Optimized Combinations of Filtrates of Trichoderma spp., Metarhizium spp., and Bacillus spp. in the Biocontrol of Rice Pests and Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains, Insects, and Plants
2.2. Single-Factor Optimization Experiment for Culture Medium Components
2.3. Plackett–Burman Design
2.4. Central Composite Design
2.5. Identifying Microbial Culture Filtrate Combinations
2.6. Determining the Characteristic Products of TMB
2.7. Observation of the Ultrastructure of C. suppressalis Larvae
2.8. RNA-Seq of C. suppressalis Larvae Treated with TMB
2.9. RNA-Seq of M. oryzae Treated with TMB
2.10. qRT-PCR Verification
2.11. Determination of the Growth Rate of M. oryzae
2.12. Determination of the Gene Expression Related to Defense Response in Rice
2.13. Determination of the Enzyme Activity Related to Defense Response in Rice
2.14. Statistical Analysis
3. Results
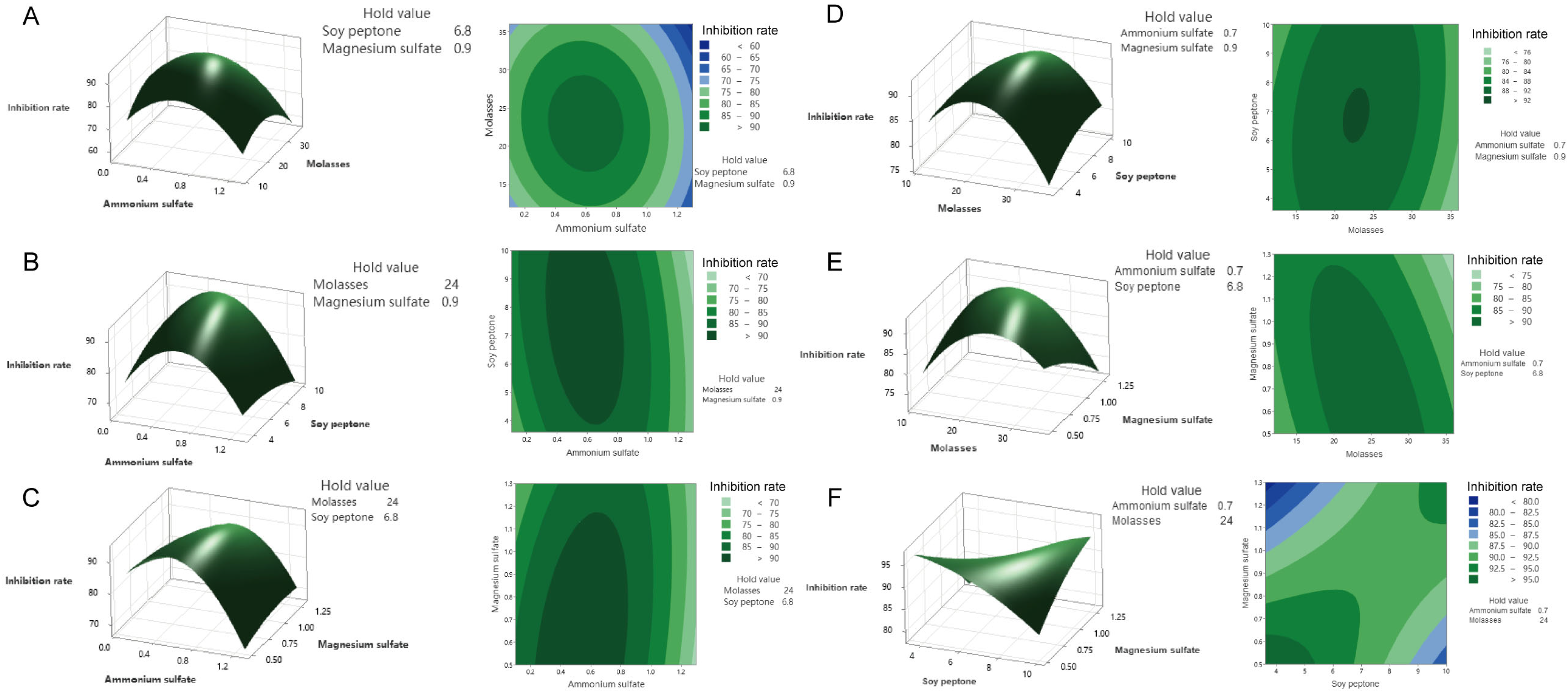
3.1. Determination of the Optimal Culture Medium to Produce a Culture Filtrate with Highly Inhibitory Effects on Pathogens and Insect Larvae
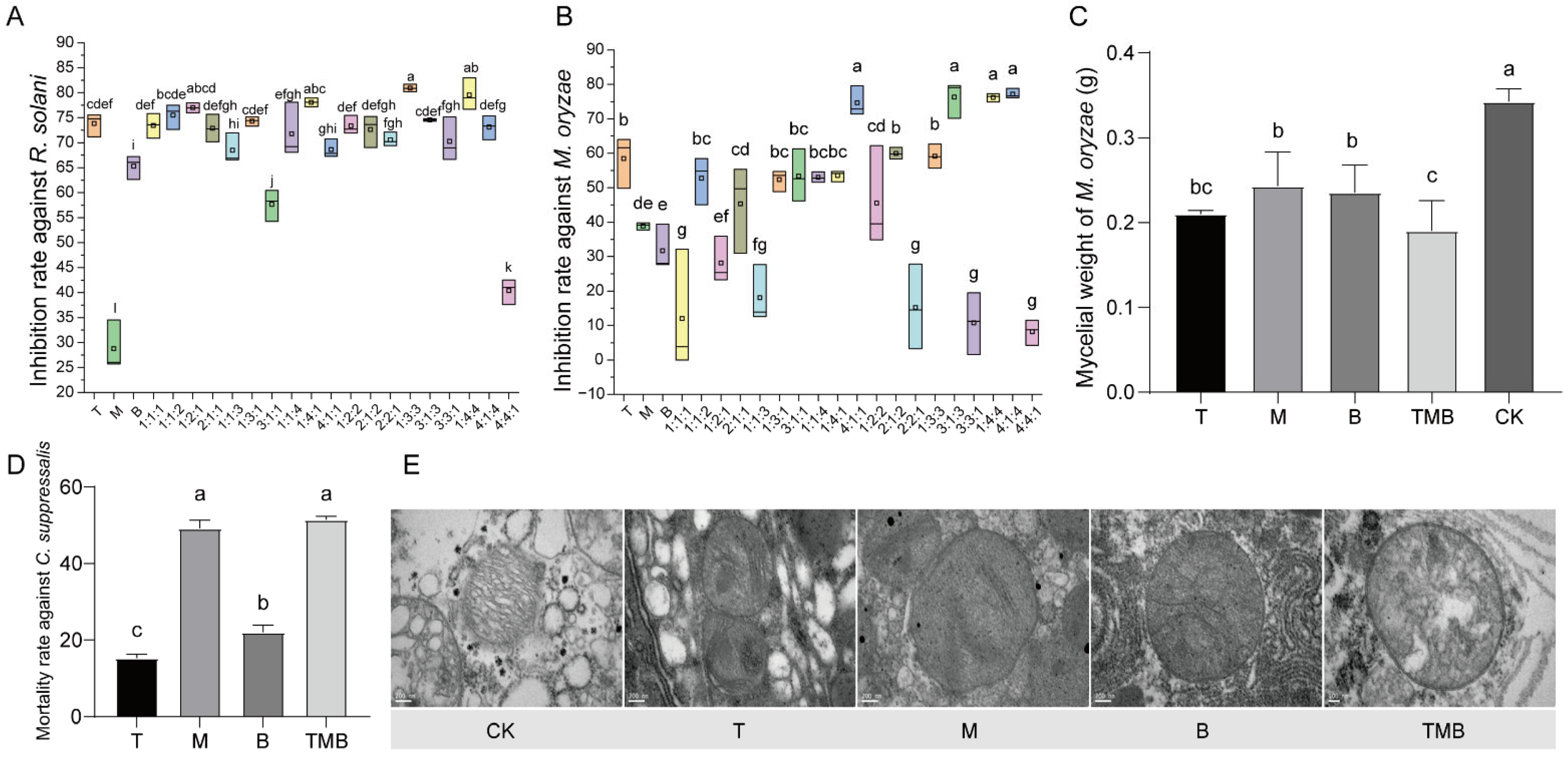
3.2. Determination of Biomarkers for Culture Filtrates
3.3. Antifungal and Insecticidal Effects of TMB
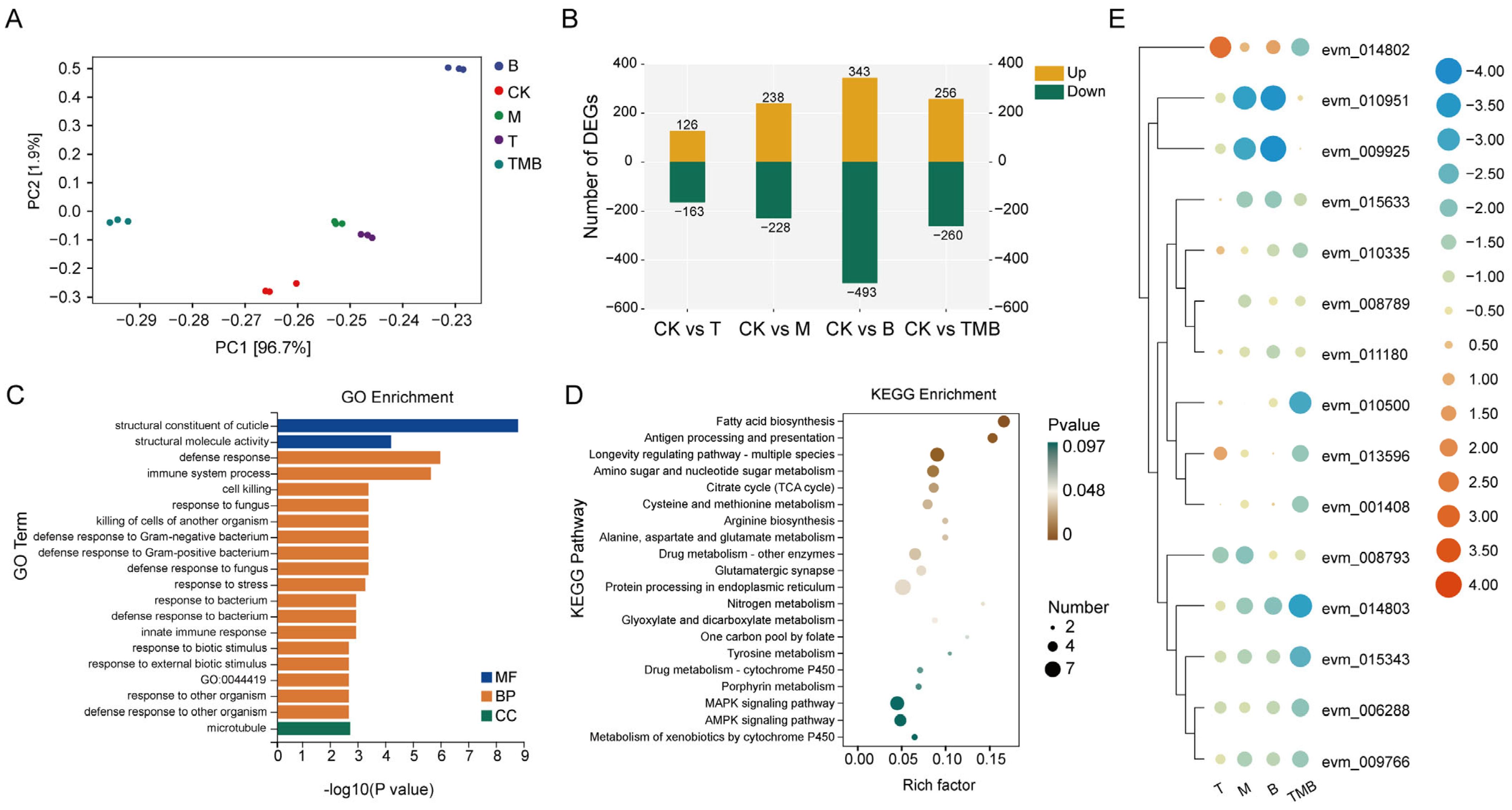
3.4. RNA-Seq Analysis of C. suppressalis Larvae After TMB Treatment
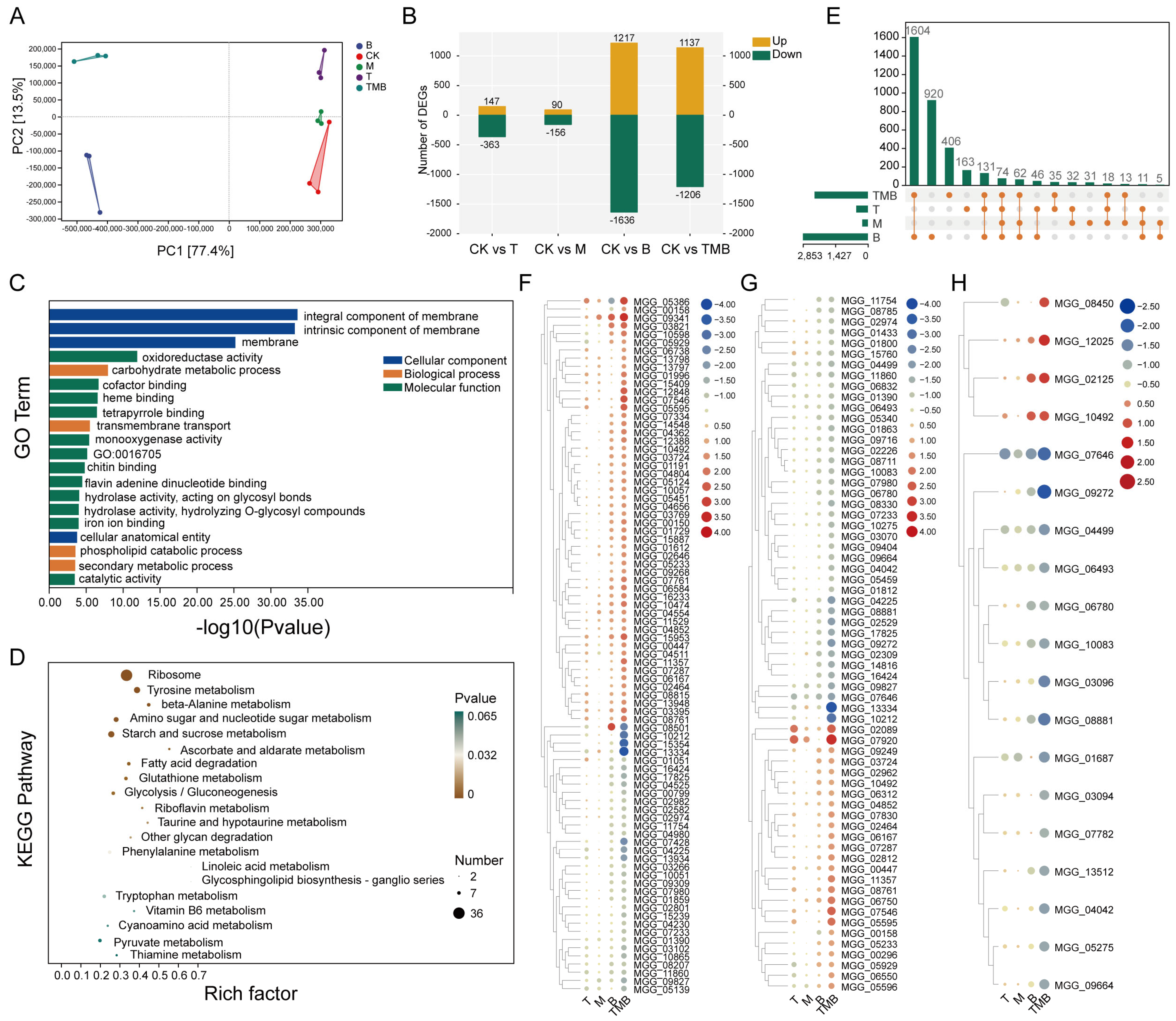
3.5. RNA-Seq Analysis of M. oryzae Treated with TMB
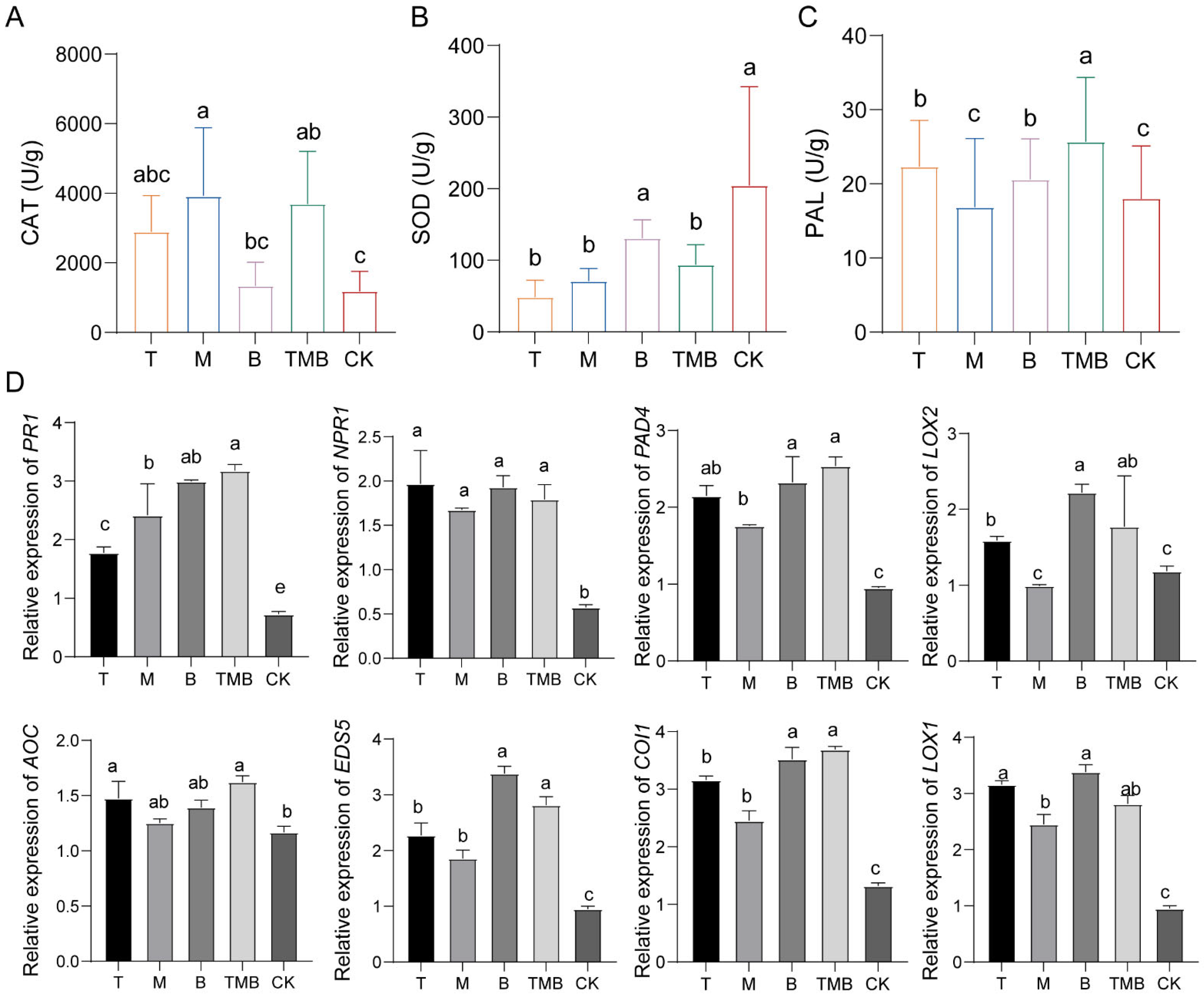
3.6. Effects of TMB on Rice Defense Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.P.; Li, Y.; Chen, F.; Wu, H.; Zhang, S. Characterization and expression of fungal defensin in Escherichia coli and its antifungal mechanism by RNA-seq analysis. Front. Microbiol. 2023, 14, 1172257. [Google Scholar] [CrossRef]
- Han, S.; Wang, Y.; Li, Y.; Zhu, R.; Gu, Y.; Li, J.; Guo, H.; Ye, W.; Nabi, H.G.; Yang, T.; et al. The OsNAC41-RoLe1-OsAGAP module promotes root development and drought resistance in upland rice. Mol. Plant 2024, 17, 1573–1593. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Tang, B.; Ryder, L.S.; MacLean, D.; Were, V.M.; Eseola, A.B.; Cruz-Mireles, N.; Ma, W.; Foster, A.J.; Oses-Ruiz, M.; et al. The transcriptional landscape of plant infection by the rice blast fungus Magnaporthe oryzae reveals distinct families of temporally co-regulated and structurally conserved effectors. Plant Cell 2023, 35, 1360–1385. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Li, S.; Wei, S.H.; Sun, W.X. Strategies to Manage Rice Sheath Blight: Lessons from Interactions between Rice and Rhizoctonia solani. Rice 2021, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, X.; Xu, H.; Liu, Y.; Lu, Z. Effects of Host Plant and Insect Generation on Shaping of the Gut Microbiota in the Rice Leaffolder, Cnaphalocrocis medinalis. Front. Microbiol. 2022, 13, 824224. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Han, K.; Guo, M.; Zou, Y.; Zhang, W.; Ma, W.; Hua, H. Cloning and functional identification of a Chilo suppressalis-inducible promoter of rice gene, OsHPL2. Pest Manag. Sci. 2020, 76, 3177–3187. [Google Scholar] [CrossRef]
- Ndayambaje, B.; Amuguni, H.; Coffin-Schmitt, J.; Sibo, N.; Ntawubizi, M.; VanWormer, E. Pesticide Application Practices and Knowledge among Small-Scale Local Rice Growers and Communities in Rwanda: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2019, 16, 4770. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, G.; Zhang, C.; Zhou, W.; Xu, X.; Shou, Q.; Yuan, F.; Li, Q.; Huang, H.; Hu, J.; et al. Pesticide exposure and forage shortage in rice cropping system prevents honey bee colony establishment. Environ. Res. 2023, 219, 115097. [Google Scholar] [CrossRef]
- Yin, Y.; Miao, J.; Shao, W.; Liu, X.; Zhao, Y.; Ma, Z. Fungicide Resistance: Progress in Understanding Mechanism, Monitoring, and Management. Phytopathology 2023, 113, 707–718. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Adeleke, B.S.; Akinola, S.A.; Fayose, C.A.; Adeyemi, U.T.; Gbadegesin, L.A.; Omole, R.K.; Johnson, R.M.; Uthman, Q.O.; Babalola, O.O. Biopesticides as a promising alternative to synthetic pesticides: A case for microbial pesticides, phytopesticides, and nanobiopesticides. Front. Microbiol. 2023, 14, 1040901. [Google Scholar] [CrossRef]
- de Sousa, T.P.; Chaibub, A.A.; Cortes, M.; Batista, T.F.C.; Bezerra, G.A.; da Silva, G.B.; de Filippi, M.C.C. Molecular identification of Trichoderma sp. isolates and biochemical characterization of antagonistic interaction against rice blast. Arch. Microbiol. 2021, 203, 3257–3268. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, M.; Zibaee, A.; Khodaparast, S.A.; Fazeli-Dinan, M. Screening and Virulence of the Entomopathogenic Fungi Associated with Chilo suppressalis Walker. J. Fungi 2021, 7, 34. [Google Scholar] [CrossRef]
- Thepbandit, W.; Srisuwan, A.; Siriwong, S.; Nawong, S.; Athinuwat, D. Bacillus vallismortis TU-Orga21 blocks rice blast through both direct effect and stimulation of plant defense. Front. Plant Sci. 2023, 14, 1103487. [Google Scholar] [CrossRef]
- Velmurugan, S.; Ashajyothi, M.; Charishma, K.; Kumar, S.; Balamurugan, A.; Javed, M.; Karwa, S.; Prakash, G.; Subramanian, S.; Gogoi, R.; et al. Enhancing defense against rice blast disease: Unveiling the role of leaf endophytic firmicutes in antifungal antibiosis and induced systemic resistance. Microb. Pathog. 2023, 184, 106326. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Leng, J.; Yu, L.; Bai, H.; Li, X.; Wisniewski, M.; Liu, J.; Sui, Y. Efficacy of the biocontrol agent Trichoderma hamatum against Lasiodiplodia theobromae on macadamia. Front. Microbiol. 2022, 13, 994422. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Lu, K.; Chen, R.; Jiang, J. Bacillus subtilis KLBMPGC81 suppresses appressorium-mediated plant infection by altering the cell wall integrity signaling pathway and multiple cell biological processes in Magnaporthe oryzae. Front. Cell. Infect. Microbiol. 2022, 12, 983757. [Google Scholar] [CrossRef] [PubMed]
- Kgosi, V.T.; Tingting, B.; Ying, Z.; Liu, H. Anti-Fungal Analysis of Bacillus subtilis DL76 on Conidiation, Appressorium Formation, Growth, Multiple Stress Response, and Pathogenicity in Magnaporthe oryzae. Int. J. Mol. Sci. 2022, 23, 5314. [Google Scholar] [CrossRef]
- Mazzola, M.; Freilich, S. Prospects for Biological Soilborne Disease Control: Application of Indigenous Versus Synthetic Microbiomes. Phytopathology 2017, 107, 256–263. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, T.; He, Y. Biological characteristics and fungicide sensitivity of Pyricularia variabilis. Open Life Sci. 2021, 16, 950–960. [Google Scholar] [CrossRef]
- Zhang, X.F.; Li, Q.Y.; Wang, M.; Ma, S.Q.; Zheng, Y.F.; Li, Y.Q.; Zhao, D.L.; Zhang, C.S. 2E,4E-Decadienoic Acid, a Novel Anti-Oomycete Agent from Coculture of Bacillus subtilis and Trichoderma asperellum. Microbiol. Spectr. 2022, 10, e0154222. [Google Scholar]
- Karuppiah, V.; Sun, J.; Li, T.; Vallikkannu, M.; Chen, J. Co-cultivation of Trichoderma asperellum GDFS1009 and Bacillus amyloliquefaciens 1841 Causes Differential Gene Expression and Improvement in the Wheat Growth and Biocontrol Activity. Front. Microbiol. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Han, X.S.; Shen, D.X.; Xiong, Q.; Bao, B.H.; Zhang, W.B.; Dai, T.T.; Zhao, Y.J.; Borriss, R.; Fan, B. The Plant-Beneficial Rhizobacterium FZB42 Controls the Soybean Pathogen Due to Bacilysin Production. Appl. Environ. Microbiol. 2021, 87, e01601-21. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lu, M.X.; Cui, Y.D.; Du, Y.Z. Selection and Evaluation of Reference Genes for Expression Analysis Using qRT-PCR in Chilo suppressalis (Lepidoptera: Pyralidae). J. Econ. Entomol. 2017, 110, 683–691. [Google Scholar]
- Shi, H.B.; Chen, N.; Zhu, X.M.; Liang, S.; Li, L.; Wang, J.Y.; Lu, J.P.; Lin, F.C.; Liu, X.H. F-box proteins MoFwd1, MoCdc4 and MoFbx15 regulate development and pathogenicity in the rice blast fungus Magnaporthe oryzae. Environ. Microbiol. 2019, 21, 3027–3045. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, X.; Tian, X.; Zhang, Z.; Wu, W.; Shang, J.; Ouyang, J.; Yao, W.; Li, S. Glycine- and Proline-Rich Protein OsGPRP3 Regulates Grain Size and Quality in Rice. J. Agric. Food Chem. 2020, 68, 7581–7590. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Borah, S.M.; Borah, P.K.; Bora, P.; Sarmah, B.K.; Lal, M.K.; Tiwari, R.K.; Kumar, R. Deciphering the antimicrobial activity of multifaceted rhizospheric biocontrol agents of solanaceous crops viz., Trichoderma harzianum MC2, and Trichoderma harzianum NBG. Front. Plant Sci. 2023, 14, 1141506. [Google Scholar] [CrossRef]
- Islam, M.R.; Chowdhury, R.; Roy, A.S.; Islam, M.N.; Mita, M.M.; Bashar, S.; Saha, P.; Rahat, R.A.; Hasan, M.; Akter, M.A.; et al. Native Trichoderma Induced the Defense-Related Enzymes and Genes in Rice against Xanthomonas oryzae pv. oryzae (Xoo). Plants 2023, 12, 1864. [Google Scholar] [CrossRef]
- Grijalba, E.P.; Espinel, C.; Cuartas, P.E.; Chaparro, M.L.; Villamizar, L.F. Metarhizium rileyi biopesticide to control Spodoptera frugiperda: Stability and insecticidal activity under glasshouse conditions. Fungal Biol. 2018, 122, 1069–1076. [Google Scholar] [CrossRef]
- Sarven, M.S.; Hao, Q.; Deng, J.; Yang, F.; Wang, G.; Xiao, Y.; Xiao, X. Biological Control of Tomato Gray Mold Caused by Botrytis Cinerea with the Entomopathogenic Fungus Metarhizium anisopliae. Pathogens 2020, 9, 213. [Google Scholar] [CrossRef]
- Gupta, R.; Keppanan, R.; Leibman-Markus, M.; Rav-David, D.; Elad, Y.; Ment, D.; Bar, M. The Entomopathogenic Fungi Metarhizium brunneum and Beauveria bassiana Promote Systemic Immunity and Confer Resistance to a Broad Range of Pests and Pathogens in Tomato. Phytopathology 2022, 112, 784–793. [Google Scholar] [CrossRef]
- Won, S.J.; Moon, J.H.; Ajuna, H.B.; Choi, S.I.; Maung, C.E.H.; Lee, S.; Ahn, Y.S. Biological Control of Leaf Blight Disease Caused by Pestalotiopsis maculans and Growth Promotion of Quercus acutissima Carruth Container Seedlings Using Bacillus velezensis CE 100. Int. J. Mol. Sci. 2021, 22, 11296. [Google Scholar] [CrossRef] [PubMed]
- Sree, K.S.; Padmaja, V. Oxidative stress induced by destruxin from Metarhizium anisopliae (Metch.) involves changes in glutathione and ascorbate metabolism and instigates ultrastructural changes in the salivary glands of Spodoptera litura (Fab.) larva. Toxicon 2008, 51, 1140–1150. [Google Scholar] [CrossRef]
- Dumas, C.; Matha, V.; Quiot, J.M.; Vey, A. Effects of destruxins, cyclic depsipeptide mycotoxins, on calcium balance and phosphorylation of intracellular proteins in lepidopteran cell lines. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1996, 114, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Lavine, M.D.; Strand, M.R. Insect hemocytes and their role in immunity. Insect Biochem. Mol. Biol. 2002, 32, 1295–1309. [Google Scholar] [CrossRef]
- Wu, S.F.; Sun, F.D.; Qi, Y.X.; Yao, Y.; Fang, Q.; Huang, J.; Stanley, D.; Ye, G.Y. Parasitization by Cotesia chilonis influences gene expression in fatbody and hemocytes of Chilo suppressalis. PLoS ONE 2013, 8, e74309. [Google Scholar] [CrossRef]
- Shahriari, M.; Zibaee, A.; Khodaparast, S.A.; Fazeli-Dinan, M.; Hoda, H.; Armand, A. Immunological interactions of Chilo suppressalis Walker (Lepidoptera: Crambidae) with the native entomopathogenic fungi. Microb. Pathog. 2021, 154, 104858. [Google Scholar] [CrossRef] [PubMed]
- Park, S.R.; Jung, D.Y.; Kim, T.W.; Lee, C.H.; Jung, J.Y. Growth arrest and DNA-damage-inducible 45 beta (GADD45beta) deletion suppresses testosterone-induced prostate hyperplasia in mice. Life Sci. 2018, 211, 74–80. [Google Scholar] [CrossRef]
- Peretz, G.; Bakhrat, A.; Abdu, U. Expression of the GADD45 homolog (CG11086) affects egg asymmetric development that is mediated by the c-Jun N-terminal kinase pathway. Genetics 2007, 177, 1691–1702. [Google Scholar] [CrossRef]
- Muellner, J.; Schmidt, K.H. Yeast Genome Maintenance by the Multifunctional PIF1 DNA Helicase Family. Genes 2020, 11, 224. [Google Scholar] [CrossRef]
- Kraft, B.D.; Pavlisko, E.N.; Roggli, V.L.; Piantadosi, C.A.; Suliman, H.B. Alveolar Mitochondrial Quality Control During Acute Respiratory Distress Syndrome. Lab. Investig. 2023, 103, 100197. [Google Scholar] [CrossRef]
- Silva-Beltran, N.P.; Boon, S.A.; Ijaz, M.K.; Mckinney, J.; Gerba, C.P. Antifungal activity and mechanism of action of natural product derivates as potential environmental disinfectants. J. Ind. Microbiol. Biotechnol. 2023, 50, kuad036. [Google Scholar] [CrossRef]
- Luo, N.; Jin, L.; Yang, C.; Zhu, Y.; Ye, X.; Li, X.; Zhang, B. Antifungal activity and potential mechanism of magnoflorine against Trichophyton rubrum. J. Antibiot. 2021, 74, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Gupta, H.; Tripathi, S.; Poluri, K.M. Biochemical and metabolomic insights into antifungal mechanism of berberine against Candida glabrata. Appl. Microbiol. Biotechnol. 2023, 107, 6085–6102. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Long, Y.; Yin, X.; Wang, W.; Zhang, R.; Mo, F.; Zhang, Z.; Chen, T.; Chen, J.; Wang, B.; et al. Antifungal activity and mechanism of tetramycin against Alternaria alternata, the soft rot causing fungi in kiwifruit. Pestic. Biochem. Physiol. 2023, 192, 105409. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Genbauffe, F.S.; Cooper, T.G. Structure and transcription of the allantoate permease gene (DAL5) from Saccharomyces cerevisiae. J. Bacteriol. 1988, 170, 266–271. [Google Scholar] [CrossRef]
- Aouida, M.; Texeira, M.R.; Thevelein, J.M.; Poulin, R.; Ramotar, D. Agp2, a Member of the Yeast Amino Acid Permease Family, Positively Regulates Polyamine Transport at the Transcriptional Level. PLoS ONE 2013, 8, e65717. [Google Scholar] [CrossRef]
- Orrapin, S.; Roytrakul, S.; Phaonakrop, N.; Thaisakun, S.; Tragoolpua, K.; Intorasoot, A.; McGill, S.; Burchmore, R.; Intorasoot, S. Fungicidal Activity of Recombinant Javanicin against Cryptococcus neoformans Is Associated with Intracellular Target(s) Involved in Carbohydrate and Energy Metabolic Processes. Molecules 2021, 26, 7011. [Google Scholar] [CrossRef]
- Zhang, P.P.; Ma, S.T. Recent development of leucyl-tRNA synthetase inhibitors as antimicrobial agents. Medchemcomm 2019, 10, 1329–1341. [Google Scholar] [CrossRef]
- Wang, N.; Liu, M.; Guo, L.; Yang, X.; Qiu, D. A Novel Protein Elicitor (PeBA1) from Bacillus amyloliquefaciens NC6 Induces Systemic Resistance in Tobacco. Int. J. Biol. Sci. 2016, 12, 757–767. [Google Scholar] [CrossRef]
- Yu, C.; Dou, K.; Wang, S.; Wu, Q.; Ni, M.; Zhang, T.; Lu, Z.; Tang, J.; Chen, J. Elicitor hydrophobin Hyd1 interacts with Ubiquilin1-like to induce maize systemic resistance. J. Integr. Plant Biol. 2020, 62, 509–526. [Google Scholar] [CrossRef]
- Shadel, G.S.; Horvath, T.L. Mitochondrial ROS signaling in organismal homeostasis. Cell 2015, 163, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Tsuda, K. Salicylic acid and jasmonic acid crosstalk in plant immunity. Essays Biochem. 2022, 66, 647–656. [Google Scholar] [PubMed]
- Joglekar, S.; Suliman, M.; Bartsch, M.; Halder, V.; Maintz, J.; Bautor, J.; Zeier, J.; Parker, J.E.; Kombrink, E. Chemical Activation of EDS1/PAD4 Signaling Leading to Pathogen Resistance in Arabidopsis. Plant Cell Physiol. 2018, 59, 1592–1607. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Chen, L.; Bai, Z.; Li, Y.; Chen, J. Optimized Combinations of Filtrates of Trichoderma spp., Metarhizium spp., and Bacillus spp. in the Biocontrol of Rice Pests and Diseases. J. Fungi 2025, 11, 471. https://doi.org/10.3390/jof11070471
Zhang X, Chen L, Bai Z, Li Y, Chen J. Optimized Combinations of Filtrates of Trichoderma spp., Metarhizium spp., and Bacillus spp. in the Biocontrol of Rice Pests and Diseases. Journal of Fungi. 2025; 11(7):471. https://doi.org/10.3390/jof11070471
Chicago/Turabian StyleZhang, Xifen, Lusheng Chen, Zhenxu Bai, Yaqian Li, and Jie Chen. 2025. "Optimized Combinations of Filtrates of Trichoderma spp., Metarhizium spp., and Bacillus spp. in the Biocontrol of Rice Pests and Diseases" Journal of Fungi 11, no. 7: 471. https://doi.org/10.3390/jof11070471
APA StyleZhang, X., Chen, L., Bai, Z., Li, Y., & Chen, J. (2025). Optimized Combinations of Filtrates of Trichoderma spp., Metarhizium spp., and Bacillus spp. in the Biocontrol of Rice Pests and Diseases. Journal of Fungi, 11(7), 471. https://doi.org/10.3390/jof11070471







