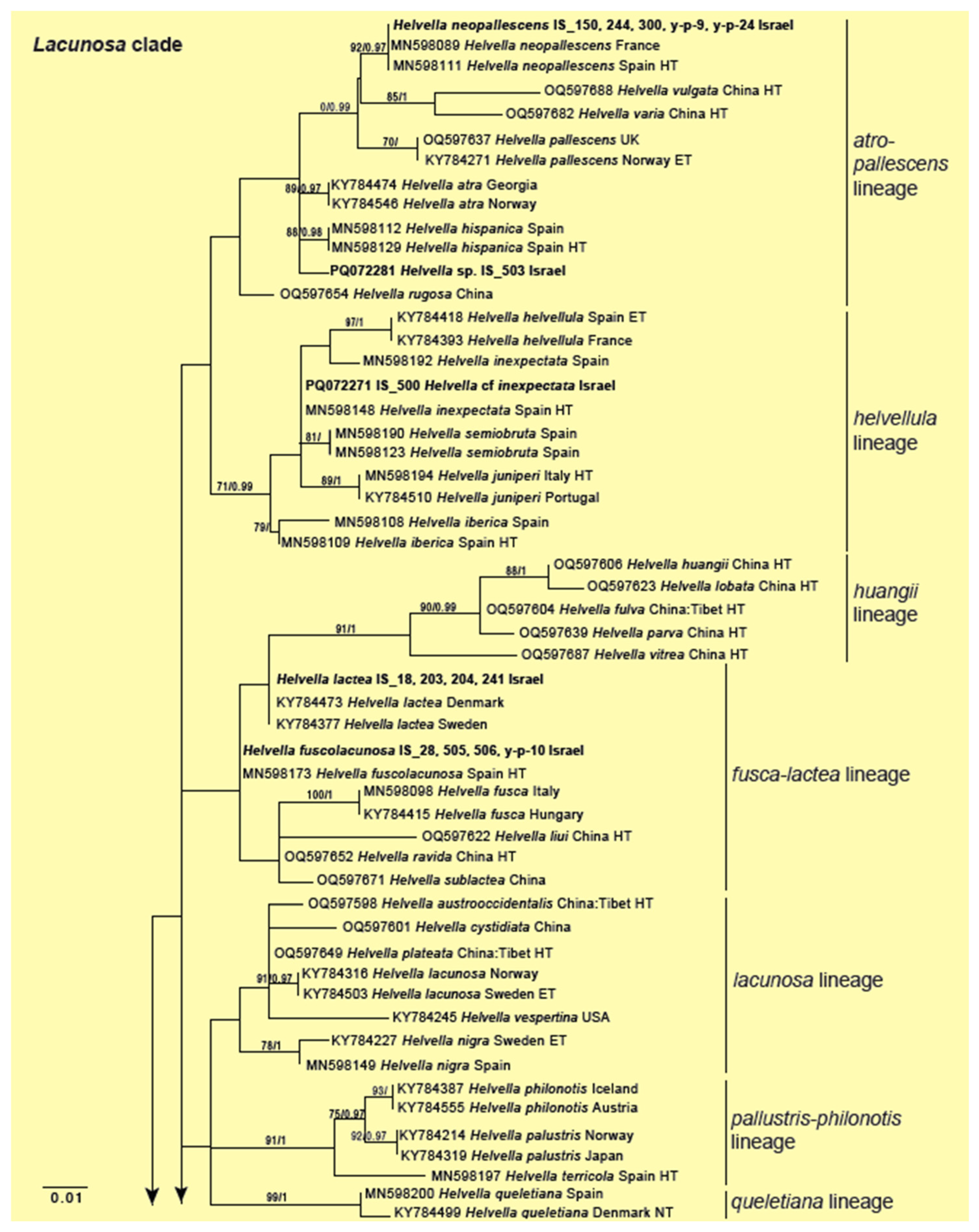
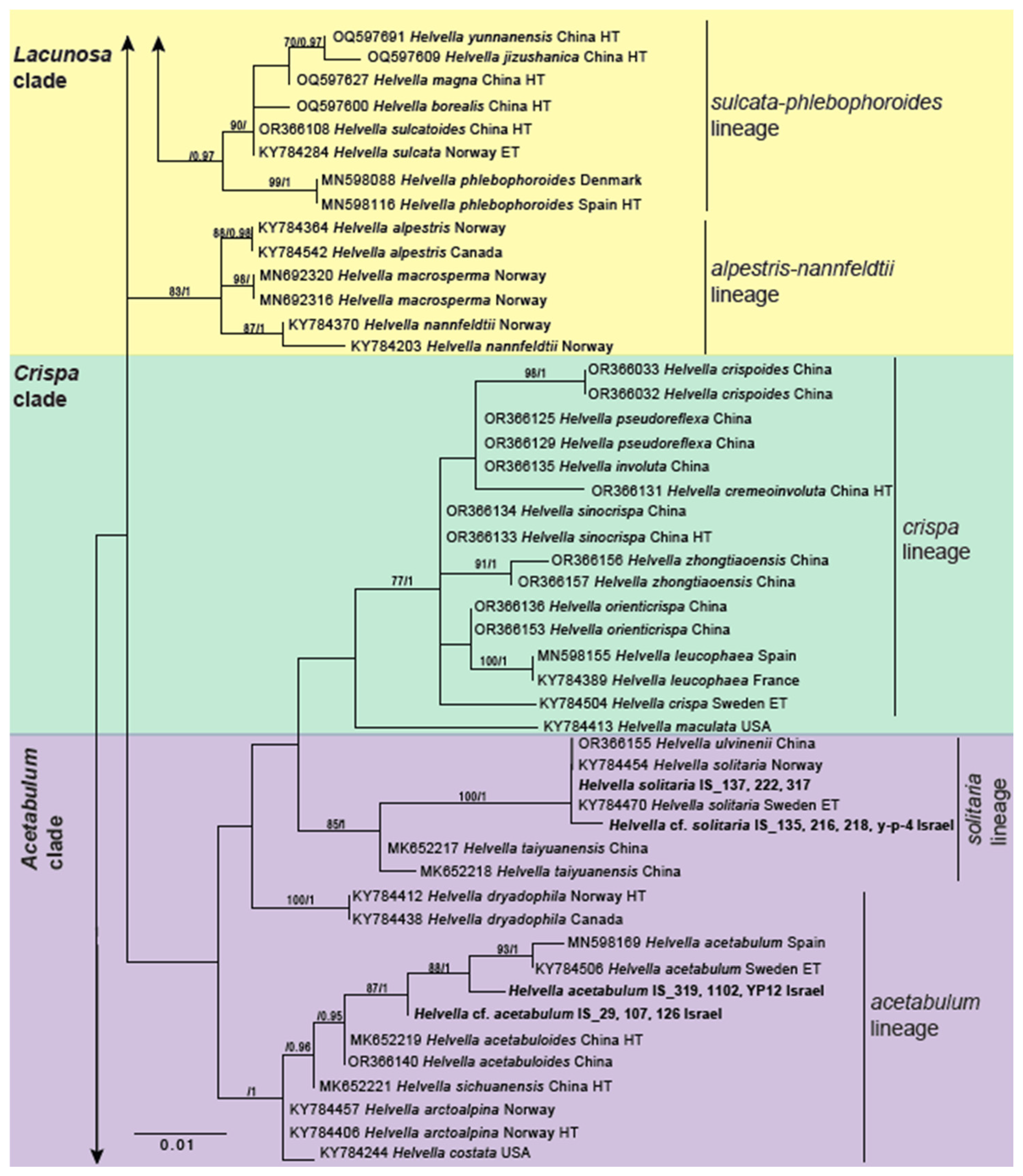
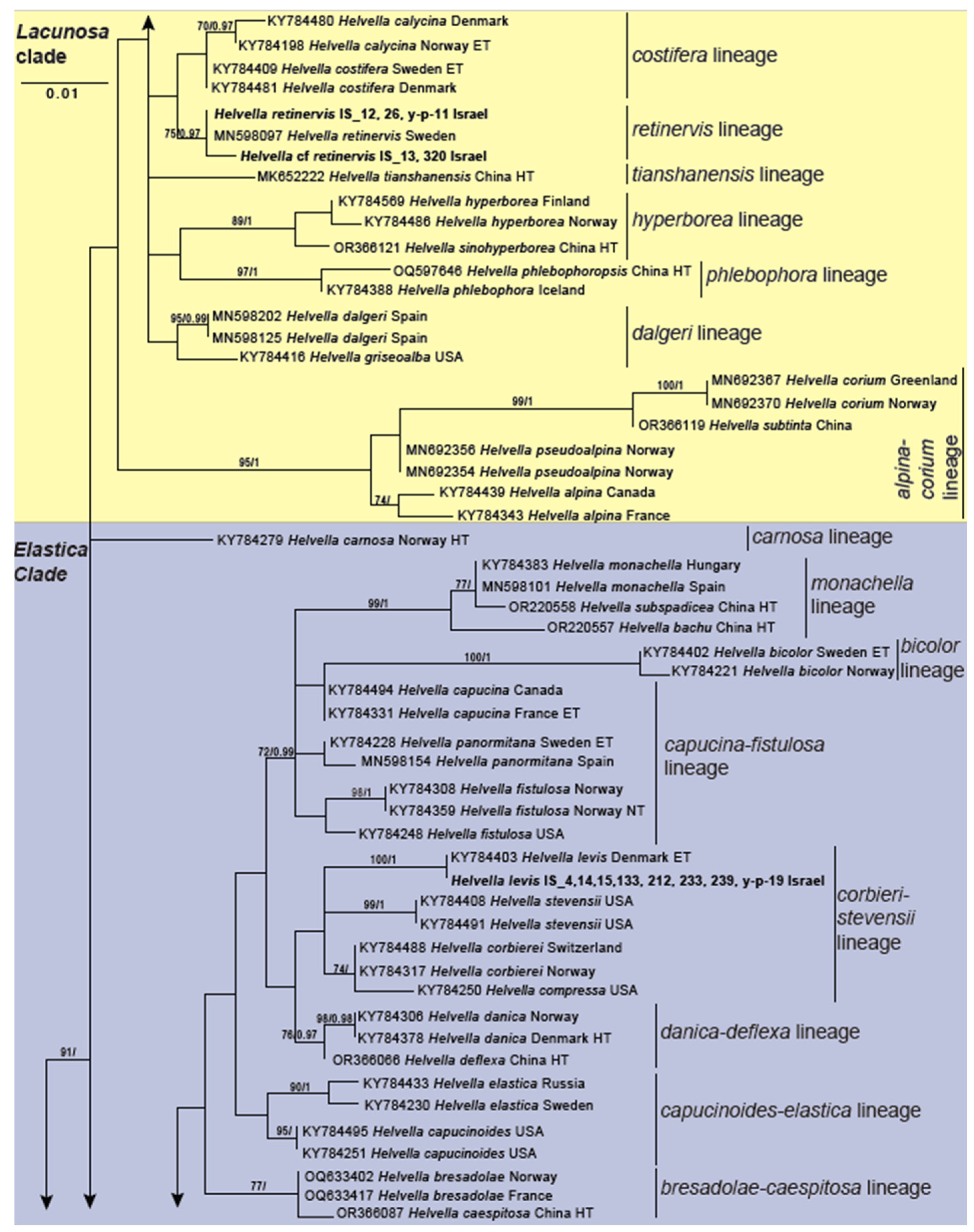
3.1. Molecular Identification
3.2. Genus Helvella
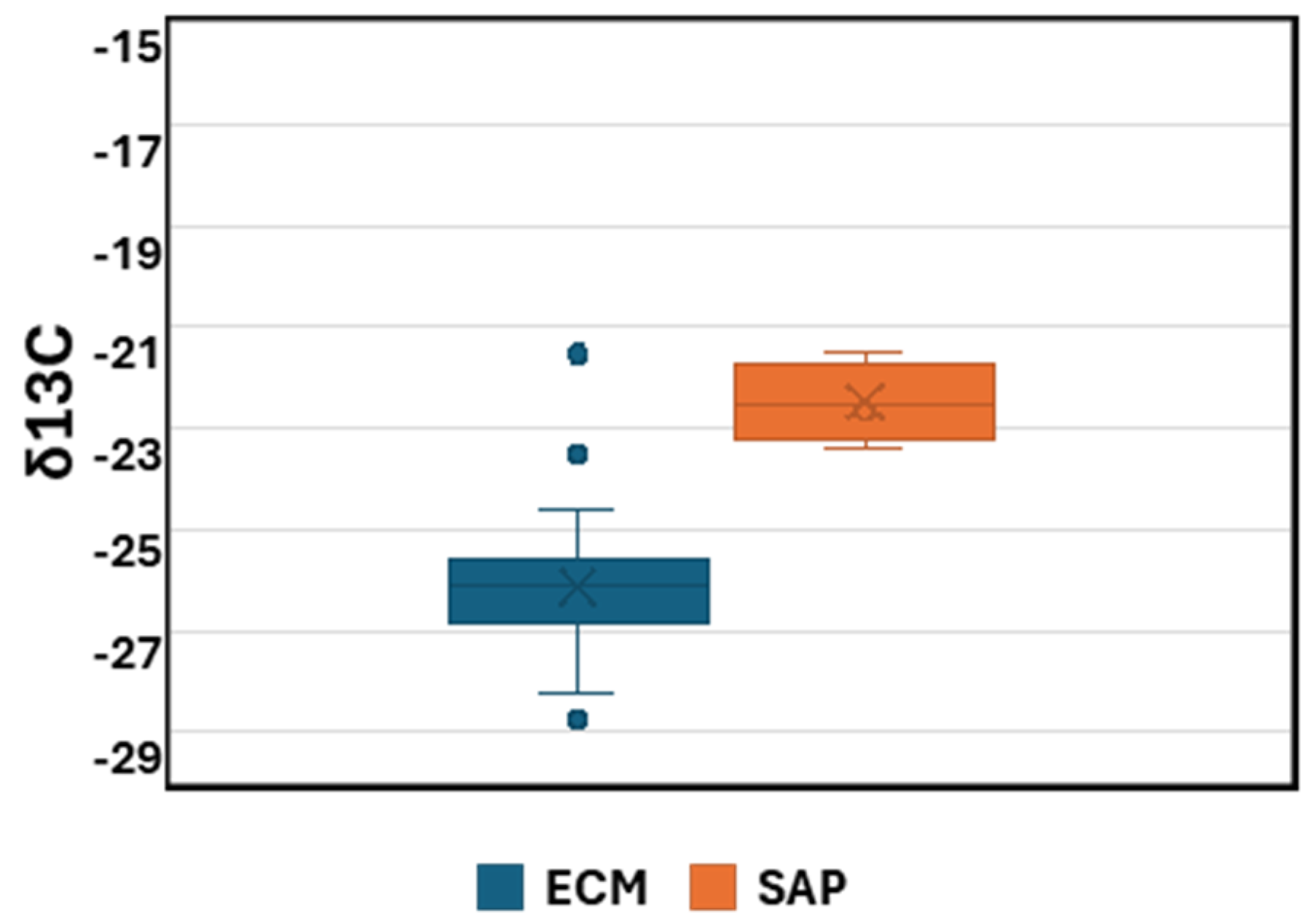
3.3. Trophic Ecology and Isotopic Analysis
4. Discussion
4.1. Diversity of Pezizales in Israel
4.2. Trophic Ecology of Pezizales in Israel
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Family | ITS GB Accession Number | ID | Habitat, Nearby Vegetation, Collected by and Date of Collection Data | Trophic Mode According to Literature Versus According QDA Model | Reported by Barseghyan and Wasser 2013? |
|---|---|---|---|---|---|---|
| Calongea prieguensis | Pezizaceae | OR142392 (ITS) | IS_y-p-28 | Forest floor; oak, bay; West Galilee Region, YS, 30 March 2022 | ECM/- | No |
| Dissingia cf. leucomelaena | Helvellaceae | OR142368 (ITS) | IS_315 | Forest floor; pine, bryophyte, grass, orchid; Merom Ha-Galil Region, EO , 15 March 2021 | ECM/- | Maybe |
| Dissingia cf. leucomelaena | Helvellaceae | OR142369 (ITS); OR141932 (28S) | IS_316 | Forest floor; pine, bryophytes, grass, orchid; Merom Ha-Galil Region, EO, 15 March 2021 | ECM/- | Maybe |
| Dissingia leucomelaena | Helvellaceae | PQ072266 | IS_252 | Forest floor; cut pine, bryophytes; grass and herbs, Merom Ha-Galil Region, EO, 15 March 2021 | ECM/ECM | Yes |
| Dissingia leucomelaena | Helvellaceae | PQ072245 | IS_106 | Trail side; bryophytes, oaks, asparagus, cyclamens, weeds; Naftali Mountains Region, EO, 07 February 2021 | ECM/ECM | Yes |
| Dissingia leucomelaena | Helvellaceae | OR141907 (28S); PQ072244 (Hsp90) | IS_102 | Trail side; bryophytes, oaks, asparagus, cyclamens, weeds; Naftali Mountains Region, EO, 07 February 2021 | ECM/ECM | Yes |
| Genea lobulata | Pyronemataceae | OR142387 (ITS) | IS_y-p-20 | Forest floor; under pine tree, bryophytes; West Galilee Region, YS, 15 March 2022 | ECM/ECM | No |
| Geopora sp. 1 | Pyronemataceae | OR142351 (ITS); OR141925 (28S) | IS_219 | Forest floor; bryophyte, bay, oak; West Galilee Region, EO and YS, 21 February 2021 | ECM/Undetermined | Maybe |
| Geopora sp. 1 | Pyronemataceae | OR142358 (ITS) | IS_238 | On limestone wall; bryophyte; West Galilee Region, YS, 14 March 2021 | ECM/Undetermined | Maybe |
| Geopora sp. 1 | Pyronemataceae | OR142336 (ITS) | IS_120 | Forest floor; oak, bryophyte; West Galilee Region, EO and YS, 16 March 2021 | ECM/ECM | Maybe |
| Geopora sumneriana | Pyronemataceae | OR142344 (ITS) | IS_151 | Forest floor; oak, pine; Golan Heights Region, EO and IS, 07 March 2021 | ECM/ECM | No |
| Helvella acetabulum | Helvellaceae | PQ072269 (Hsp90) | IS_319 | Forest floor; oak; West Galilee Region, EO and YS, 16 March 2021 | ECM/ECM | Yes |
| Helvella acetabulum | Helvellaceae | OR142373 (ITS) | IS_1102 | Forest floor; oak; West Galilee Region, YS, 15 March 2022 | ECM/ECM | Yes |
| Helvella acetabulum | Helvellaceae | PQ072277 (Hsp90) | IS_y-p-12 | Valley bank; bryophytes; West Galilee Region, YS, 14 March 2022 | ECM/ECM | Yes |
| Helvella cf. acetabulum | Helvellaceae | PQ072243 (Hsp90) | IS_29 | Side of trail; Rhamnus sp, Cercis siliquastrum, oak, asparagus, Retama sp, bryophytes; Naftali Mountains Region, EO, 26 January 2021 | ECM/ECM | Yes |
| Helvella cf. acetabulum | Helvellaceae | OR141909 (28S); PQ072246 (Hsp90) | IS_107 | On slope near trail; Rhamnus sp, oak, bryophyte; Naftali Mountains Region, EO, 14 February 202 | ECM/ECM | Yes |
| Helvella cf. acetabulum | Helvellaceae | OR141911 (28S); PQ072248 (Hsp90) | IS_126 | Forest floor; bay, oak; West Galilee Region, EO and YS, 16 March 2021 | ECM/- | Yes |
| Helvella cf. calycina | Helvellaceae | OR142353 (ITS) | IS_231 | Forest floor; oak; West Galilee Region, EO and YS, 01 March 2021 | ECM/Undetermined | No |
| Helvella cf. inexpectata | Helvellaceae | OR142370 (ITS); PQ072271 (Hsp90) | IS_500 | Forest floor; bryophytes, oak, pine, weeds; West Galilee Region, EO and YS, 11 February 2021 | ECM/ECM | No |
| Helvella cf. poculiformis | Helvellaceae | PQ072255 (Hsp90) | IS_210 | Forest floor; oak; Emek Yizrael Region, YS, 18 February 2021 | ECM/ECM | No |
| Helvella cf. poculiformis | Helvellaceae | OR141910 (28S); PQ072247 (Hsp90) | IS_125 | Forest floor rich soil; bay, oak; West Galilee Region, EO and YS, 16 March 2021 | ECM/ECM | No |
| Helvella cf. retinervis | Helvellaceae | PQ072270 (Hsp90) | IS_320 | Forest floor; oak; West Galilee Region, EO and YS, 16 March 2021 | ECM/ECM | No |
| Helvella cf. retinervis | Helvellaceae | OR141895 (28S); PQ072239 (Hsp90) | IS_13 | Forest floor; oak; West Galilee Region, EO and YS, 16 March 2021 | ECM/ECM | No |
| Helvella cf. solitaria | Helvellaceae | OR141923 (28S); PQ072259 (Hsp90) | IS_216 | Forest floor; oak, bay; West Galilee Region, EO and YS, 22 February 2021 | ECM/ECM | No |
| Helvella cf. solitaria | Helvellaceae | OR142350 (ITS); OR141924 (28S); PQ072260 (Hsp90) | IS_218 | Forest floor; oak, bay; West Galilee Region, EO and YS, 22 February 2021 | ECM/ECM | No |
| Helvella cf. solitaria | Helvellaceae | PQ072273 (Hsp90) | IS_y-p-4 | Valley floor; bryophytes; West Galilee Region, YS, 13 March 2022 | ECM/ECM | No |
| Helvella cf. solitaria | Helvellaceae | OR141914 (28S); PQ072250 (Hsp90) | IS_135 | Valley bed; bryophyte; West Galilee Region, EO and YS, 16 March 2021 | ECM/ECM | No |
| Helvella fuscolacunosa | Helvellaceae | OR142371 (ITS); PQ072272 (Hsp90) | IS_505 | Forest floor; oak, bay; West Galilee Region, EO and YS, 11 February 2021 | ECM/Undetermined | No |
| Helvella fuscolacunosa | Helvellaceae | OR141901 (28S); PQ072242 (Hsp90) | IS_28 | On side of trail; Rhamnus sp, Cercis siliquastrum, oak, asparagus, Retama sp, bryophytes; Naftali Mountains Region, EO and YS, 26 January 2021 | ECM/ECM | No |
| Helvella fuscolacunosa | Helvellaceae | OR142372 (ITS) | IS_506 | Forest floor; oak, bay; West Galilee Region, EO and YS, 11 February 2021 | ECM/ECM | No |
| Helvella fuscolacunosa | Helvellaceae | OR142381 (ITS); PQ072275 (Hsp90) | IS_y-p-10 | Valley bank; bryophytes, oak; West Galilee Region YS, 14 March 2022 | ECM/ECM | No |
| Helvella lactea | Helvellaceae | OR142347 (ITS); PQ072253 (Hsp90) | IS_203 | Forest floor; oak; West Galilee Region, EO and YS, 29 March 2021 | ECM/- | No |
| Helvella lactea | Helvellaceae | OR142360 (ITS); PQ072264 (Hsp90) | IS_241 | Forest floor; oak; West Galilee Region, EO and YS, 14 March 2021 | ECM/ECM | No |
| Helvella lactea | Helvellaceae | PQ072254 (Hsp90) | IS_204 | Forest floor; oak; West Galilee Region, EO and YS, 29 January 2021 | ECM/- | No |
| Helvella lactea | Helvellaceae | OR142323 (ITS) | IS_18 | Forest floor; oak; West Galilee Region, YS, 25 March 2020 | ECM/- | No |
| Helvella levis | Helvellaceae | OR141921 (28S); PQ072257 (Hsp90) | IS_212 | Forest floor; oak, bay; West Galilee Region, EO and YS, 22 February 2021 | ECM/ECM | No |
| Helvella levis | Helvellaceae | OR142359 (ITS); OR141929 (28S); PQ072263 (Hsp90) | IS_239 | Forest floor; bay, oak; West Galilee Region, YS, 14 March 2021 | ECM/ECM | No |
| Helvella levis | Helvellaceae | OR141913 (28S); PQ072249 (Hsp90) | IS_133 | Valley bed; oak; West Galilee Region, EO and YS, 16 March 2021 the closest match is H. stevensii which is in the lineage of H. levis | ECM/ECM | No |
| Helvella levis | Helvellaceae | PP974222 (ITS) | IS_y-p-19 | Forest floor; oak; West Galilee Region, YS, 15 March 2022 | ECM/ECM | No |
| Helvella levis | Helvellaceae | OR141896 (28S) | IS_15 | Forest floor; oak; West Galilee Region, SM, 22 March 2020 | ECM/ECM | No |
| Helvella levis | Helvellaceae | PQ072237 (Hsp90) | IS_4 | Forest floor; pine, herbs; Carmel Forest, CC, 12 March 2020 | ECM/ECM | No |
| Helvella levis | Helvellaceae | OR142321 (ITS); PQ072240 (Hsp90) | IS_14 | Limestone soil; bryophytes; West Galilee Region, SM, 22 March 2022 | ECM/Undetermined | No |
| Helvella levis | Helvellaceae | OR142354 (ITS); PQ072262 (Hsp90) | IS_233 | Forest floor; Laurus nobilis, Quercus calliprinos, Clematis cirrhosa; West Galilee Region, YS, 07 March 2021 | ECM/- | No |
| Helvella neopallescens | Helvellaceae | OR142362 (ITS); OR141930 (28S); PQ072265 (Hsp90) | IS_244 | Forest floor (pig track); bay, oak; West Galilee Region, YS, 14 March 2021 | ECM/ECM | No |
| Helvella neopallescens | Helvellaceae | OR142380 (ITS); PQ072274 (Hsp90) | IS_y-p-9 | Valley bank; bryophytes; West Galilee Region, YS, 14 March 2022 | ECM/ECM | No |
| Helvella neopallescens | Helvellaceae | OR142390 (ITS); PQ072280 (Hsp90) | IS_y-p-24 | Forest floor; oak, magnolia; West Galilee Region, YS, 17 March 2022 | ECM/ECM | No |
| Helvella neopallescens | Helvellaceae | OR142365 (ITS); OR141931 (28S); PQ072267 (Hsp90) | IS_300 | Forest floor; oak; Golan Heights, YS, 08 February 2021 | ECM/ECM | No |
| Helvella neopallescens | Helvellaceae | PQ072252 (Hsp90) | IS_150 | Forest floor; bryophytes, oak, ferns; Golan Heights Region, CC, 03 March 2021 | ECM/ECM | No |
| Helvella poculiformis | Helvellaceae | OR141922 (28S); PQ072258 (Hsp90) | IS_213 | Forest floor; oak, bay; West Galilee Region, EO and YS, 22 February 2021 | ECM/- | No |
| Helvella poculiformis | Helvellaceae | OR142386 (ITS); PQ072279 (Hsp90) | IS_y-p-18 | Dry stream bank; bryophyte, oak; West Galilee Region, YS, 15 March 2022 | ECM/ECM | No |
| Helvella poculiformis | Helvellaceae | OR142382 (ITS); PQ072278 (Hsp90) | IS_y-p-13 | Valley bank; bryophytes; West Galilee Region, YS, 14 March 2022 | ECM/ECM | No |
| Helvella poculiformis | Helvellaceae | OR141920 (28S); PQ072256 (Hsp90) | IS_211 | Forest floor; oak; Emek Yizrael Region, YS, 18 February 2021 | ECM/- | No |
| Helvella retinervis | Helvellaceae | OR141899 (28S); PQ072241 (Hsp90) | IS_26 | Forest floor; oak; West Galilee Region, YS, 01 February 2021 | ECM/ECM | No |
| Helvella retinervis | Helvellaceae | PQ072276 (Hsp90) | IS_y-p-11 | Valley bank; bryophytes, oak; West Galilee Region, YS, 14 March 2022 | ECM/ECM | No |
| Helvella retinervis | Helvellaceae | OR141894 (28S); PQ072238 (Hsp90) | IS_12 | Forest floor; oak, pine; West Galilee Region, SM, 22 March 2022 | ECM/ECM | No |
| Helvella solitaria | Helvellaceae | OR141926 (28S); PQ072261 (Hsp90) | IS_222 | Forest floor; bay, oak; West Galilee Region, EO and YS, 23 February 2021 | ECM/ECM | No |
| Helvella solitaria | Helvellaceae | PQ072268 (Hsp90) | IS_317 | Forest floor; Pistacia, oak; West Galilee Region, EO and YS, 13 March 2021 | ECM/ECM | No |
| Helvella solitaria | Helvellaceae | OR141915 (28S); PQ072251 (Hsp90) | IS_137 | Valley bed; bryophyte; West Galilee Region, EO and YS, 16 March 2021 | ECM/ECM | No |
| Helvella sp. | Helvellaceae | OR141936 (28S) | IS_y-p-17 | Dry stream bank; oak; West Galilee Region, YS, 15 March 2022. Note: Close match to H. levis (99%). | ECM/ECM | Maybe |
| Helvella sp. 1 | Helvellaceae | PQ072281(Hsp90) | IS_503 | Forest floor; Cistus sp, Calicotome villosa, weeds; West Galilee Region, EO and YS, 11 February 2021, Note: close to H. atra and H. hispanica | ECM/ECM | Maybe |
| Helvella sp. | Helvellaceae | OR141892 (28S) | IS_8 | On steep forest wall; bryophytes, oak; West Galilee Region, SM, 22 March 2020 | ECM/ECM | Maybe |
| Helvella sp. | Helvellaceae | OR141934 (28S) | IS_y-p-6 | Valley floor; bryophytes; West Galilee Region, YS, 14 March 2022, Note: close to H. pallascens and H. vulgate | ECM/ECM | Maybe |
| Humaria sp. 1 | Pyronemataceae | OR142340 (ITS) | IS_124 | Forest floor on rich soil; bay; oak; West Galilee Region, EO and YS, 22 March 2021 | ECM/ECM | Maybe |
| Humaria sp. 2 | Pyronemataceae | OR142325 (ITS); OR141898 (28S) | IS_22 | Forest floor; oak, bryophyte; Golan Heights, EO, 22 December 2020, Note: close to H. hemisphaerica | ECM/- | Maybe |
| Legaliana sp.1 | Pezizaceae | OR142343 (ITS); OR141912 (28S) | IS_132 | Valley bed; oak; West Galilee Region, EO&YS, 16 March 2021 | ECM/ECM | Maybe |
| Legaliana sp.1 | Pezizaceae | OR142377 (ITS) | IS_y-p-2 | Valley floor; oak; bryophyte; West Galilee Region, YS, 22 March 2022 | ECM/ECM | Maybe |
| Legaliana sp.1 | Pezizaceae | PP972214 (28S) | IS_6 | Forest floor; oak; West Galilee Region, SM, 14 March 2020, Note: similar to P. badiofusca | ECM/Undetermined | Maybe |
| Legaliana sp.2 | Pezizaceae | OR142374 (ITS) | IS_1105 | Forest floor; oak; West Galilee Region, YS, 31 March 2021 | ECM/ECM | Maybe |
| Legaliana sp.2 | Pezizaceae | OR142391 (ITS); OR141937 (28S) | IS_y-p-26 | Forest floor; oak; West Galilee Region, YS, 20 March 2022 | ECM/ECM | Maybe |
| Morchella sp. | Morchellaceae | OR142322 (ITS) | IS_17 | Forest floor soil; Lower Galilee Region, LZ and SM, 30 March 2020. Note: ITS sequence is 98.9% similar to M. vulgaris; 98.5% similar to M. elata; | ECM/- | Maybe |
| Otidea adorniae | Pyronemataceae | OR142341 (ITS) | IS_128 | Forest floor; bay, oak; West Galilee Region, EO and YS, 16 March 2021 | ECM/ECM | No |
| Otidea bufonia | Pyronemataceae | OR142393 (ITS) | IS_y-p-35 | Forest floor; oak; West Galilee Region, YS, 23 November 2023 | ECM/- | No |
| Paragalactinia cf. michelii | Pezizaceae | OR142357 (ITS) | IS_237 | Limestone wall; bryophyte; West Galilee Region, EO and YS, 14 March 2021 | ECM/ECM | Yes |
| Paragalactinia cf. hypoleuca | Pezizaceae | OR142363 (ITS) | IS_245 | Forest floor (pig track); bay, oak; West Galilee Region, EO and YS, 14 March 2021 | ECM/- | No |
| Paragalactinia cf. hypoleuca | Pezizaceae | OR142364 (ITS) | IS_251 | Forest floor; oak, Merom Ha-Galil Region YS, 15 March 2021 | ECM/ECM | No |
| Paragalactinia cf. hypoleuca | Pezizaceae | OR142349 (ITS); OR141918 (28S) | IS_207 | No data | ECM/Undetermined | No |
| Paragalactinia cf. hypoleuca | Pezizaceae | OR142389 (ITS) | IS_y-p-23 | Dry stream bank; bryophytes; West Galilee Region, YS, 16 March 2022 | ECM/ECM | No |
| Paragalactinia cf. hypoleuca | Pezizaceae | OR142378 (ITS) | IS_y-p-3 | Valley floor; oak, bryophyte; West Galilee Region, YS, 13 March 2022 | ECM/ECM | No |
| Paragalactinia cf. hypoleuca | Pezizaceae | OR142366 (ITS) | IS_301 | Forest floor; oak; Golan Hights Region, EO and SM, 08 February 2021 | ECM/ECM | No |
| Paragalactinia cf. hypoleuca | Pezizaceae | OR142379 (ITS) | IS_y-p-8 | Valley bank; bryophytes, oak; West Galilee Region, YS, 14 March 2022 | ECM/ECM | No |
| Paragalactinia cf. hypoleuca | Pezizaceae | OR142346 (ITS); OR141916 (28S) | IS_202 | Forest floor; oak; West Galilee Region, EO and YS, 21 January 2021 | ECM/Undetermined | No |
| Paragalactinia succosa | Pezizaceae | OR141933 (28S) | IS_y-p-5 | Valley floor; bryophytes, cyclamen; West Galilee Region, YS, 13 March 20223 | ECM/- | Yes |
| Paragalactinia sp. | Pezizaceae | ITS, no GB number | IS-1108 | Forest floor; oak, West Galilee Region, YS, 31 March 2021 | ECM/- | Maybe |
| Paragalactinia sp. 1 | Pezizaceae | OR141928 (28S) | IS_224 | Forest floor; bryophytes, West Galilee Region, EO and YS, 23 March 2021 | ECM/- | Maybe |
| Peziza cf. azureoides | Pezizaceae | OR142318 (ITS) | IS_7 | On steep forest wall; bryophytes; West Galilee Region, SM, 22 March 2020 | ECM/- | No |
| Phaeopezia apiculata | Pezizaceae | OR142334 (ITS); OR141906 (28S) | IS_101 | Trail side; bryophytes, oaks, asparagus, cyclamens, weeds; Naftali Mountains Region, EO, 07 February 2021 | ECM/Undetermined | No |
| Phylloscypha phyllogena | Pezizaceae | OR142384 (ITS) | IS_y-p-15 | Valley slope; bryophytes; West Galilee Region, YS, 15 March 2022 | ECM/ECM | No |
| Phylloscypha sp. | Pezizaceae | PP974221 (ITS) | IS_1109 | Valley slope; bryophytes; West Galilee Region | ECM/Undetermined | Maybe |
| Sarcosphaera sp. 1 | Pezizaceae | OR142319 (ITS) | IS_9 | Limestone soil; bryophytes; West Galilee Region, SM, 22 March 2020 Note: close to S. crassa | ECM/ECM | Maybe |
| Sarcosphaera sp. 1 | Pezizaceae | OR141893 (28S) | IS_10 | Forest floor; bryophytes; West Galilee Region, SM, 22 March 2020. Note: close to S. coronaria | ECM/- | Maybe |
| Sepultariella semiimmersa | Pyronemataceae | OR142388 (ITS) | IS_y-p-22 | Dry stream bed; oak, pine; West Galilee Region, YS, 16 March 2022 | ECM/ECM | No |
| Tarzetta cf quercus-ilicis | Pyronemataceae | OR142352 (ITS); OR141927 (28S) | IS_223 | Forest floor; oak; West Galilee Region, EO and YS, 23 February 2021 | ECM/ECM | No |
| Tarzetta cf quercus-ilicis | Pyronemataceae | OR142348 (ITS); OR141917 (28S) | IS_205 | Forest floor; bryophytes; West Galilee Region, YS, 29 January 2021 | ECM/Undetermined | No |
| Trichophaea cf. woolhopeia | Pyronemataceae | OR142383 (ITS) | IS_y-p-14 | Valley bank; bryophytes; West Galilee Region, YS, 14 March 2022, Note: close to T. woolhopeia (96.7%) | ECM/Saprobe | Maybe |
| Anthracobia sp. | Pyronemataceae | OR142326 (ITS) | IS_23 | Forest floor; burned pine; Carmel Forest Region, SM, 01 January 2021 | Saprobe/Saprobe | Maybe |
| Daleomyces sp. | Pezizaceae | OR142342 (ITS) | IS_129 | Forest floor; Jerusalem pine, Pistacia lentiscus, buckthorn, Clematis cirrhosa, Smilax aspera; West Galilee Region, EO and YS, 16 March 2021 | Saprobe/ECM | No species |
| Daleomyces bicolor | Pezizaceae | OR142376 (ITS) | IS_y-p-1 | Forest floor; oak, pine; West Galilee Region, YS, 02 February 2022 | Saprobe/ECM | No |
| Elaiopezia sp. | Pezizaceae | OR142385 (ITS); OR141935 (28S) | IS_y-p-16 | Dry stream bank; bryophyte, oak; West Galilee Region, YS, 03 March 2022 | Saprobe/ECM | Maybe |
| Geopyxis majalis | Pyronemataceae | OR142339 (ITS) | IS_123 | On soil next to path near base of a limestone wall; weeds, bryophytes, cedar; Jerusalem pine; West Galilee Region, EO and YS, 16 March 2021 | Saprobe-endophyte/Undetermined | No species |
| Geopyxis majalis | Pyronemataceae | OR142320 (ITS) | IS_11 | On forest floor; pine; West Galilee Region, SM, 22 March 2020 | Saprobe-endophye/Saprobe | No species |
| Melastiza sp. | Pyronemataceae | OR142329 (ITS); OR141900 (28S) | IS_27 | Irrigated hamra soil, compost; West Galilee Region, YS and CC, 01 March 2021 | Saprobe/ECM | Maybe |
| Peziza varia | Pezizaceae | OR142338 (ITS) | IS_122 | Forest floor, sandy limestone soil, cut branches; fig, cedar; West Galilee Region, EO and YS, 16 March 2021 | Saprobe/Saprobe | Yes |
| Peziza varia | Pezizaceae | OR142317 (ITS) | IS_3 | Forest floor; Emek Yizrael Region, DL, 07 March 2020 | Saprobe/- | Yes |
| Peziza sp. 1 sensu stricto | Pezizaceae | OR142324 (ITS); OR141897 (28S) | IS_21 | Forest floor, near oak, Golan Heights Region, EO, 24 December 2020, Note: similar to P. arvernensis | Saprobe/- | Maybe |
| Peziza sp. 2 sensu stricto | Pezizaceae | OR142367 (ITS) | IS_303 | Forest floor in clearing, annuals; grass; EO, 08 March 2021, Note: closest to P. subvesiculosa | Saprobe/- | Maybe |
| Sarcoscypha coccinea | Sarcoscyphaceae | OR142327 (ITS) | IS_24 | Dead oak branch; oak; West Galilee Region, EO and SM & YS, 05 January 2021 | Saprobe/Undetermined | Yes |
| Sarcoscypha coccinea | Sarcoscyphaceae | OR142330 (ITS); OR141902 (28S) | IS_30 | Dead oak branch; oak; Golan Hights Region, 03 January 2021 | Saprobe/- | Yes |
| Sarcoscypha coccinea | Sarcoscyphaceae | OR142345 (ITS) | IS_201 | Dead oak branch; oak; West Galilee Region, EO and YS, 21 January 2021 | Saprobe/- | Yes |
| Sarcoscypha coccinea | Sarcoscyphaceae | OR142332 (ITS); OR141904 (28S) | IS_32 | No data | Saprobe/- | Yes |
| Sarcoscypha coccinea | Sarcoscyphaceae | OR142333 (ITS); OR141905 (28S) | IS_33 | No data | Saprobe/- | Yes |
| Scutellinia sp. 1 | Pyronemataceae | OR142337 (ITS) | IS_121 | Sandy limestone soil in alluvial area next to cut branches; fig, cedar; West Galilee Region, EO and YS, 03 March 2021 | Saprobe/- | Maybe |
| Scutellinia sp. 2 | Pyronemataceae | OR142316 (ITS) | IS_2 | Scutellinia sp., OR14 2316 (ITS), dead branch, pine, Gilboa Mountains, DL, 07 March 2020 | Saprobe/- | Maybe |
Appendix B

References
- Hansen, K.; Pfister, D.H. Systematics of the Pezizomycetes—The operculate discomycetes. Mycologia 2006, 98, 1029–1040. [Google Scholar] [PubMed]
- Pfister, D.H.; Healy, R. Pezizomycetes. In Encyclopedia of Mycology; Zaragoza, O., Casadevall, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 1, pp. 295–309. [Google Scholar]
- Hansen, K.; Sandal, S.K.; Dissing, H. New and rare species of Pezizales from calcareous woodlands in Denmark. Nord. J. Bot. 1998, 18, 611–626. [Google Scholar] [CrossRef]
- Petersen, P.M. The ecology of Danish soil inhabiting Pezizales with emphasis on edaphic conditions. Nord. J. Bot. 1984, 4, 816. [Google Scholar] [CrossRef]
- Leonardi, M.; Iotti, M.; Oddis, M.; Lalli, G.; Pacioni, G.; Leonardi, P.; Maccherini, S.; Perini, C.; Salerni, E.; Zambonelli, A. Assessment of ectomycorrhizal fungal communities in the natural habitats of Tuber magnatum (Ascomycota, Pezizales). Mycorrhiza 2013, 23, 349–358. [Google Scholar] [CrossRef]
- Ori, F.; Hall, I.; Gianchino, C.; Iotti, M.; Zambonelli, A. Truffles and morels: Two different evolutionary strategies of fungal-plant interactions in the Pezizales. In Plant Microbe Interface; Varma, A., Tripathi, S., Prasad, R., Eds.; Springer: Berlin, Germany, 2019; pp. 69–93. [Google Scholar]
- Tedersoo, L.; May, T.W.; Smith, M.E. Ectomycorrhizal lifestyle in fungi: Global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 2010, 20, 217–263. [Google Scholar] [CrossRef]
- Lebreton, A.; Zeng, Q.; Miyauchi, S.; Kohler, A.; Dai, Y.C.; Martin, F.M. Evolution of the mode of nutrition in symbiotic and saprotrophic fungi in forest ecosystems. Annu. Rev. Ecol. Evol. System. 2021, 52, 385–404. [Google Scholar] [CrossRef]
- Nsele, N.N.; Padayachee, T.; Nelson, D.R.; Syed, K. Pezizomycetes genomes reveal diverse P450 complements characteristic of saprotrophic and ectomycorrhizal lifestyles. J. Fungi 2023, 9, 830. [Google Scholar] [CrossRef]
- Dirks, A.C.; Methven, A.S.; Miller, A.N.; Orozco-Quime, M.; Maurice, S.; Bonito, G.; Van Wyk, J.; Ahrendt, S.; Kuo, A.; Andreopoulos, W.; et al. Phylogenomic insights into the taxonomy, ecology, and mating systems of the lorchel family Discinaceae (Pezizales, Ascomycota). Mol. Phylogenet. Evol. 2025, 205, 108286. [Google Scholar] [CrossRef]
- Selosse, M.A.; Martos, F.; Perry, B.A.; Padamsee, M.; Roy, M.; Pailler, T. Saprotrophic fungal mycorrhizal symbionts in achlorophyllous orchid: Finding treasures among the ‘molecular scraps’? Plant Signal. Behav. 2010, 5, 349–353. [Google Scholar] [CrossRef]
- Hibbett, D.S.; Ohman, A.; Glotzer, D.; Nuhn, M.; Kirk, P.; Nilsson, R.H. Progress in molecular and morphological taxon discovery in Fungi and options for formal classification of environmental sequences. Fungal Biol. Rev. 2011, 25, 38–47. [Google Scholar] [CrossRef]
- Truong, C.; Mujic, A.B.; Healy, R.; Kuhar, F.; Furci, G.; Torres, D.; Niskanen, T.; Sandoval-Leiva, P.A.; Fernández, N.; Escobar, J.M.; et al. How to know the fungi: Combining field inventories and DNA-barcoding to document fungal diversity. New Phytol. 2017, 214, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Bonito, G.; Smith, M.E.; Nowak, M.; Healy, R.A.; Guevara, G.; Cazares, E.; Kinoshita, A.; Nouhra, E.; Dominguez, L.; Tedersoo, L.; et al. Historical biogeography and diversification of truffles in the Tuberaceae and their newly identified Southern Hemisphere sister lineage. PLoS ONE 2013, 8, e52765. [Google Scholar] [CrossRef]
- Kraisitudomsook, N.; Healy, R.A.; Pfister, D.H.; Truong, C.; Nouhra, E.; Kuhar, F.; Mujic, A.B.; Trappe, J.M.; Smith, M.E. Resurrecting the genus Geomorium: Systematic study of fungi in the genera Underwoodia and Gymnohydnotrya (Pezizales) with the description of three new South American species. Persoonia 2019, 44, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Hobbie, E.A.; Högberg, P. Nitrogen isotopes link mycorrhizal fungi and plants to nitrogen dynamics. New Phytol. 2012, 196, 367–382. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Sánchez, F.S.; Rygiewicz, P.T. Carbon use, nitrogen use, and isotopic fractionation of ectomycorrhizal and saprotrophic fungi in natural abundance and 13C-labelled cultures. Mycol. Res. 2004, 108, 725–736. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Weber, N.S.; Trappe, J.M. Mycorrhizal vs saprotrophic status of fungi: The isotopic evidence. New Phytol. 2001, 150, 601–610. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Macko, S.A.; Shugart, H.H. Insights into nitrogen and carbon dynamics of ectomycorrhizal and saprotrophic fungi from isotopic evidence. Oecologia 1999, 118, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Argiroff, W.A.; Zak, D.R.; Pellitier, P.T.; Upchurch, R.A.; Belke, J.P. Decay by ectomycorrhizal fungi couples soil organic matter to nitrogen availability. Ecol. Lett. 2022, 25, 391–404. [Google Scholar] [CrossRef]
- Talbot, J.M.; Bruns, T.D.; Smith, D.P.; Branco, S.; Glassman, S.I.; Erlandson, S.; Vilgalys, R.; Peay, K.G. Independent roles of ectomycorrhizal and saprotrophic communities in soil organic matter decomposition. Soil Biol. Biochem. 2013, 57, 282–291. [Google Scholar] [CrossRef]
- Henn, M.R.; Gleixner, G.; Chapela, I.H. Growth-dependent stable carbon isotope fractionation by basidiomycete fungi: δ13C pattern and physiological process. Appl. Environ. Microbiol. 2002, 68, 4956–4964. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Rice, S.F.; Weber, N.S.; Smith, J.E. Isotopic evidence indicates saprotrophy in post-fire Morchella in Oregon and Alaska. Mycologia 2016, 108, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Healy, R.A.; Arnold, A.E.; Bonito, G.; Huang, Y.L.; Lemmond, B.; Pfister, D.H.; Smith, M.E. Endophytism and endolichenism in Pezizomycetes: The exception or the rule? New Phytol. 2022, 233, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, S.; Kiss, E.; Kuo, A.; Drula, E.; Kohler, A.; Sánchez-García, M.; Morin, E.; Andreopoulos, B.; Barry, K.W.; Bonito, G.; et al. Large-scale genome sequencing of mycorrhizal fungi provides insights into the early evolution of symbiotic traits. Nat. Commun. 2020, 11, 5125. [Google Scholar] [CrossRef] [PubMed]
- Selosse, M.A.; Schneider-Maunoury, L.; Martos, F. Time to re-think fungal ecology? Fungal ecological niches are often prejudged. New Phytol. 2018, 217, 968. [Google Scholar]
- Mayor, J.R.; Schuur, E.A.G.; Henkel, T.W. Elucidating the nutritional dynamics of fungi using stable isotopes. Ecol. Lett. 2009, 12, 171–183. [Google Scholar] [CrossRef]
- Kadmon, R.; Danin, A. Floristic variation in Israel: A GIS analysis. Flora 1997, 192, 341–345. [Google Scholar] [CrossRef]
- Levin, N.; Shmida, A. Determining conservation hotspots across biogeographic regions using rainfall belts: Israel as a case study. Isr. J. Ecol. Evol. 2007, 53, 33–58. [Google Scholar] [CrossRef]
- Binyamini, N. Larger Fungi of Israel. Ascomycotina, Basidiomycotina; Ramot Publishing Co.: Tel Aviv, Israel, 2007. [Google Scholar]
- Nemlich, H.; Avizohar-Hershenzon, Z. Pezizales of Israel. I. Morchellaceae and Helvellaceae. Isr. J. Bot. 1972, 21, 153–163. [Google Scholar]
- Barseghyan, G.S.; Wasser, S.P. Species diversity of operculate discomycetes in Israel. Isr. J. Plant Sci. 2008, 56, 341–348. [Google Scholar] [CrossRef]
- Barseghyan, G.S.; Wasser, S.P. Species diversity of the genera Morchella St. Amans and Helvella L. ex St. Amans (Ascomycota, Pezizales) in Israeli mycobiota. Nova Hedwig. 2008, 87, 315–336. [Google Scholar] [CrossRef]
- Barseghyan, G.S.; Wasser, S.P. Operculated Discomycetes (Pezizales, Ascomycota) of Israel; Volz, P.A., Nevo, E., Eds.; Koeltz Scientific Books: Königstein, Germany, 2013. [Google Scholar]
- Ferdman, Y.; Sitrit, Y.; Li, Y.F.; Roth-Bejerano, N.; Kagan-Zur, V. Cryptic species in the Terfezia boudieri complex. Antonie Van Leeuwenhoek 2009, 95, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Kagan-Zur, V.; Roth-Bejerano, N. Studying the brown desert truffles of Israel. Isr. J. Plant Sci. 2008, 56, 309–314. [Google Scholar] [CrossRef]
- Morte, A.; Kagan-Zur, V.; Navarro-Ródenas, A.; Sitrit, Y. Cultivation of desert truffles—A crop suitable for arid and semi-arid zones. Agronomy 2021, 11, 1462. [Google Scholar] [CrossRef]
- Turgeman, T.; Sitrit, Y.; Danai, O.; Luzzati, Y.; Bustan, A.; Roth-Bejerano, N.; Kagan-Zur, V.; Masaphy, S. Introduced Tuber aestivum replacing introduced Tuber melanosporum: A case study. Agrofor. Syst. 2012, 84, 337–343. [Google Scholar] [CrossRef]
- Masaphy, S.; Zabari, L.; Gander, G. Fruiting of morels in Israel. Mushroom Biol. Biotechnol. 2007, 213, 209. [Google Scholar]
- Orlofsky, E.; Zabari, L.; Bonito, G.; Masaphy, S. Changes in soil bacteria functional ecology associated with Morchella rufobrunnea fruiting in a natural habitat. Environ. Microbiol. 2021, 23, 6651–6662. [Google Scholar] [CrossRef]
- Tietel, Z.; Masaphy, S. Chemotyping of three Morchella species reveals species- and age-related aroma volatile biomarkers. LWT 2022, 154, 112587. [Google Scholar] [CrossRef]
- Tedersoo, L.; Smith, M.E. Lineages of ectomycorrhizal fungi revisited: Foraging strategies and novel lineages revealed by sequences from belowground. Fungal Biol. Rev. 2013, 27, 83–99. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Ed.; Academic Press: Cambridge, MA, USA, 1990; pp. 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Skrede, I.; Carlsen, T.; Schumacher, T. A synopsis of the saddle fungi (Helvella: Ascomycota) in Europe—Species delimitation, taxonomy and typification. Persoonia 2017, 39, 201–253. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.J.; Ashton, B.; Buxton, S.; Cheung, M.; Cooper, A.; Duran, C.; Field, M.; Heled, J.; Kearse, M.; Markowitz, S.; et al. Geneious v5.6. Available online: http://www.geneious.com (accessed on 4 December 2024).
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Fungal Barcoding Consortium; Bolchacova, E.; Voigt, K.; et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed]
- Skrede, I.; Gonzalvo, L.B.; Mathiesen, C.; Schumacher, T. The genera Helvella and Dissingia (Ascomycota:Pezizomycetes) in Europe—Notes on species from Spain. Fung. Syst. Evol. 2020, 6, 65–93. [Google Scholar] [CrossRef]
- Mao, N.; Xu, Y.Y.; Zhang, Y.X.; Huang, X.B.; Hou, C.L.; Fan, L. Phylogeny and species diversity of the genus Helvella with emphasis on eighteen new species from China. Fungal Syst. Evol. 2023, 12, 111–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.C.; Zhuang, W.Y.; Zhao, R.L. Species diversity of Helvella lacunosa clade (Pezizales, Ascomycota) in China and description of sixteen new species. J. Fungi 2023, 9, 697. [Google Scholar] [CrossRef]
- Yu, F.M.; Lei, L.; Luangharn, T.; Zhao, Q.; Zhu, Y.A. Four new additions to Helvella (Helvellaceae, Pezizales) from Northern Thailand. Front. Microbiol. 2023, 14, 1182025. [Google Scholar] [CrossRef]
- Katoh, K.; Toh, H. Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics 2010, 26, 1899–1900. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogeneticinference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- Põlme, S.; Abarenkov, K.; Nilsson, R.H.; Lindahl, B.D.; Clemmensen, K.E.; Kauserud, H.; Nguyen, N.; Kjøller, R.; Bates, S.T.; Baldrian, P.; et al. FungalTraits: A user-friendly traits database of fungi and fungus-like stramenopiles. Fungal Divers. 2020, 105, 1–16. [Google Scholar] [CrossRef]
- Van Vooren, N. Reinstatement of old taxa and publication of new genera for naming some lineages of the Pezizaceae (Ascomycota). Ascomycete.org 2020, 12, 179–192. [Google Scholar]
- Moreno-Arroyo, B.; Gómez, J.; Calonge, F.D. Pachyphloeus prieguensis, sp. nov. (Ascomycotina), encontrada en España. Bol. Soc. Micol. Madr. 1996, 21, 85–92. [Google Scholar]
- Healy, R.A.; Bonito, G.M.; Trappe, J.M. Calongea, a new genus of truffles in the Pezizaceae (Pezizales). An. Jard. Bot. Madr. 2009, 66, 25–32. [Google Scholar] [CrossRef]
- Sow, A.; Van Wyk, J.; Lemmond, B.; Healy, R.; Smith, M.E.; Bonito, G. Effective field collection of Pezizales ascospores for procuring diverse fungal isolates. Diversity 2024, 16, 165. [Google Scholar] [CrossRef]
- Dissing, H. The genus Helvella in Europe, with special emphasis on the species found in Norden. Dan. Bot. Ark. 1966, 25, 1–172. [Google Scholar]
- Tedersoo, L.; Bahram, M.; Põlme, S.; Kõljalg, U.; Yorou, N.S.; Wijesundera, R.; Ruiz, L.V.; Vasco-Palacios, A.M.; Quang Thu, P.; Suija, A.; et al. Global diversity and geography of soil fungi. Science 2014, 346, 2356688. [Google Scholar] [CrossRef]
- Hansen, K.; Læssøe, T.; Pfister, D.H. Phylogenetic diversity in the core group of Peziza inferred from ITS sequences and morphology. Mycol. Res. 2002, 106, 879–902. [Google Scholar] [CrossRef]
- Hansen, K.; LoBuglio, K.F.; Pfister, D.H. Evolutionary relationships of the cup-fungus genus Peziza and Pezizaceae inferred from multiple nuclear genes: RPB2, β-tubulin, and LSU rDNA. Mol. Phylogenet. Evol. 2005, 36, 1–23. [Google Scholar] [CrossRef]
- Tedersoo, L.; Suvi, T.; Larsson, E.; Kõljalg, U. Diversity and community structure of ectomycorrhizal fungi in a wooded meadow. Mycol. Res. 2006, 110, 734–748. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Agerer, R. Nitrogen isotopes in ectomycorrhizal sporocarps correspond to belowground exploration types. Plant Soil 2010, 327, 71–83. [Google Scholar] [CrossRef]
- Deane-Coe, K.K. Cyanobacteria associations in temperate forest bryophytes revealed by δ15N analysis. J. Torrey Bot. Soc. 2015, 143, 50–57. [Google Scholar] [CrossRef]
- Korotkin, H.B.; Swenie, R.A.; Miettinen, O.; Budke, J.M.; Chen, K.H.; Lutzoni, F.; Smith, M.E.; Matheny, P.B. Stable isotope analyses reveal previously unknown trophic mode diversity in the Hymenochaetales. Am. J. Bot. 2018, 105, 1869–1887. [Google Scholar] [CrossRef] [PubMed]
- Ponce, Á.; Salerni, E.; D’Aguanno, M.N.; Perini, C. Wood-decay fungi fructifying in Mediterranean deciduous oak forests: A community composition, richness and productivity study. Forests 2023, 14, 1326. [Google Scholar] [CrossRef]
- Hansen, K.; Perry, B.A.; Dranginis, A.W.; Pfister, D.H. A phylogeny of the highly diverse cup-fungus family Pyronemataceae (Pezizomycetes, Ascomycota) clarifies relationships and evolution of selected life history traits. Mol. Phylogenet. Evol. 2013, 67, 311–335. [Google Scholar] [CrossRef]
- Santiago, K.A.A.; Ting, A.S.Y. Endolichenic fungi from common lichens as new sources for valuable bio-active compounds. In Natural Bio-Active Compounds: Volume 1: Production and Applications; Akhtar, M.S., Swamy, M.K., Sinniah, U.R., Eds.; Springer: Singapore, 2019; pp. 105–127. [Google Scholar]
- Kohler, A.; Martin, F. The evolution of the mycorrhizal lifestyles—A genomic perspective. In Molecular Mycorrhizal Symbiosis; Martin, F., Ed.; Wiley Online Library: Hoboken, NJ, USA, 2016; pp. 87–106. [Google Scholar]
- Khan, F.K.; Sánchez-García, M.; Johannesson, H.; Ryberg, M. High rate of gene family evolution in proximity to the origin of ectomycorrhizal symbiosis in Inocybaceae. New Phytol. 2024, 244, 219–234. [Google Scholar] [CrossRef]
- Megersa, S.; Yohannes, Y.; Dejene, T.; Martín-Pinto, P. Natural forests support higher mycological diversity and more edible mushroom species than plantation forests in Ethiopia. For. Int. J. For. Res. 2024, 98, 136–147. [Google Scholar] [CrossRef]
- Borovička, J.; Kolařík, M.; Halasů, V.; Perini, C.; Parker, A.D.; Gryndler, M.; Cohen, J.D.; Hršelová, H.; Pastorino, R.; Žigová, A.; et al. Taxonomic revision of the genus Sarcosphaera (Ascomycota: Pezizales) in Europe and North America revealed unexpected diversity. Mycol. Prog. 2024, 23, 69. [Google Scholar] [CrossRef]


| Species | Family | GB Accession no | Sample ID | Reported by Barseghyan and Wasser [34] |
|---|---|---|---|---|
| a. Ectomycorrhizal species | ||||
| Calongea prieguensis | Pezizaceae | OR142392 (ITS) | IS_YP28 | No |
| Dissingia cf. leucomelaena | Helvellaceae | OR142369 (ITS); OR141932 28S | IS_316 | Maybe |
| Dissingia leucomelaena | Helvellaceae | OR141907(28S); PQ072244 (Hsp90) | IS_102 | Yes |
| Genea lobulata | Pyronemataceae | OR142387 (ITS) | IS_YP20 | No |
| Geopora sp. | Pyronemataceae | OR142351(ITS)OR141925 (28S) | IS_219 | Maybe |
| Geopora sumneriana | Pyronemataceae | OR142344 (ITS) | IS_151 | No |
| Helvella acetabulum | Helvellaceae | OR142373 (ITS) | IS-1102 | Yes |
| Helvella cf. acetabulum | Helvellaceae | OR141909(28S); PQ072246 (Hsp90) | IS_107 | Yes |
| Helvella cf. calycina | Helvellaceae | OR142353 (ITS) | IS_231 | No |
| Helvella cf. inexpectata | Helvellaceae | OR142370 (ITS) PQ072271 (Hsp90) | IS_500 | No |
| Helvella cf. poculiformis | Helvellaceae | OR141910(28S); PQ072247 (Hsp90) | IS_125 | No |
| Helvella cf. retinervis | Helvellaceae | OR141895(28S); PQ072239 (Hsp90) | IS_13 | No |
| Helvella cf. solitaria | Helvellaceae | OR142350(ITS); OR141924(28S); PQ072260 (Hsp90) | IS_218 | No |
| Helvella fuscolacunosa | Helvellaceae | OR142371(ITS); PQ072272 (Hsp90) | IS_YP10 | No |
| Helvella lactea | Helvellaceae | OR142347(ITS); PQ072253 (Hsp90) | IS_203 | No |
| Helvella levis | Helvellaceae | OR142359(ITS); OR141929(28S); PQ072263 (Hsp90) | IS_239 | No |
| Helvella neopallescens | Helvellaceae | OR142362(ITS); OR141930(28S); PQ072265 (Hsp90) | IS_244 | No |
| Helvella poculiformis | Helvellaceae | OR141922(28S); PQ072258 (Hsp90) | IS_213 | No |
| Helvella retinervis | Helvellaceae | OR141899(28S); PQ072241 (Hsp90) | IS_26 | No |
| Helvella solitaria | Helvellaceae | OR141926(28S); PQ072261 (Hsp90) | IS_222 | No |
| Helvella sp. | Helvellaceae | PQ072281 (Hsp90) | IS_503 | Maybe |
| Humaria sp. | Pyronemataceae | OR142340 (ITS) | IS_124 | Maybe |
| Legaliana sp. 1 | Pezizaceae | OR142343(ITS); OR141912 (28S) | IS_132 | Maybe |
| Legaliana sp. 2 | Pezizaceae | OR142391 (ITS); OR141937 28S | IS_YP26 | Maybe |
| Otidea adorniae | Otideaceae | OR142341 (ITS) | IS_128 | No |
| Otidea bufonia | Otideaceae | OR142393 (ITS) | IS_YP35 | No |
| Paragalactinia cf. michelii | Pezizaceae | OR142357 (ITS) | IS_237 | Yes |
| Paragalactinia cf. hypoleuca | Pezizaceae | OR142349(ITS); OR141918 (28S) | IS_207 | No |
| Paragalactinia succosa | Pezizaceae | OR141933 (2S) | IS_YP5 | Yes |
| Paragalactinia sp. | Pezizaceae | OR141928 (28S) | IS_224 | Maybe |
| Peziza cf. azureoides | Pezizaceae | OR142318 (ITS) | IS_7 | No |
| Phylloscypha phyllogena | Pezizaceae | OR142384 (ITS) | IS_YP15 | No |
| Phylloscypha sp. | Pezizaceae | PP974221 (ITS) | IS_1109 | Maybe |
| Sarcosphaera sp. | Pezizaceae | OR142319 (ITS) | IS_9 | Maybe |
| Sepultariella semiimmersa | Pyronemataceae | OR142388 (ITS) | IS_YP22 | No |
| Tarzetta cf. quercus-ilicis | Tarzettaceae | OR142352(ITS); OR141927 (28S) | IS_223 | No |
| Trichophaea cf. woolhopeia | Pyronemataceae | OR142383 (ITS) | IS_YP14 | No |
| b. Saprotrophic species | ||||
| Anthracobia sp. | Pyronemataceae | OR142326 (ITS) | IS_23 | Maybe |
| Elaiopezia sp. | Pezizaceae | OR142385(ITS); OR141935 (28S) | IS_YP16 | Maybe |
| Daleomyces sp. | Pezizaceae | OR142342 (ITS) | IS_129 | Maybe |
| Daleomyces bicolor | Pezizaceae | OR142376 (ITS) | IS_YP1 | No |
| Geopyxis majalis | Tarzettaceae | OR142339 (ITS) | IS_123 | No * |
| Melastiza sp. | Pyronemataceae | OR142329(ITS); OR141900 (28S) | IS_27 | Maybe |
| Morchella sp. | Morchellaceae | OR142322 (ITS) | IS_17A | Maybe |
| Peziza varia | Pezizaceae | OR142338 (ITS) | IS_122 | Yes |
| Peziza sp. sensu stricto | Pezizaceae | OR142324(ITS); OR141897 (28S) | IS_21 | Maybe |
| Phaeopezia apiculata | Pezizaceae | OR142334(ITS); OR141906 (28S) | IS_101 | No |
| Sarcoscypha coccinea | Sarcoscyphaceae | OR142330(ITS); OR141902 (28S) | IS_30 | Yes |
| Scutellinia sp. | Pyronemataceae | OR142337 (ITS) | IS_121 | Maybe |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masaphy, S.; Bonito, G.; Orlofsky, E.; Van Wyk, J.; Lemmond, B.; Healy, R.; Smith, M.E.; Segal, Y.; Zabari, L. Pezizales in Israel: Molecular Phylogenetic and δ13Cδ15N Stable Isotope Data Reveal New Records and Potential Discrepancies in Their Trophic Ecology. J. Fungi 2025, 11, 414. https://doi.org/10.3390/jof11060414
Masaphy S, Bonito G, Orlofsky E, Van Wyk J, Lemmond B, Healy R, Smith ME, Segal Y, Zabari L. Pezizales in Israel: Molecular Phylogenetic and δ13Cδ15N Stable Isotope Data Reveal New Records and Potential Discrepancies in Their Trophic Ecology. Journal of Fungi. 2025; 11(6):414. https://doi.org/10.3390/jof11060414
Chicago/Turabian StyleMasaphy, Segula, Gregory Bonito, Ezra Orlofsky, Judson Van Wyk, Benjamin Lemmond, Rosanne Healy, Matthew E. Smith, Yaniv Segal, and Limor Zabari. 2025. "Pezizales in Israel: Molecular Phylogenetic and δ13Cδ15N Stable Isotope Data Reveal New Records and Potential Discrepancies in Their Trophic Ecology" Journal of Fungi 11, no. 6: 414. https://doi.org/10.3390/jof11060414
APA StyleMasaphy, S., Bonito, G., Orlofsky, E., Van Wyk, J., Lemmond, B., Healy, R., Smith, M. E., Segal, Y., & Zabari, L. (2025). Pezizales in Israel: Molecular Phylogenetic and δ13Cδ15N Stable Isotope Data Reveal New Records and Potential Discrepancies in Their Trophic Ecology. Journal of Fungi, 11(6), 414. https://doi.org/10.3390/jof11060414









