1. Introduction
Invasive aspergillosis (IA) is a life-threatening opportunistic fungal infection that predominantly affects immunocompromised individuals. However, emerging evidence indicates that IA can also develop in patients with only mild or localized immune dysfunction, such as those with diabetes mellitus, chronic lung disease, or advanced age [
1,
2]. Although the lungs are the most common site of involvement, extrapulmonary manifestations—including those affecting the paranasal sinuses, orbital apex, and intracranial compartments—are increasingly recognized, albeit rare [
3].
The orbital apex is a complex anatomical region containing the optic canal and superior orbital fissure, where cranial nerves and vascular structures converge. Infections in this area may lead to orbital apex syndrome, characterized by ophthalmoplegia, ptosis, and visual disturbances. Fungal infections in this region, especially those caused by Aspergillus species, pose a diagnostic challenge due to their insidious onset, subtle radiological findings, and nonspecific clinical manifestations.
In immunocompetent patients, the disease may progress more slowly and mimic non-infectious inflammatory disorders such as sarcoidosis, granulomatosis with polyangiitis, or tuberculous granulomas [
4,
5]. Imaging findings such as low signal intensity on T2-weighted MRI or bone erosion on CT may offer early diagnostic clues, but these are not pathognomonic. Moreover, the decision to perform a biopsy in this anatomically delicate region is often deferred, especially when inflammatory markers are unremarkable, resulting in delayed diagnosis.
The risk of administering corticosteroids or other immunosuppressive treatments before confirming the infectious etiology can lead to rapid deterioration, particularly if fungal pathogens are present. Prompt identification and initiation of antifungal therapy are essential, yet often delayed due to the deceptive clinical picture. Recent reviews have emphasized the importance of including fungal infections in the differential diagnosis of orbital apex lesions, even in patients without overt immunosuppression [
6,
7].
This report presents a rare case of invasive orbital apex aspergillosis with contiguous intracranial extension in an immunocompetent patient. The case underscores the importance of maintaining a high index of suspicion for fungal infections even in patients without classic risk factors, and highlights the critical role of early biopsy and targeted antifungal therapy in optimizing patient outcomes.
2. Case Presentation
The patient was a 50-year-old Japanese woman with well-controlled type 2 diabetes mellitus (HbA1c 5.8%) and no history of immunosuppressive therapy. She was a non-smoker and worked as an office administrator. Three months prior to presentation, she began experiencing mild retro-orbital pain, fatigue, and intermittent low-grade fever. At that time, her symptoms were attributed to nonspecific viral illness. However, she gradually developed right-sided ptosis and complained of diplopia on lateral gaze, prompting ophthalmologic referral.
Initial neurological examination revealed partial right oculomotor nerve palsy with preserved pupillary light reflex and no afferent pupillary defect. Visual acuity was 1.0 (20/20) bilaterally. Fundoscopic examination was unremarkable, with no signs of papilledema or retinal changes. Laboratory findings were within normal limits, including white blood cell count (6800/μL), C-reactive protein (0.2 mg/dL), and erythrocyte sedimentation rate (12 mm/h). Autoimmune and infectious panels, including ANA, ACE, quantiferon, and HIV, were negative.
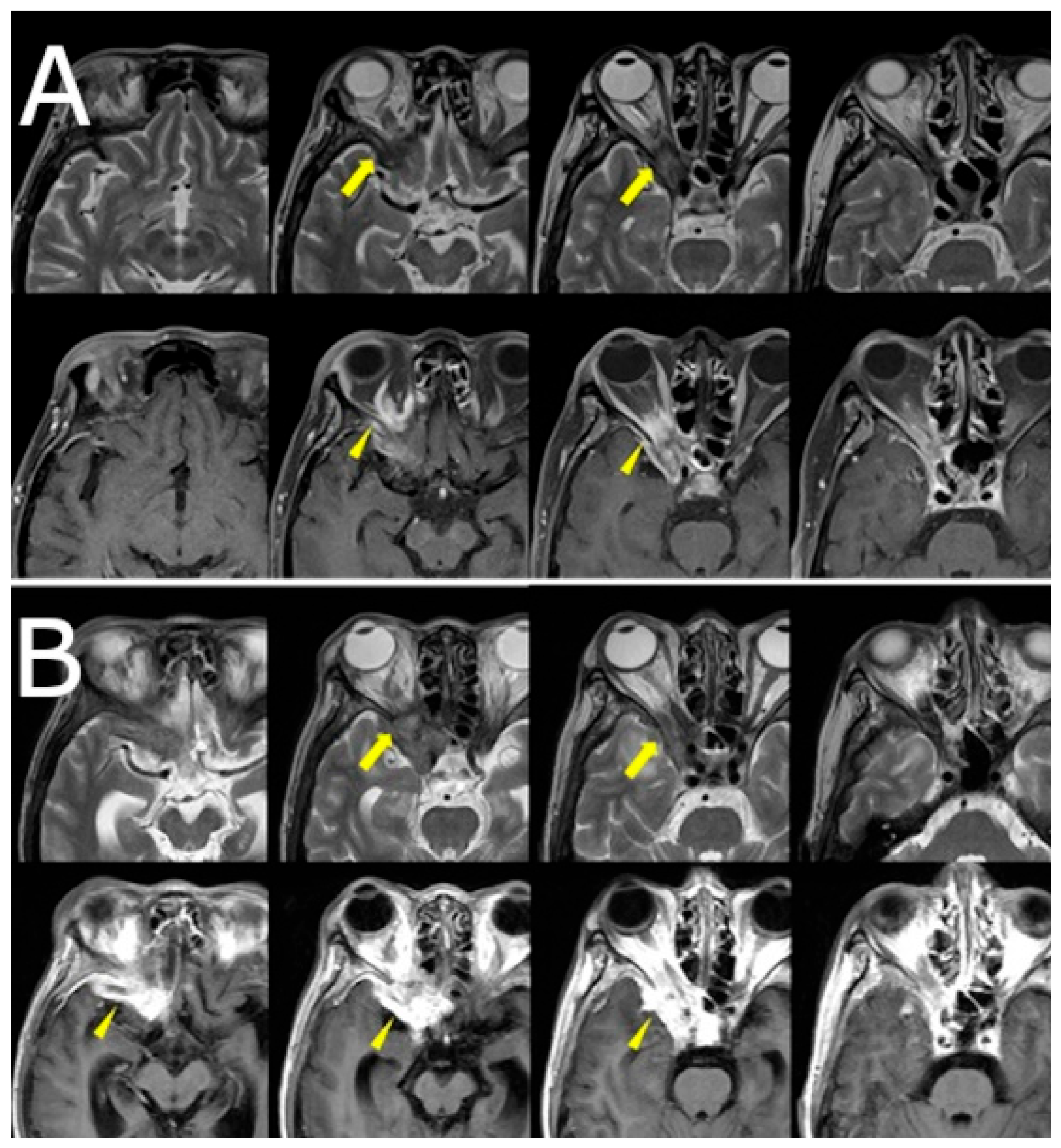
Orbital and brain MRI demonstrated a faint low-signal lesion in the right orbital apex on T2-weighted images, with mild gadolinium enhancement on fat-suppressed T1-weighted images. No surrounding edema or sinus involvement was noted. The lesion was interpreted by the neuroradiologist as likely granulomatous in nature, with a differential that included sarcoidosis and idiopathic orbital inflammation. Given the lesion’s deep location and absence of systemic findings, biopsy was deferred, and clinical observation was initiated (
Figure 1A).
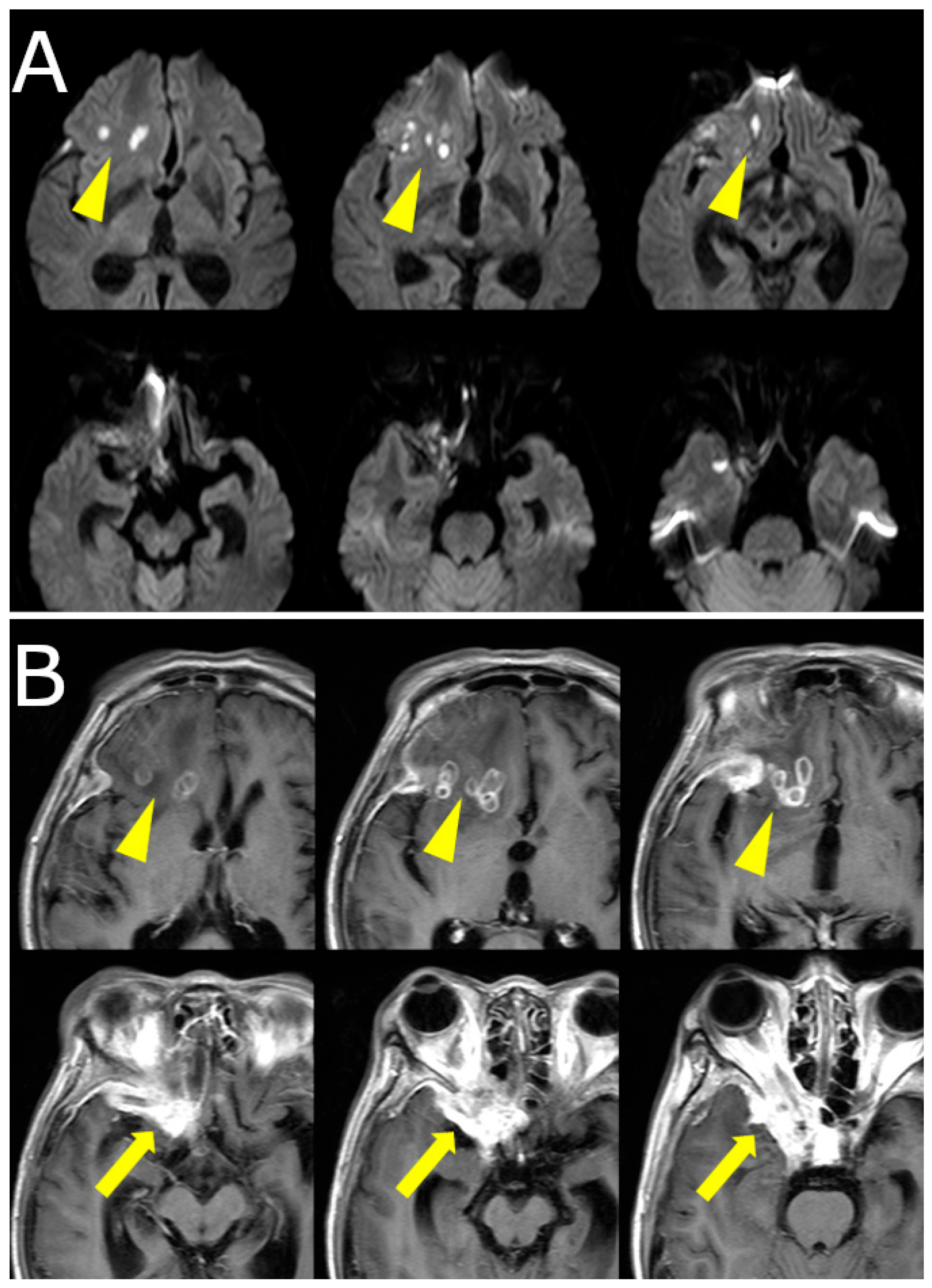
Over the following weeks, her symptoms progressed. She reported increasing difficulty in eye movement, blurred vision in the right eye, and worsening retro-orbital pain. Visual acuity in the right eye decreased to 0.3 (20/60), while the left eye remained unaffected. New findings included proptosis and chemosis without conjunctival injection. Repeat MRI revealed lesion progression into the right cavernous sinus and frontal lobe, with peripheral ring enhancement and central diffusion restriction, suggesting abscess formation. Edema in the right frontal lobe was also observed (
Figure 1B,
Figure 2).
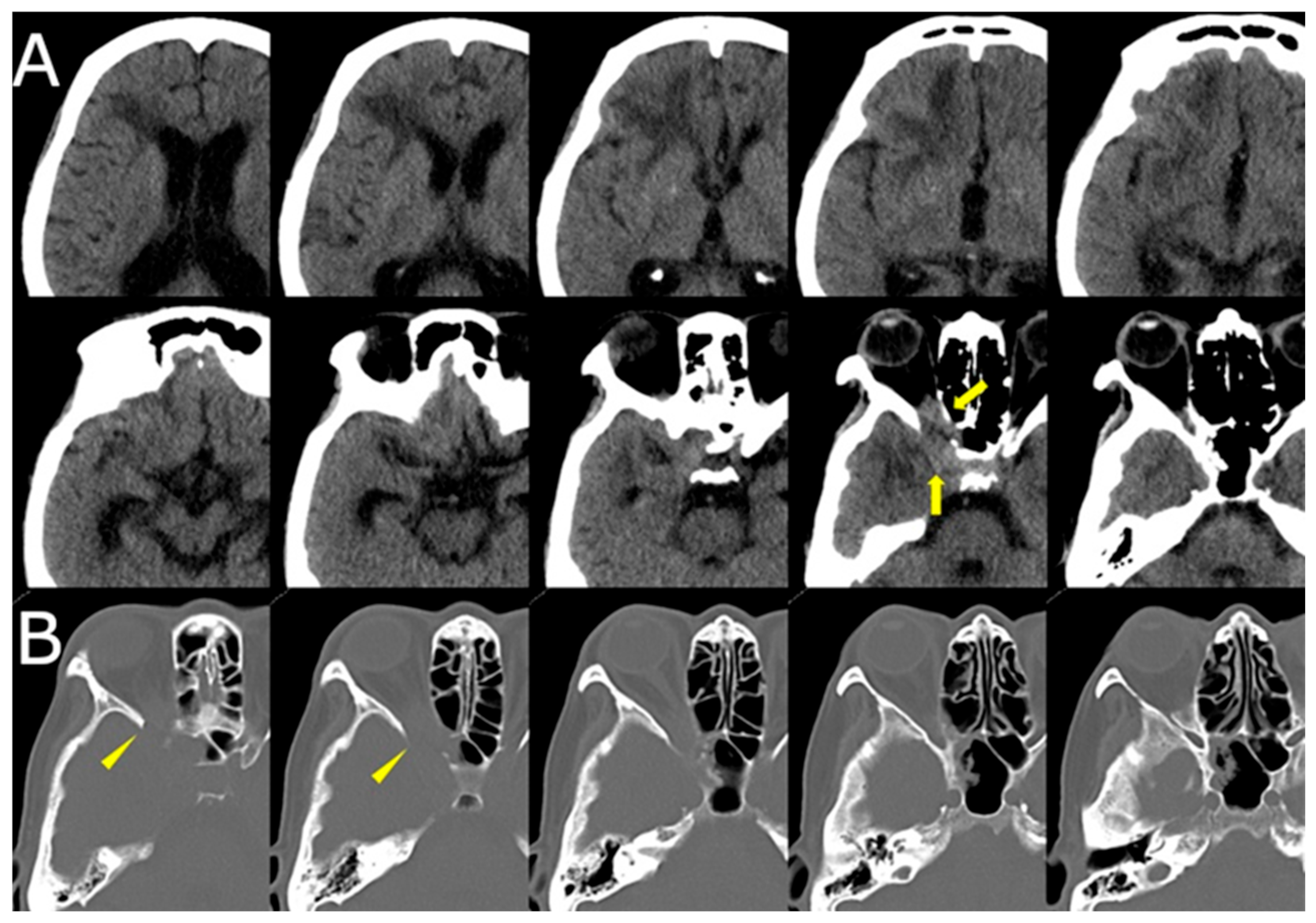
CT of the sinuses demonstrated opacification of the right ethmoid and sphenoid sinuses, with bony erosion of the medial orbital wall and sinus roof (
Figure 3). These findings raised suspicion for an invasive fungal process.
Empirical treatment with anti-tuberculosis therapy (isoniazid 300 mg/day, rifampicin 450 mg/day, ethambutol 750 mg/day, and pyrazinamide 1000 mg/day) and broad-spectrum antibiotics (ceftriaxone 2 g/day) was initiated. However, the patient experienced gastrointestinal side effects including nausea and anorexia, and her symptoms continued to worsen.
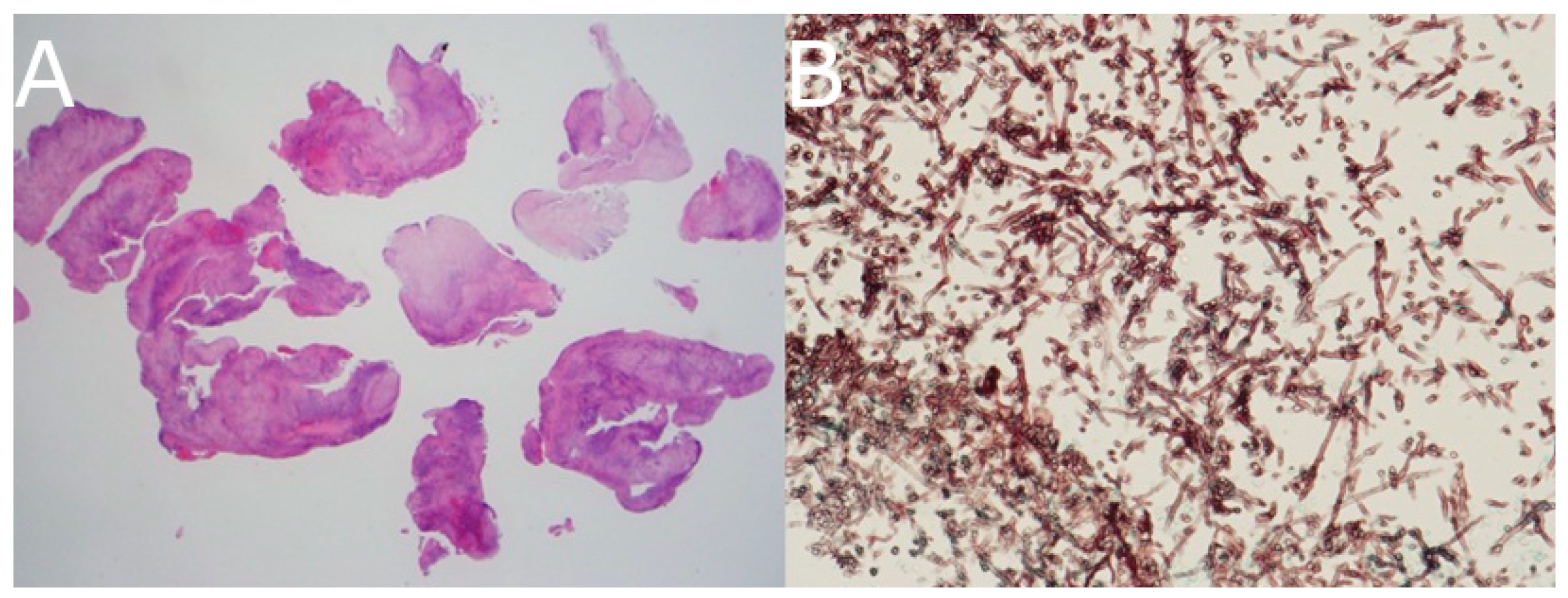
Transnasal endoscopic biopsy of the sphenoid sinus was performed. Histopathology revealed necrotizing granulomatous inflammation. Grocott methenamine silver staining showed septated fungal hyphae with acute-angle branching, confirming the diagnosis of Aspergillus species (
Figure 4).
Antifungal therapy was initiated with intravenous voriconazole (6 mg/kg every 12 h for the first two doses, followed by 4 mg/kg every 12 h). The patient showed gradual improvement in ocular symptoms and resolution of fever. Follow-up MRI after one and three months showed a marked reduction in lesion size. The voriconazole course was continued for 12 weeks, during which liver function remained within acceptable limits, and no severe side effects were reported. At six-month follow-up, the patient remained symptom-free with normal visual acuity and full ocular motility.
3. Discussion
Invasive fungal infections (IFIs) of the orbital apex with contiguous intracranial involvement are rare and frequently misdiagnosed, particularly in non-neutropenic hosts. While traditionally considered diseases of the severely immunocompromised, increasing case reports—such as those by Sharma et al. and DeShazo et al.—demonstrate that even patients with localized immunosuppressive risk factors (e.g., diabetes mellitus) are susceptible to aggressive disease progression [
1,
2,
3]. In our case, the patient was immunocompetent aside from well-controlled diabetes and presented with imaging findings that were initially subtle and non-specific.
The differential diagnosis for orbital apex lesions is broad, including granulomatous diseases (e.g., sarcoidosis and tuberculosis), neoplasms (e.g., lymphoma and meningioma), and idiopathic orbital inflammatory syndrome (IOIS). These entities often overlap radiologically, complicating the diagnosis. Sarcoidosis tends to be bilateral and systemic with elevated ACE levels, whereas tuberculosis often presents with basal meningeal enhancement and positive interferon-gamma release assays (IGRAs). In our case, negative IGRA and the absence of systemic granulomatous signs made tuberculosis less likely, yet empiric anti-TB treatment was initiated due to the ambiguous clinical picture. MRI plays a central role in early detection, but fungal lesions may lack definitive characteristics. Typical T2 hypointensity due to paramagnetic elements like iron and manganese can be absent early in the disease. Gadolinium enhancement may appear mild or irregular, as observed in our case. CT, particularly in bone windows, can reveal erosive changes in the sinus walls or orbital apex, suggestive of invasive fungal sinusitis. Diffusion-weighted imaging (DWI) is particularly valuable for detecting fungal abscesses through diffusion restriction patterns, while PET/CT may help distinguish neoplastic from infectious lesions, albeit with limited specificity and accessibility [
4,
5,
6,
7].
Histopathological confirmation remains essential. In our patient, transnasal sphenoid sinus biopsy was critical in establishing the diagnosis. Grocott and PAS staining revealed acute-angle branching hyphae typical of Aspergillus, allowing prompt initiation of antifungal therapy. While fungal cultures or molecular diagnostics such as ITS sequencing can provide species identification and susceptibility, clinical urgency often necessitates empiric therapy [
8].
Voriconazole remains the treatment of choice due to its superior efficacy and CNS penetration. Therapeutic drug monitoring (TDM) is recommended to maintain therapeutic levels and minimize toxicity such as visual disturbances or hepatotoxicity. Although TDM was not performed in our case, the patient tolerated the drug well and exhibited significant clinical improvement without adverse effects [
2]. This reinforces the importance of early treatment, particularly when diagnosis is delayed.
This case also highlights the importance of multidisciplinary collaboration. Involvement of infectious diseases, ophthalmology, otolaryngology, and radiology facilitated appropriate decision-making and expedited biopsy and treatment. As emphasized in recent guidelines, IFIs in non-neutropenic patients remain a diagnostic challenge and require high clinical suspicion, early recognition, and prompt therapy to optimize outcomes [
9].
4. Conclusions
Clinicians should maintain a high index of suspicion for invasive fungal infections in patients presenting with progressive orbital apex lesions, particularly when imaging is atypical or symptoms persist despite empirical treatment. Diagnostic consideration should be heightened in the following scenarios:
Gradual worsening of cranial neuropathies (e.g., ptosis and ophthalmoplegia);
Poor response to corticosteroids or anti-tuberculosis therapy;
T2-hypointense orbital lesions with subtle enhancement on MRI;
Evidence of bony erosion or sinus involvement on CT;
Elevated serum fungal markers (β-D-glucan and galactomannan).
This case underscores the importance of early biopsy and multidisciplinary collaboration in achieving accurate diagnosis and effective treatment. It also highlights that invasive aspergillosis can develop even in immunocompetent patients, especially those with diabetes mellitus. Prompt recognition and targeted antifungal therapy are essential to prevent irreversible complications, such as vision loss or intracranial dissemination. Our report contributes to the growing awareness of this diagnostic pitfall and emphasizes a systematic approach for earlier identification and intervention.