Monitoring of Fungal Diversity and Microclimate in Nine Different Museum Depots
Abstract
1. Introduction
2. Materials and Methods
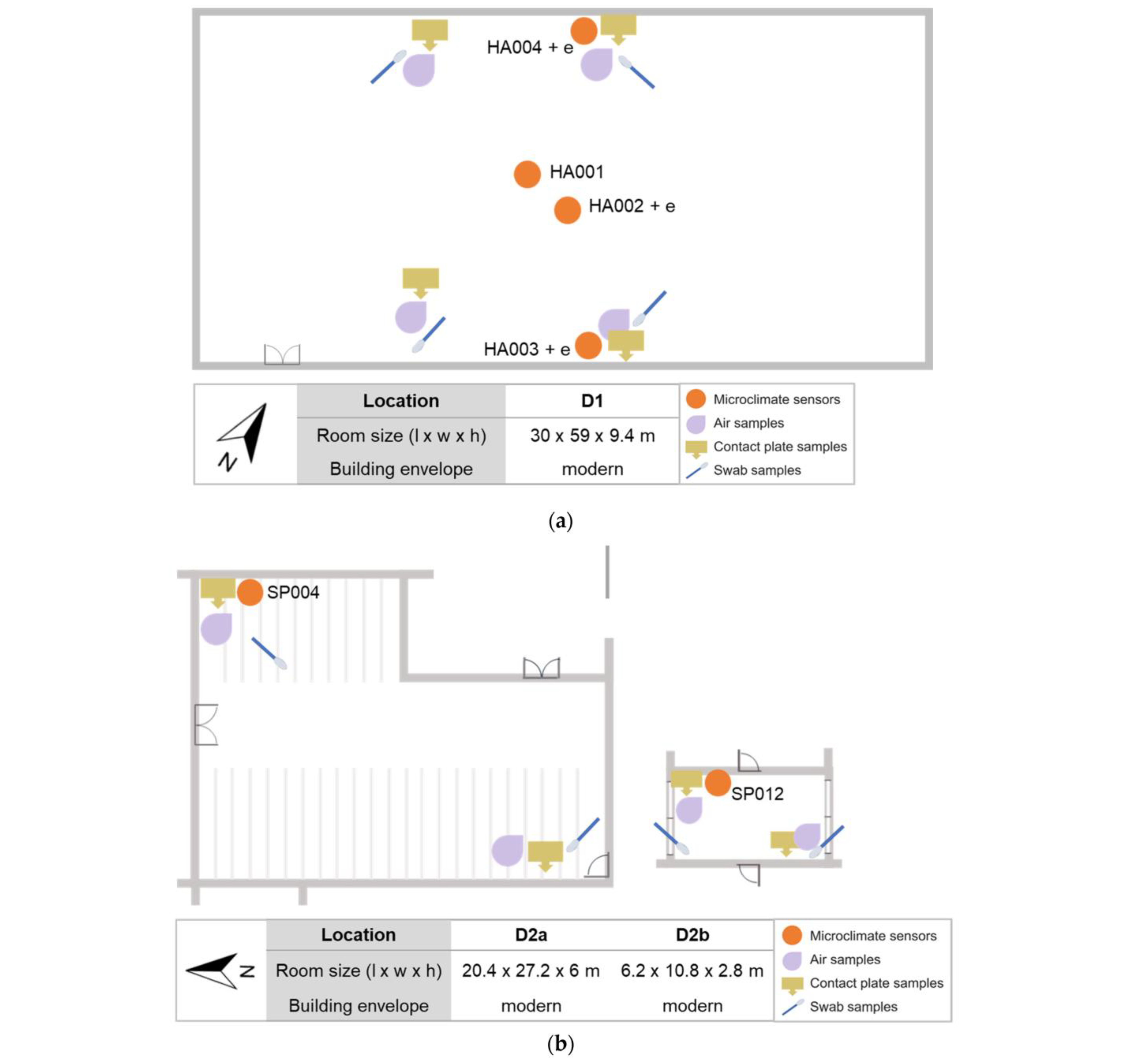
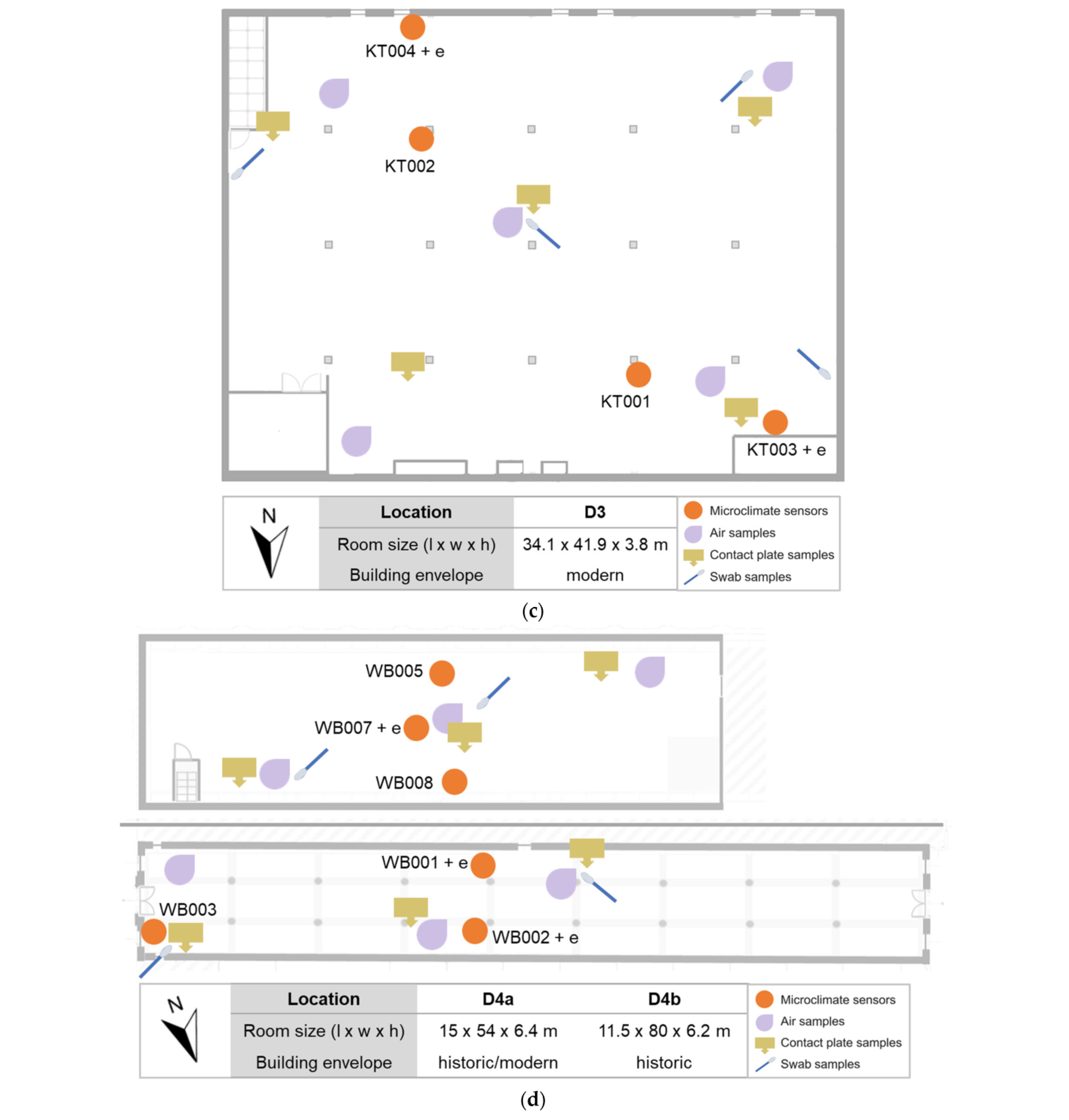
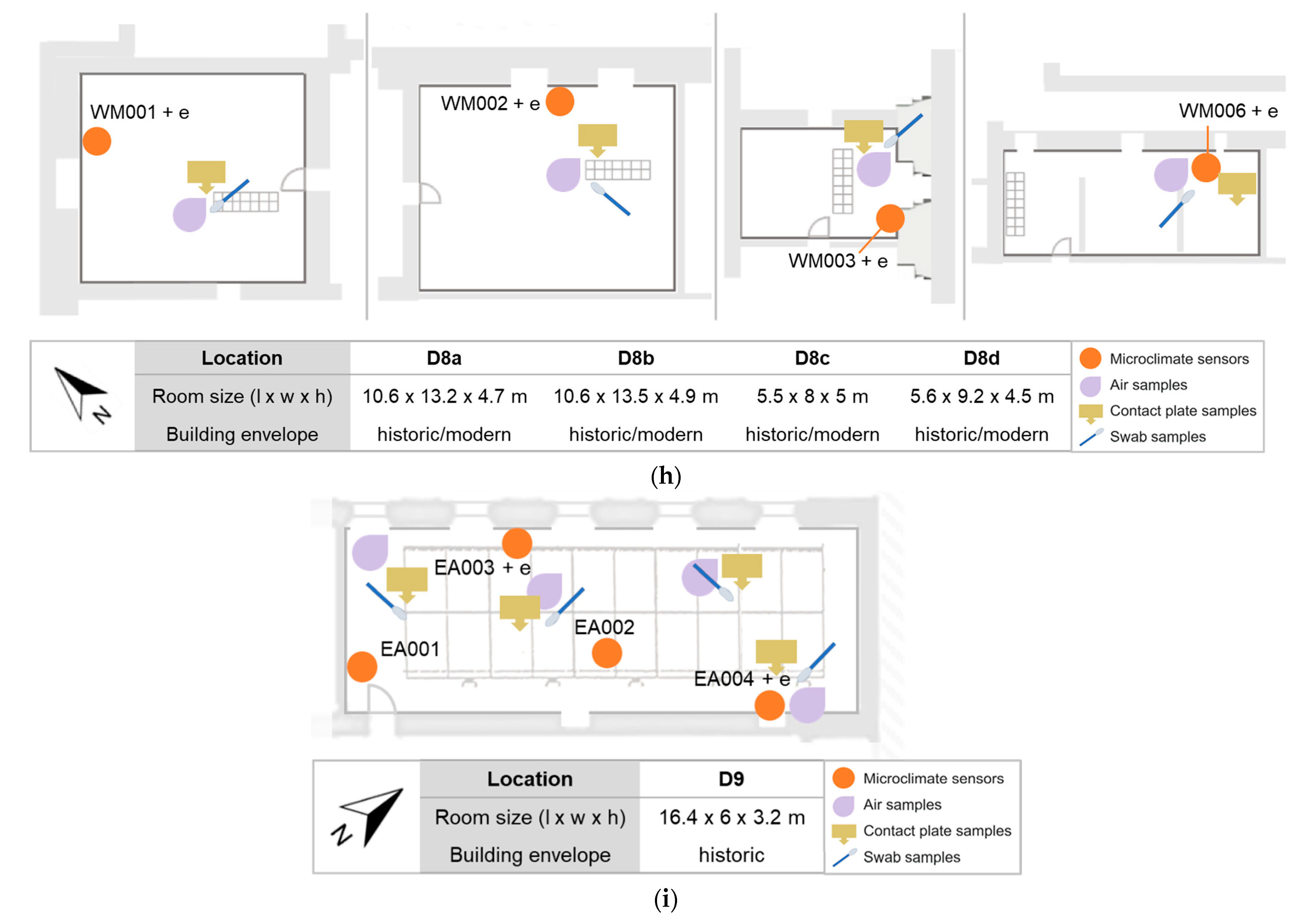
2.1. Description of Sites
2.2. Indoor and Outdoor Microclimate
2.3. Microbiological Profiles
2.3.1. Sampling
2.3.2. Cultivation Plate Analysis
2.3.3. Metagenomic (Metataxonomic) Analysis
2.4. Data Analysis and Statistics
3. Results
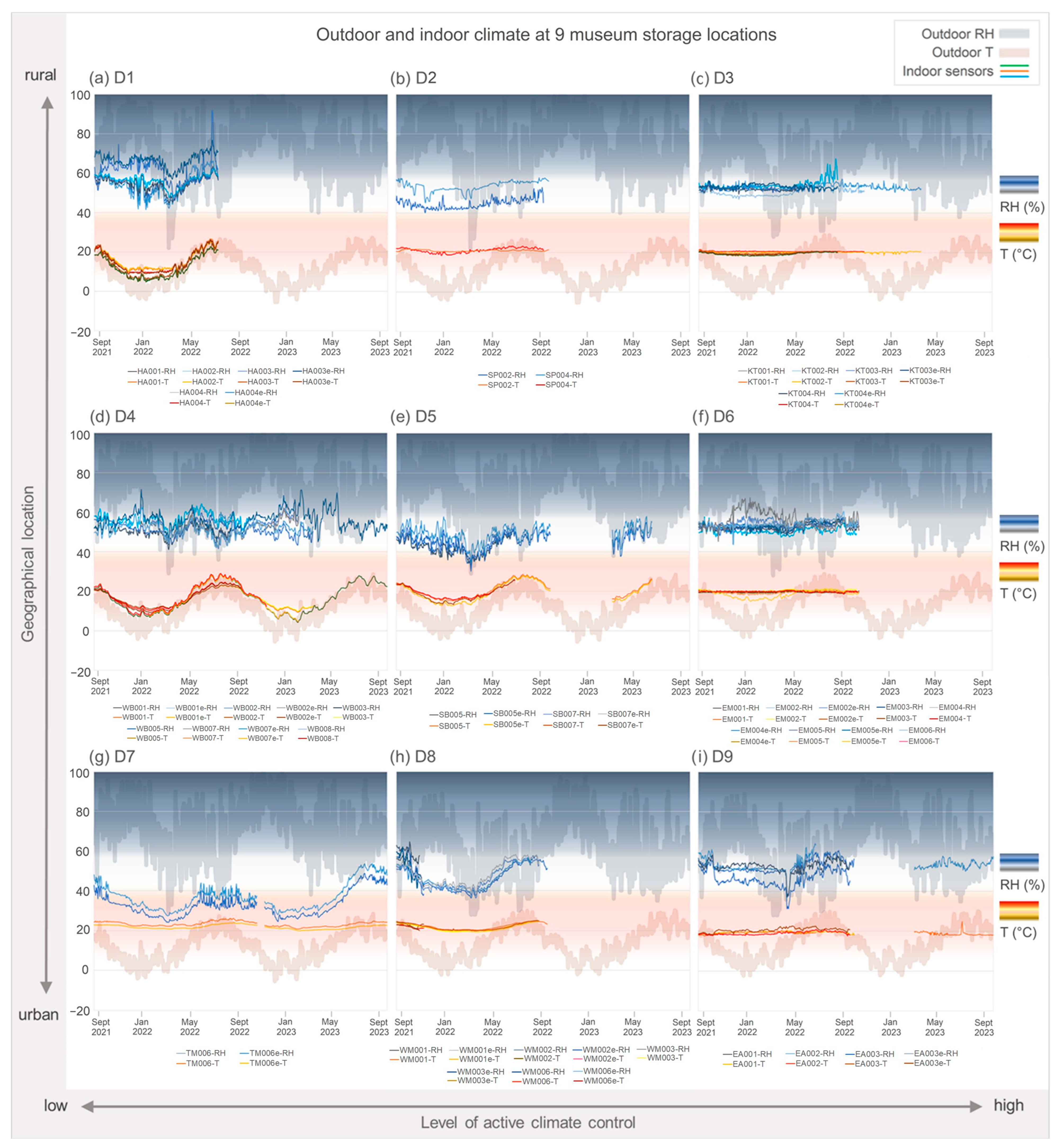
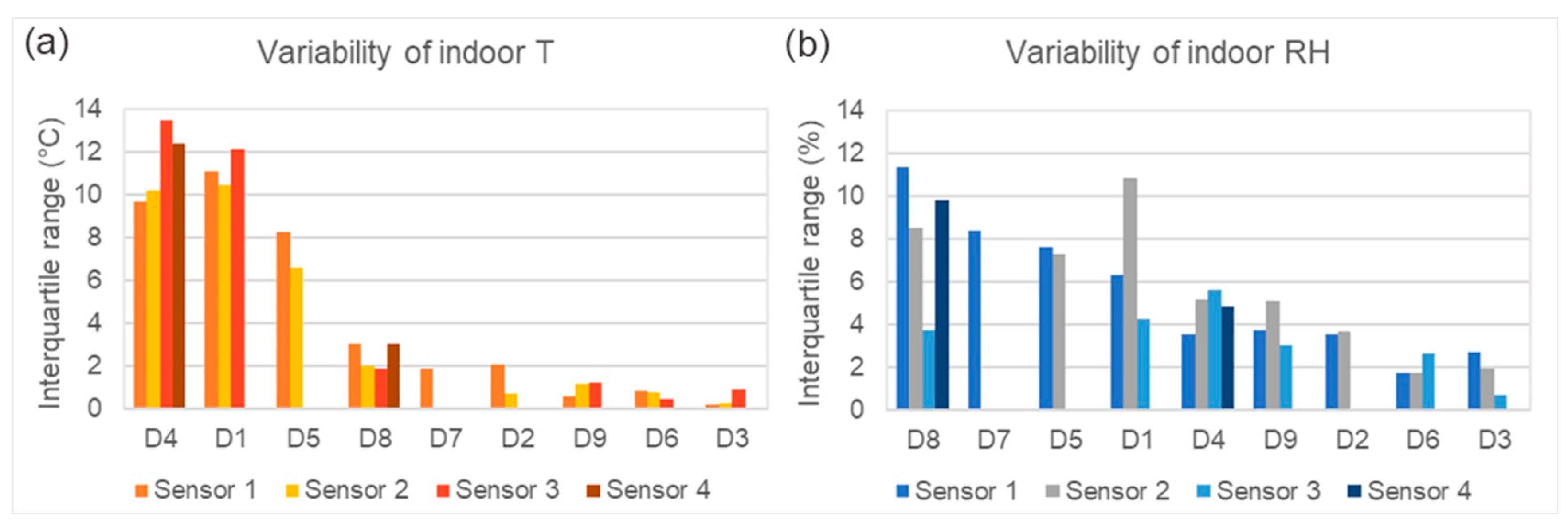
3.1. Climate Data—T and RH
3.2. Microbiological Data
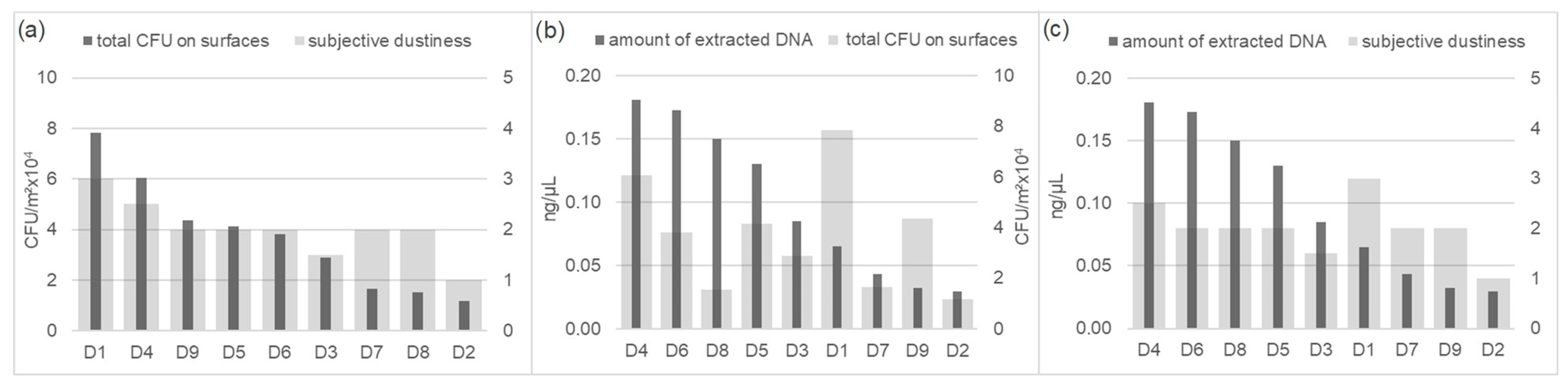
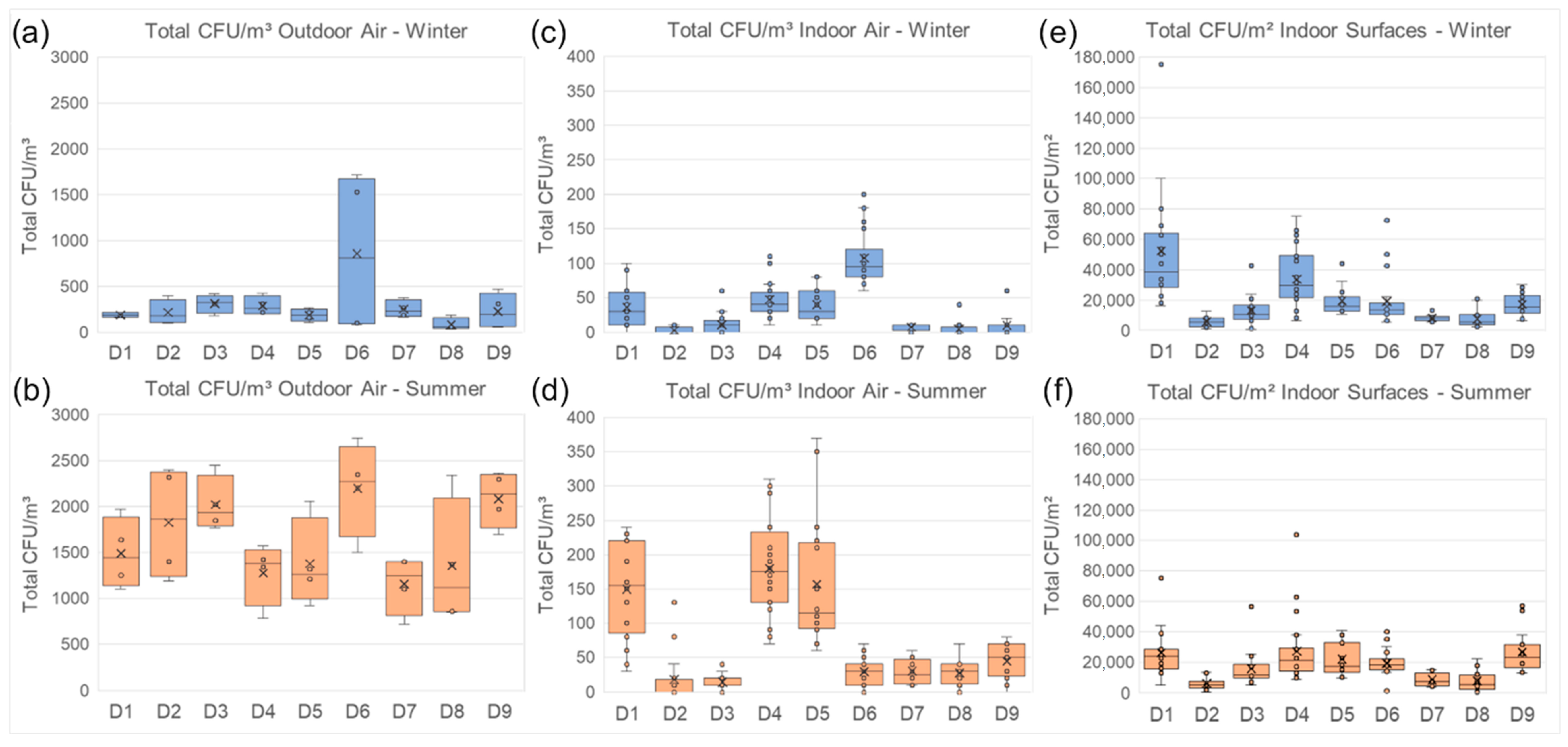
3.2.1. Cultivation Data
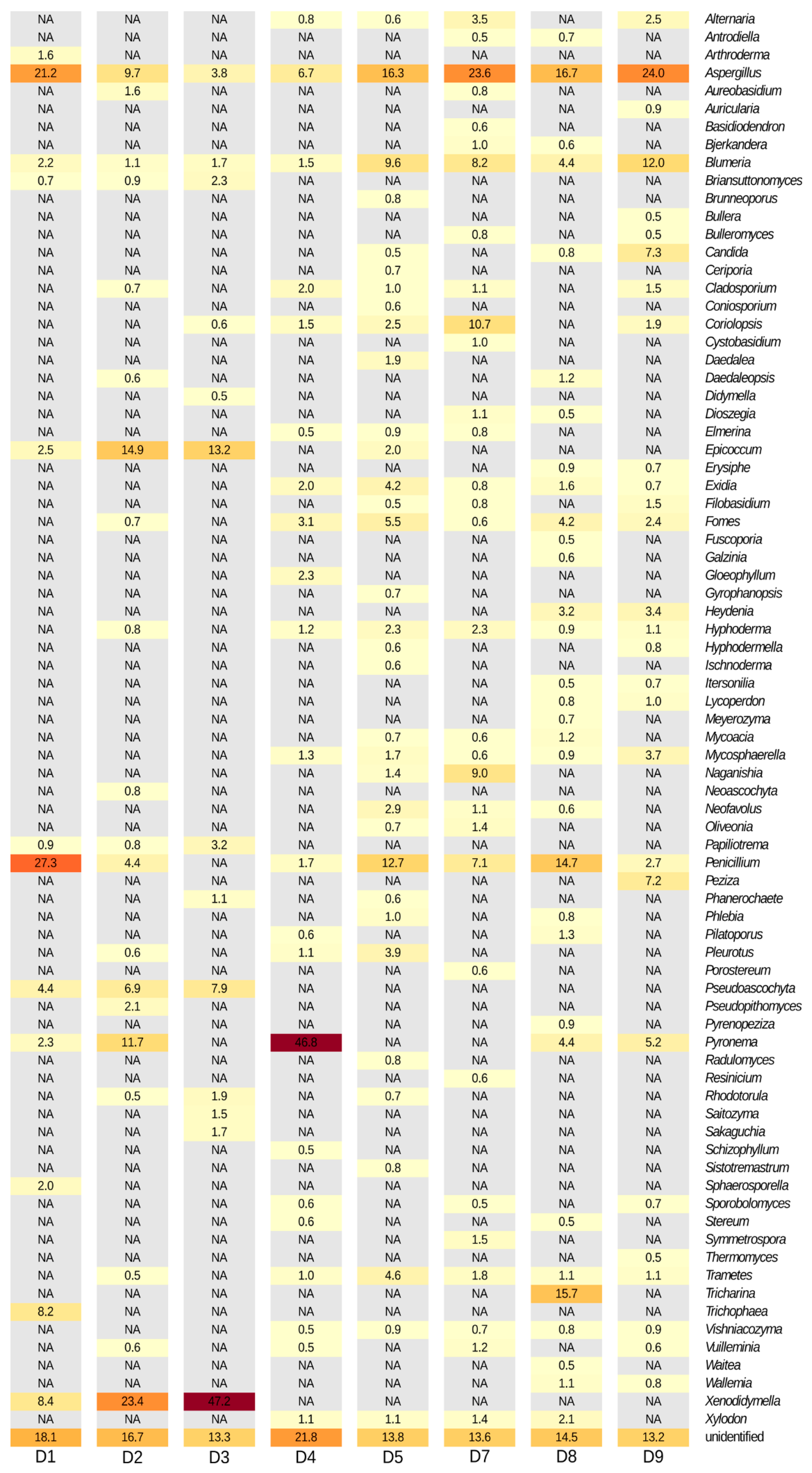
3.2.2. Metagenomic Data
4. Discussion
4.1. Microclimate
4.2. Fungal Profiles
4.3. Climate Variability and Microbial Diversity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Loddo, M. Depot versus museum: What is the future of art museum collections? In Metamorphosis. The Transformation of Dutch Museums; Roos, J., Hoebink, D., Eds.; TU Delft—Heritage & Architecture on Behalf of the Rondeltappe Bernoster Kemmers Foundation: Delft, The Netherlands, 2019. [Google Scholar]
- Holl, K.; Kilian, R.; Klemm, L.; Lengsfeld, K.; Bichlmair, S.; Krus, M. Sustainable Museum Storage Buildings for Long-term Preservation. Stud. Conserv. 2018, 63, S1, S366–S368. [Google Scholar] [CrossRef]
- Knudsen, L.R.; Lundbye, S.R. Performance of Danish low-energy museum storage buildings. In ICOM-CC 18th Triennial Conference Preprints, Copenhagen, Denmark, 4–8 September 2017; Bridgland, J., Ed.; ICOM Committee for Conservation: Paris, France, 2018. [Google Scholar]
- Padfield, T.; Borchersen, K. (Eds.) Museum Microclimates, Contributions to the Conference in Copenhagen 19–23 November 2007; The National Museum of Denmark: Copenhagen, Denmark, 2007. [Google Scholar]
- Ashley-Smith, J.; Burmester, A.; Eibl, M. Climate for Collections: Standards and Uncertainties. Postprints of the Munich Climate Conference 7 to 9 November 2012; Doerner Institute: Munich, Germany; Archetype Publications: London, UK, 2013. [Google Scholar]
- Camuffo, D. Microclimate for Cultural Heritage: Measurement, Risk Assessment, Conservation, Restoration, and Maintenance of Indoor and Outdoor Monuments; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Camuffo, D.; Bertolin, C.; Bonazzi, A.; Campana, F.; Merlo, C. Past, present and future effects of climate change on a wooden inlay bookcase cabinet: A new methodology inspired by the novel European Standard EN 1575:2010. J. Cult. Herit. 2014, 15, 26–35. [Google Scholar] [CrossRef]
- Gadd, G.; Fomina, M.; Pinzari, F. Fungal biodeterioration and preservation of cultural heritage, artwork, and historical artifacts: Extremophily and adaptation. Microbiol. Mol. Biol. Rev. 2024, 88, e0020022. [Google Scholar] [CrossRef] [PubMed]
- Sterflinger, K.; Pinzari, F. The revenge of time: Fungal deterioration of cultural heritage with particular reference to books, paper and parchment. Environ. Microbiol. 2012, 14, 559–566. [Google Scholar] [CrossRef]
- Bertalan, S.; Wood Lee, M.; Olcott Price, L. Mold/Fungi., Chap. 12. In Paper Conservation Catalog, 9th ed.; American Institute for Conservation Book and Paper Group: Washington, DC, USA, 1994; Available online: https://cool.culturalheritage.org/coolaic/sg/bpg/pcc/12_mold-fungi.pdf (accessed on 5 March 2025).
- Florian, M.-L.E. Fungal Facts: Solving Fungal Problems in Heritage Collections; Archetype Publications: London, UK, 2002; ISBN 1873132638. [Google Scholar]
- Gutarowska, B. (Ed.) A Modern Approach to Biodeterioration Assessment and the Disinfection of Historical Book Collections; Institute of Fermentation Technology and Microbiology Lodz University of Technology: Łódź, Poland, 2016. [Google Scholar]
- Bastholm, C.J.; Madsen, A.; Andersen, B.; Frisvad, J.; Richter, J. The mysterious mould outbreak—A comprehensive fungal colonisation in a climate-controlled museum repository challenges the environmental guidelines for heritage collections. J. Cult. Herit. 2022, 55, 78–87. [Google Scholar] [CrossRef]
- Bastholm, C.J.; Madsen, A.M.; Frisvad, J.C.; Richter, J. Xerophilic fungi in museum repositories challenge our perception of healthy buildings and the preservation of cultural heritage. In Healthy Buildings 2021—Europe, Proceedings of the 17th International Healthy Buildings Conference, Oslo, Norway, 21–23 June 2021; ISIAQ International Society of Indoor Air Quality: Oslo, Norway, 2021; pp. 260–268. [Google Scholar]
- Krus, M.; Kilian, R.; Sedlbauer, K. Mould growth prediction by computational simulation on historic buildings. In Museum Microclimates, Contributions to the Conference in Copenhagen 19–23 November 2007; Padfield, T., Borchersen, K., Eds.; National Museum of Denmark: Copenhagen, Denmark, 2007; ISBN 978-87-7602-080-4. [Google Scholar]
- Peleg, M. A New Look at Models of the Combined Effect of Temperature, pH, Water Activity, or Other Factors on Microbial Growth Rate. Food Eng. Rev. 2021, 14, 31–44. [Google Scholar] [CrossRef]
- Vereecken, E.; Saelens, D.; Roels, S. A comparison of different mould prediction models. In Proceedings of the Building Simulation 2011: 12th Conference of International Building Performance Simulation Association, Sydney, Australia, 14–16 November 2011; pp. 1934–1941. [Google Scholar]
- Anderlik, L. Entwicklung und Implementierung eines Algorithmus zur Vorhersage der Schimmelpilzbildung im Rahmen der Hausautomation. Master Thesis, Technische Universität München (TUM), Munich, Germany, 2017. [Google Scholar]
- IPCC. Sections 2–3. In Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report. of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 42–90. [Google Scholar]
- Leissner, J.; Kilian, R.; Kotova, L.; Jacob, D.; Mikolajewicz, U.; Broström, T.; Ashley-Smith, J.; Schellen, H.; Martens, M.; van Schijndel, J.; et al. Climate for Culture: Assessing the impact of climate change on the future indoor climate in historic buildings using simulations. Herit. Sci. 2015, 3, 38–52. [Google Scholar] [CrossRef]
- European Commission; Sabbioni, C.; Brimblecombe, P.; Cassar, M. Chapter 4.2 Climate Induced Decay of Outdoor Wooden Structures by Fungal Growth. In The Atlas of Climate Change Impact on European Cultural Heritage—Scientific Analysis and Management Strategies; Sabbioni, C., Brimblecombe, P., Cassar, M., Eds.; Anthem Press: London, UK, 2010; pp. 44–45. [Google Scholar]
- Lankester, P.; Brimblecombe, P. The Impact of Future Climate on Historic Interiors. Sci. Total Environ. 2012, 417–418, 248–254. [Google Scholar] [CrossRef]
- Damialis, A.; Mohammad, A.B.; Halley, J.M.; Gange, A.C. Fungi in a changing world: Growth rates will be elevated, but spore production may decrease in future climates. Int. J. Biometeorol. 2015, 59, 1157–1167. [Google Scholar] [CrossRef]
- Borrego, S.; Molina, A.; Santana, A. Mold on Stored Photographs and Maps: A Case Study. In Topics in Photographic Preservation; Photographic Materials Group (PMG) of the American Institute for Conservation (AIC): Washington, DC, USA, 2015; Volume 16, pp. 109–120. [Google Scholar]
- Borrego, S.; Molina, A.; Santana, A. Fungi in Archive Repositories Environments and the Deterioration of the Graphics Documents. EC Microbiol. 2017, 11, 205–226. [Google Scholar]
- De Carvalho, H.P.; Mesquita, N.; Trovão, J.; Rodríguez, S.F.; Pinheiro, A.C.; Gomes, V.; Alcoforado, A.; Gil, F.; Portugal, A. Fungal contamination of paintings and wooden sculptures inside the storage room of a museum: Are current norms and reference values adequate? J. Cult. Herit. 2018, 34, 268–276. [Google Scholar] [CrossRef]
- Bastholm, C.J.; Andersen, B.; Frisvad, J.C.; Oestergaard, S.K.; Nielsen, J.L.; Madsen, A.M.; Richter, J. A novel contaminant in museums? A cross-sectional study on xerophilic Aspergillus growth in climate-controlled repositories. Sci. Total Environ. 2024, 20, 173880. [Google Scholar] [CrossRef] [PubMed]
- Grabek-Lejko, D.; Tekiela, A.; Kasprzyk, I. Risk of biodeterioration of cultural heritage objects, stored in the historical and modern repositories in the Regional Museum in Rzeszow (Poland). A case study. Int. Biodeter. Biodegr. 2017, 123, 46–55. [Google Scholar] [CrossRef]
- Savković, Ž.; Stupar, M.; Unković, N.; Ivanović, Z.; Blagojević, J.; Popović, S.; Vukojević, J.; Ljaljević Grbić, M. Diversity and seasonal dynamics of culturable airborne fungi in a cultural heritage conservation facility. Int. Biodeter. Biodegr. 2021, 157, 105163. [Google Scholar] [CrossRef]
- Querner, P.; Sterflinger, K.; Derksen, K.; Leissner, J.; Landsberger, B.; Hammer, A.; Brimblecombe, P. Climate Change and Its Effects on Indoor Pests (Insect and Fungi) in Museums. Climate 2022, 10, 103. [Google Scholar] [CrossRef]
- Derksen, K.; Brimblecombe, P.; Piñar, G.; Waldherr, M.; Graf, A.; Haltrich, M.; Querner, P.; Sterflinger, K. Fungal Biodeterioration Risk in Monastic Libraries without Climate Control. Microorganisms 2024, 12, 1450. [Google Scholar] [CrossRef]
- Ellis, M.B. Dematiaceous Hyphomycetes; Commonwealth Mycological Institute: Kew Surrey, UK, 1971. [Google Scholar]
- Domsch, K.H.; Gams, W.; Anderson, T.-H. Compendium of Soil Fungi, 2nd ed.; IHW-Verlag: Eching, Germany, 2007; ISBN 978-3-930167-69-2. [Google Scholar]
- de Hoog, G.S.; Guarro, J. (Eds.) Atlas of Clinical Fungi; Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 1995. [Google Scholar]
- Klich, M.A. Identification of Common Aspergillus Species, 1st ed.; Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2002; ISBN 90-70351-46-3. [Google Scholar]
- Pitt, J.I. A Laboratory Guide to Common Penicillium Species, 3rd ed.; Food Science Australia: North Ryde, Australia, 2000; ISBN 0643048375. [Google Scholar]
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi, 2nd ed.; Westerdijk Laboratory Manual Series: Utrecht, The Netherlands, 2019; ISBN 978-94-91751-18-9. [Google Scholar]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. ISBN 9780123721808. [Google Scholar]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Fungal Barcoding Consortium. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- Neuhaus, E. A critical look at the use of HVAC systems in the museum environment. In Climate for Collections: Standards and Uncertainties. Postprints of the Munich Climate Conference 7 to 9 November 2012; Ashley-Smith, J., Burmester, A., Eibl, M., Eds.; Doerner Institut: Munich, Germany; Archetype Publications: London, UK, 2013. [Google Scholar]
- Padfield, T.; Jensen, L.A. Humidity buffering of building interiors by absorbent materials. In Proceedings of the 9th Nordic Symposium on Building Physics, Tampere, Finland, 29 May–2 June 2011; pp. 475–482. [Google Scholar]
- Andretta, M.; Coppola, F.; Seccia, L. Investigation on the interaction between the outdoor environment and the indoor microclimate of a historical library. J. Cult. Herit. 2015, 17, 75–86. [Google Scholar] [CrossRef]
- Kupczak, A.; Sadłowska-Sałęga, A.; Krzemień, L.; Sobczyk, J.; Radoń, J.; Kozłowski, R. Impact of paper and wooden collections on humidity stability and energy consumption in museums and libraries. Energy Build. 2018, 158, 77–85. [Google Scholar] [CrossRef]
- Davies, R.R. Viable Molds in House Dust. Trans. Brit. mycol. Soc. 1960, 43, 617–630. [Google Scholar] [CrossRef]
- Weikl, F.; Tischer, C.; Probst, A.J.; Heinrich, J.; Markevych, I.; Jochner, S.; Pritsch, K. Fungal and Bacterial Communities in Indoor Dust Follow Different Environmental Determinants. PLoS ONE 2016, 11, e0154131. [Google Scholar] [CrossRef] [PubMed]
- Karbowska-Berent, J.; Górny, R.; Strzelczyk, A.; Wlazło, A. Airborne and dust borne microorganisms in selected Polish libraries and archives. Build. Environ. 2011, 46, 1872–1879. [Google Scholar] [CrossRef]
- Ruga, L.; Orlandi, F.; Fornaciari, M. Viable and not viable spore concentrations in National Gallery of Umbria (Italy). Aerobiologia 2021, 37, 363–372. [Google Scholar] [CrossRef]
- Aguilera, F.; Valenzuela, L.; Montejo, E. Analysis of airborne fungal spore content in different indoor microenvironments in the Cathedral of Jaén (South Spain). Aerobiologia 2022, 38, 501–517. [Google Scholar] [CrossRef]
- Afshari, A.; Anderson, H.; Cohen, A.; De Oliveira Fernandes, E.; Douwes, J.; Górny, R.; Hirvonen, M.-R.; Jaakkola, J.; Kirchner, S.; Kurnitski, J.; et al. WHO Guidelines for Indoor Air Quality: Dampness and Mould; WHO Regional Office for Europe: Geneva, Switzerland, 2009; ISBN 7989289041683. [Google Scholar]
- Rao, C.Y.; Burge, H.A.; Chang, J.C. Review of quantitative standards and guidelines for fungi in indoor air. J. Air Waste Manag. Assoc. 1996, 46, 899–908. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Sequeira, S.O.; Macedo, M.F. Fungi in archives, libraries, and museums: A review on paper conservation and human health. Crit. Rev. Microbiol. 2019, 45, 686–700. [Google Scholar] [CrossRef]
- Lloyd, H.; Bendix, C.; Brimblecombe, P.; Thickett, D. Dust in historic libraries. In Museum Microclimates, Contributions to the conference in Copenhagen 19–23 November 2007; Padfield, T., Borchersen, K., Eds.; The National Museum of Denmark: Copenhagen, Denmark, 2007; pp. 135–144. [Google Scholar]
- Žilka, M.; Hrabovský, M.; Dušička, J.; Zahradníková, E.; Gahurová, D.; Ščevková, J. Comparative analysis of airborne fungal spore distribution in urban and rural environments of Slovakia. Environ. Sci. Pollut. Res. Int. 2024, 31, 63145–63160. [Google Scholar] [CrossRef]
- Talley, S.M.; Coley, P.D.; Kursar, T.A. The effects of weather on fungal abundance and richness among 25 communities in the Intermountain West. BMC Ecol. 2002, 2, 7. [Google Scholar] [CrossRef]
- Haas, D.; Ilieva, M.; Fritz, T.; Galler, H.; Habib, J.; Kriso, A.; Kropsch, M.; Ofner-Kopeinig, P.; Reinthaler, F.F.; Strasser, A.; et al. Background concentrations of airborne, culturable fungi and dust particles in urban, rural and mountain regions. Sci. Total Environ. 2023, 892, 164700. [Google Scholar] [CrossRef]
- Boddy, L.; Büntgen, U.; Egli, S.; Gange, A.C.; Heegaard, E.; Kirk, P.M.; Mohammad, A.; Kauserud, H. Climate variation effects on fungal fruiting. Fungal Ecol. 2013, 10, 20–33. [Google Scholar] [CrossRef]
- Kauserud, H.; Heegaard, E.; Semenov, M.A.; Boddy, L.; Halvorsen, R.; Stige, L.C.; Sparks, T.H.; Gange, A.C.; Stenseth, N.C. Climate change and spring-fruiting fungi. Proc. Biol. Sci. 2010, 277, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Diez, J.; Kauserud, H.; Andrew, C.; Heegaard, E.; Krisai-Greilhuber, I.; Senn-Irlet, B.; Høiland, K.; Egli, S.; Büntgen, U. Altitudinal upwards shifts in fungal fruiting in the Alps. Proc. Biol. Sci. 2020, 287, 20192348. [Google Scholar] [CrossRef]
- Baldrian, P.; Bell–Dereske, L.; Lepinay, C.; Větrovský, T.; Kohout, P. Fungal communities in soils under global change. Stud. Mycol. 2022, 103, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Bidartondo, M.I.; Ellis, C.; Kauserud, H.; Kennedy, P.G.; Lilleskov, E.A.; Suz, L.M.; Andrew, C. Climate change: Fungal responses and effects. In State of the World’s Fungi. Report; Willis, K.J., Ed.; Royal Botanic Gardens: Kew, UK, 2018; pp. 62–69. [Google Scholar]
- Dijksterhuis, J. Fungal spores: Highly variable and stress-resistant vehicles for distribution and spoilage. Food Microbiol. 2018, 81, 2–11. [Google Scholar] [CrossRef]
- Ulevicius, V.; Peciulyte, D.; Lugauskas, A.; Andriejauskienė, J. Field study on changes in viability of airborne fungal propagules exposed to UV radiation. Environ. Toxicol. 2004, 19, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Segers, F.J.J.; van Laarhoven, K.A.; Huinink, H.P.; Adan, O.C.G.; Wösten, H.A.B.; Dijksterhuis, J. The indoor fungus Cladosporium halotolerans survives humidity dynamics markedly better than Aspergillus niger and Penicillium rubens despite less growth at lowered steady-state water activity. Appl. Environ. Microbiol. 2016, 82, 5089–5098. [Google Scholar] [CrossRef]
- Sterflinger, K.; Voitl, C.; Lopandic, K.; Piñar, G.; Tafer, H. Big sound and extreme fungi—Xerophilic, halotolerant Aspergilli and Penicillia with low optimal temperature as invaders of historic pipe organs. Life 2018, 8, 22. [Google Scholar] [CrossRef]
- Micheluz, A.; Manente, S.; Tigini, V.; Prigione, V.P.; Pinzari, F.; Ravagnan, G.; Varese, G.C. The extreme environment of a library: Xerophilic fungi inhabiting indoor niches. Int. Biodeter. Biodegr. 2015, 99, 1–7. [Google Scholar] [CrossRef]
- Zalar, P.; Graf Hriberšek, D.; Gostinčar, C.; Breskvar, M.; Džeroski, S.; Matul, M.; Novak Babič, M.; Čremožnik Zupančič, J.; Kujović, A.; Gunde-Cimerman, N.; et al. Xerophilic fungi contaminating historically valuable easel paintings from Slovenia. Front. Microbiol. 2023, 14, 1258670. [Google Scholar] [CrossRef]
- Sabatini, L.; Sisti, M.; Campana, R. Evaluation of fungal community involved in the bioderioration process of wooden artworks and canvases in Montefeltro area (Marche, Italy). Microbiol. Res. 2018, 207, 203–210. [Google Scholar] [CrossRef]
- Polo, A.; Cappitelli, F.; Villa, F.; Pinzari, F. Biological invasion in the indoor environment: The spread of Eurotium halophilicum on library materials. Int. Biodeter. Biodegr. 2017, 118, 34–44. [Google Scholar] [CrossRef]
- Montanari, M.; Melloni, V.; Pinzari, F.; Innocenti, G. Fungal biodeterioration of historical library materials stored in Compactus movable shelves. Int. Biodeter. Biodegr. 2012, 75, 83–88. [Google Scholar] [CrossRef]
- Văcar, C.L.; Mircea, C.; Pârvu, M.; Podar, D. Diversity and Metabolic Activity of Fungi Causing Biodeterioration of Canvas Paintings. J. Fungi 2022, 8, 589. [Google Scholar] [CrossRef] [PubMed]
- Borrego, S.; Guiamet, P.; Gómez de Saravia, S.; Batistini, P.; Garcia, M.; Lavin, P.; Perdomo, I. The quality of air at archives and the biodeterioration of photographs. Int. Biodeter. Biodegr. 2010, 64, 139–145. [Google Scholar] [CrossRef]
- Borrego, S.; Lavin, P.; Perdomo, I.; Gómez de Saravia, S.; Guiamet, P. Determination of indoor air quality in archives and biodeterioration of the documentary heritage. Int. Sch. Res. Not. 2012, 2012, 680598. [Google Scholar] [CrossRef]
- Poyatos, F.; Morales, F.; Nicholson, A.W.; Giordano, A. Physiology of biodeterioration on canvas paintings. J. Cell. Physiol. 2018, 233, 2741–2751. [Google Scholar] [CrossRef]

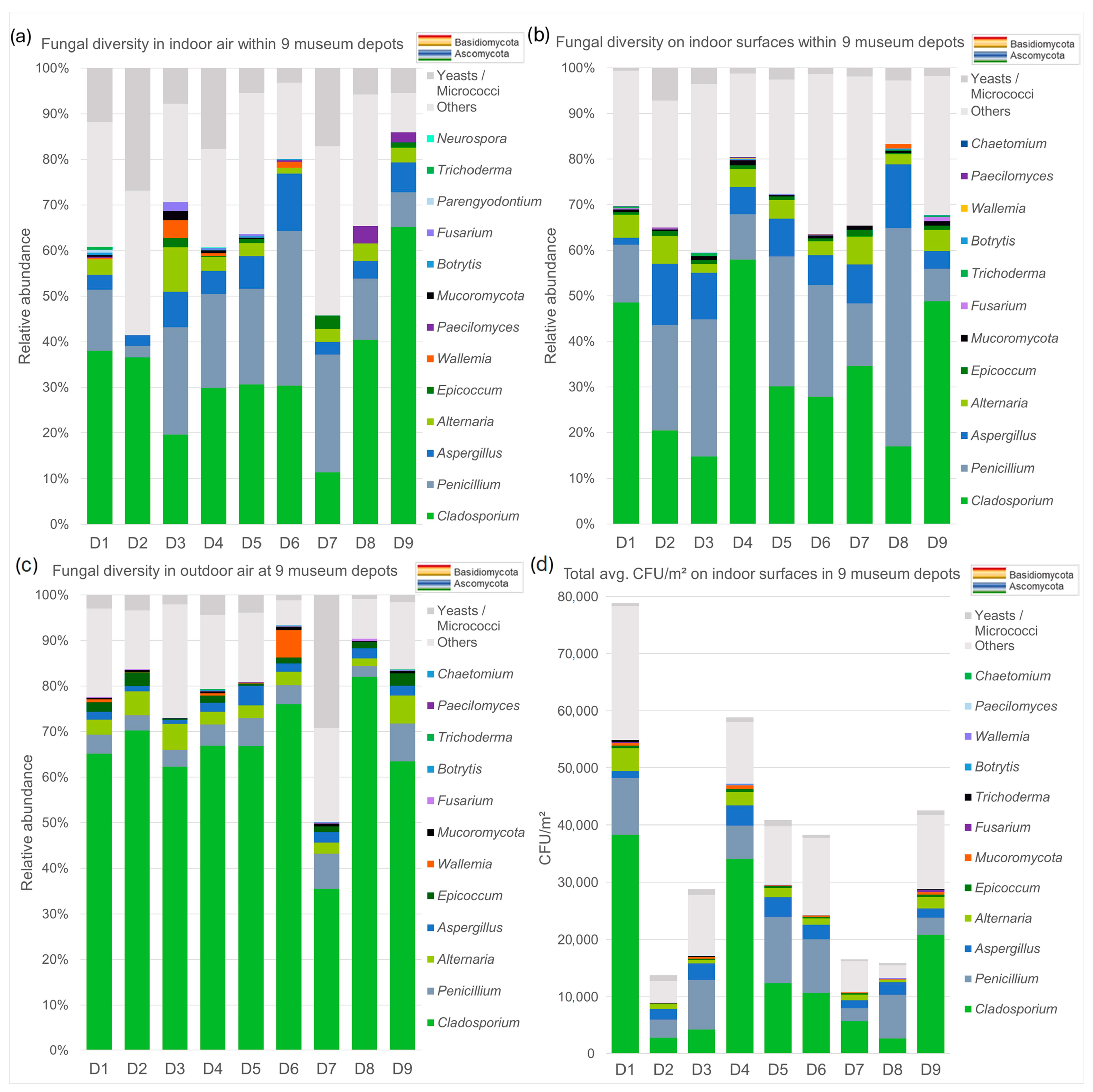
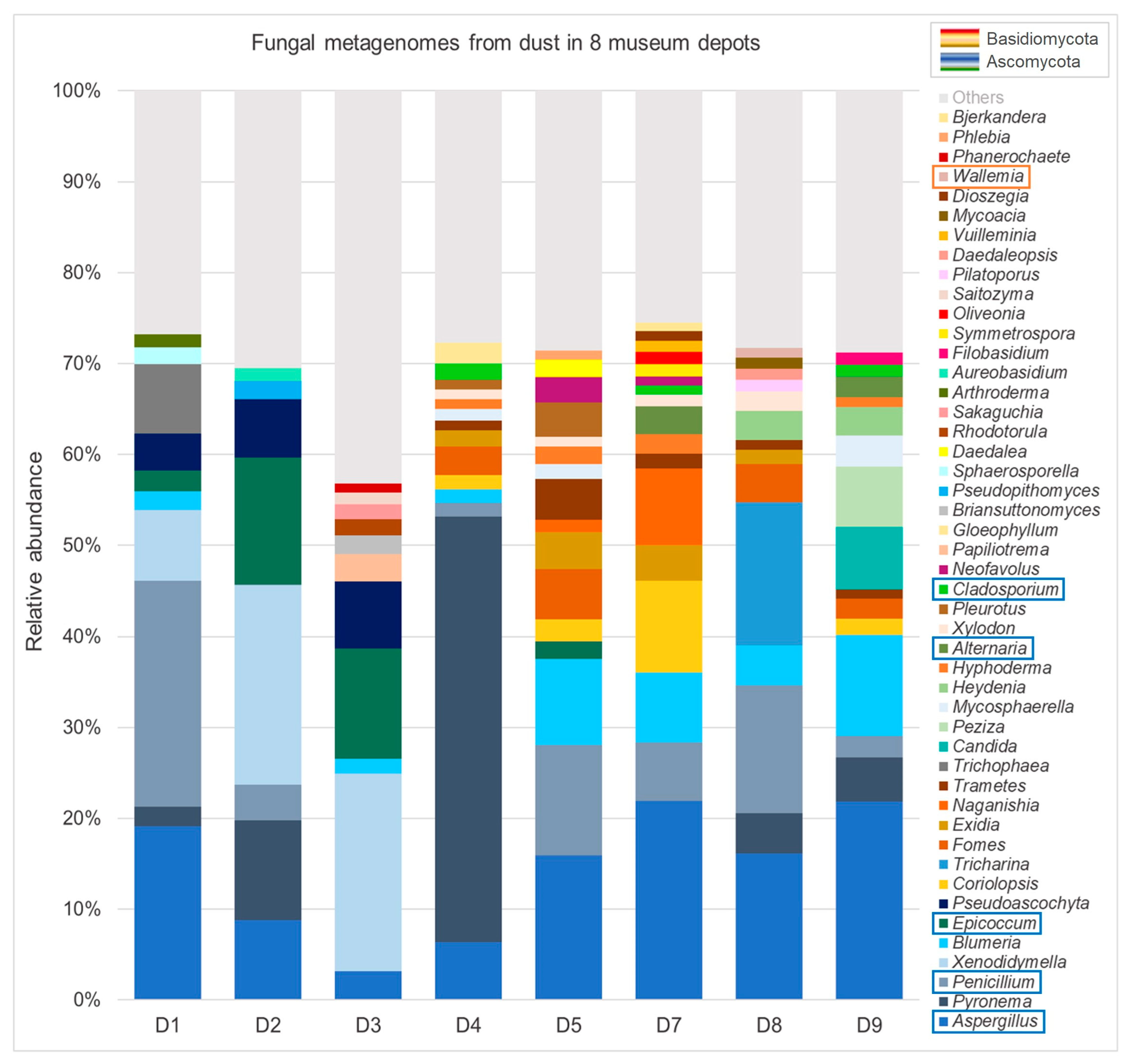

| Location | D1 | D2 | D3 | D4 | D5 | D6 | D7 | D8 | D9 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Indoor ACC | none | HVAC | HVAC | none/(D) | none/(H) | HVAC | none/(D) | HVAC | HVAC | |
| Average T (°C) | Indoor | 15.3 ± 4.6 | 21.1 ± 0.7 | 20.1 ± 0.3 | 18.0 ± 4.7 | 20.0 ± 3.2 | 19.9 ± 0.3 | 23.7 ± 1.0 | 22.1 ± 1.2 | 19.3 ± 0.5 |
| Outdoor | 11.6 ± 7.0 | 11.6 ± 7.0 | 12.5 ± 7.1 | 11.6 ± 6.8 | 11.6 ± 6.8 | 12.7 ± 7.0 | 11.6 ± 6.8 | 13.8 ± 7.1 | 13.8 ± 7.1 | |
| Average RH (%) | Indoor | 54.9 ± 3.5 | 48.9 ± 2.0 | 52.4 ± 1.2 | 52.1 ± 2.5 | 43.2 ± 3.5 | 52.7 ± 1.3 | 31.5 ± 3.5 | 50.9 ± 4.3 | 52.7 ± 2.6 |
| Outdoor | 70.0 ± 12.9 | 70.0 ± 12.9 | 62.7 ± 12.8 | 67.8 ± 12.4 | 67.8 ± 12.4 | 62.6 ± 12.4 | 67.8 ± 12.4 | 57.5 ± 11.7 | 57.5 ± 11.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derksen, K.; Brimblecombe, P.; Piñar, G.; Waldherr, M.; Graf, A.B.; Querner, P.; Sterflinger, K. Monitoring of Fungal Diversity and Microclimate in Nine Different Museum Depots. J. Fungi 2025, 11, 478. https://doi.org/10.3390/jof11070478
Derksen K, Brimblecombe P, Piñar G, Waldherr M, Graf AB, Querner P, Sterflinger K. Monitoring of Fungal Diversity and Microclimate in Nine Different Museum Depots. Journal of Fungi. 2025; 11(7):478. https://doi.org/10.3390/jof11070478
Chicago/Turabian StyleDerksen, Katharina, Peter Brimblecombe, Guadalupe Piñar, Monika Waldherr, Alexandra Bettina Graf, Pascal Querner, and Katja Sterflinger. 2025. "Monitoring of Fungal Diversity and Microclimate in Nine Different Museum Depots" Journal of Fungi 11, no. 7: 478. https://doi.org/10.3390/jof11070478
APA StyleDerksen, K., Brimblecombe, P., Piñar, G., Waldherr, M., Graf, A. B., Querner, P., & Sterflinger, K. (2025). Monitoring of Fungal Diversity and Microclimate in Nine Different Museum Depots. Journal of Fungi, 11(7), 478. https://doi.org/10.3390/jof11070478







