Candida glabrata Prosthetic Joint Infection Managed with Ibrexafungerp
Abstract
1. Background
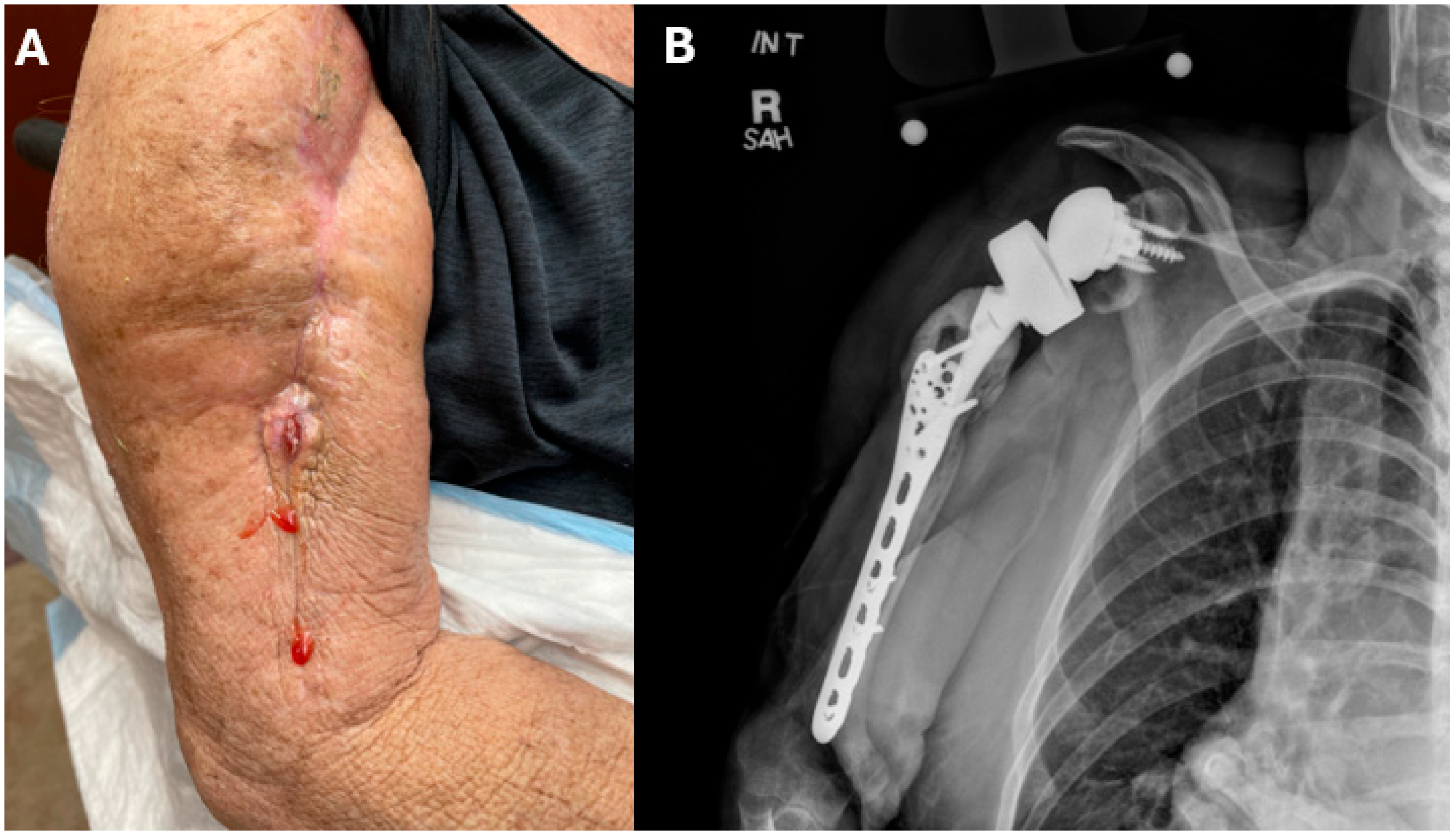
2. Case
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IV | Intravenous |
| PJI | Prosthetic Joint Infection |
| MALDI-TOF | Matrix-Assisted Laser Desorption/Ionization Time-of-Flight (Mass Spectrometry). |
| MIC | Minimum Inhibitory Concentration |
References
- Ricotta, E.E.; Lai, Y.L.; Babiker, A.; Strich, J.R.; Kadri, S.S.; Lionakis, M.S.; Prevots, D.R.; Adjemian, J. Invasive Candidiasis Species Distribution and Trends, United States, 2009–2017. J. Infect. Dis. 2021, 223, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Herrera, E.; Frias-De-Leon, M.G.; Hernandez-Castro, R.; Garcia-Salazar, E.; Arenas, R.; Ocharan-Hernandez, E.; Ro-driguez-Cerdeira, C. Antifungal Resistance in Clinical Isolates of Candida glabrata in Ibero-America. J. Fungi 2021, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Healey, K.R.; Jimenez Ortigosa, C.; Shor, E.; Perlin, D.S. Genetic Drivers of Multidrug Resistance in Candida glabrata. Front. Microbiol. 2016, 7, 1995. [Google Scholar] [CrossRef] [PubMed]
- McCarty, T.P.; White, C.M.; Pappas, P.G. Candidemia and Invasive Candidiasis. Infect. Dis. Clin. 2021, 35, 389–413. [Google Scholar] [CrossRef] [PubMed]
- Spec, A.; Pullman, J.; Thompson, G.R., III; Powderly, W.G.; Tobin, E.H.; Vazquez, J.; Wring, S.A.; Angulo, D.; Helou, S.; Pappas, P.G.; et al. MSG-10: A Phase 2 study of oral ibrexafungerp (SCY-078) following initial echinocandin therapy in non-neutropenic patients with invasive candidiasis. J. Antimicrob. Chemother. 2019, 74, 3056–3062. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. BREXAFEMME® (ibrexafungerp). SCYNEXIS Inc. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/214900s002lbl.pdf (accessed on 11 December 2024).
- Mesquida, A.; Díaz-García, J.; Sánchez-Carrillo, C.; Muñoz, P.; Escribano, P.; Guinea, J. In vitro activity of ibrexafungerp against Candida species isolated from blood cultures. Determination of wild-type populations using the EUCAST method. Clin. Microbiol. Infect. 2022, 28, e1-140.e4. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Alexander, B.D.; Pappas, P.; Patterson, T.F.; Hoenigl, M.; Miceli, M.H.; Vergidis, P.; Spec, A.; Thompson, G.R.; Ostrosky-Zeichner, L.; et al. An Open-label Study of Ibrexafungerp in Patients with Refractory Fungal Infections (FURI). In Proceedings of the ESCMID Global, Vienna, Austria, 11–15 April 2025. [Google Scholar]
- Lee, I.; Fishman, N.O.; Zaoutis, T.E.; Morales, K.H.; Weiner, M.G.; Synnestvedt, M.; Nachamkin, I.; Lautenbach, E. Risk factors for fluconazole-resistant Candida glabrata bloodstream infections. Arch. Intern. Med. 2009, 169, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Durán-Valle, M.T.; Gago, S.; Gómez-López, A.; Cuenca-Estrella, M.; Diez-Canseco, L.J.; Gomez-Garces, J.L.; Zaragoza, O. Recurrent episodes of candidemia due to Candida glabrata with a mutation in hot spot 1 of the FKS2 gene developed after prolonged therapy with caspofungin. Antimicrob. Agents Chemother. 2012, 56, 3417–3419. [Google Scholar] [CrossRef] [PubMed]
- Nunnally, N.S.; Etienne, K.A.; Angulo, D.; Lockhart, S.R.; Berkow, E.L. In Vitro Activity of Ibrexafungerp, a Novel Glucan Synthase Inhibitor against Candida glabrata Isolates with FKS Mutations. Antimicrob. Agents Chemother. 2019, 63, e01692-19. [Google Scholar] [CrossRef] [PubMed]
- Barros, N.; Rosenblatt, R.E.; Phipps, M.M.; Fomin, V.; Mansour, M.K. Invasive fungal infections in liver diseases. Hepatol. Commun. 2023, 7, e0216. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.; Benjamin, D.K., Jr.; Calandra, T.F.; Edwards, J.E., Jr.; Filler, S.G.; Fisher, J.F.; Kullberg, B.J.; Ostrosky-Zeichner, L.; et al. Infectious Diseases Society of America. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect Dis. 2009, 48, 503–535. [Google Scholar] [CrossRef] [PubMed]
| Antifungal | Index Surgery MIC (µg/mL) | Recurrent Infection MIC (µg/mL) | CLSI Breakpoint (µg/mL) Susceptible | CLSI ECV (µg/mL) Wild-Type |
|---|---|---|---|---|
| Amphotericin B | 1 | 0.5 | - | ≤2 |
| Anidulafungin | 0.25 | 0.5 | ≤0.12 | - |
| Caspofungin | 1 | 2 | ≤0.12 | - |
| Micafungin | 0.12 | 0.25 | ≤0.06 | - |
| Fluconazole | 128 | 256 | ≤32 1 | - |
| Itraconazole | 1 | >16 | - | ≤4 |
| Posaconazole | 2 | >8 | - | ≤1 |
| Voriconazole | 1 | 4 | - | 0.25 |
| Ibrexafungerp | 1 | 1 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadarevic, E.; McHugh, J.; Vergidis, P. Candida glabrata Prosthetic Joint Infection Managed with Ibrexafungerp. J. Fungi 2025, 11, 442. https://doi.org/10.3390/jof11060442
Nadarevic E, McHugh J, Vergidis P. Candida glabrata Prosthetic Joint Infection Managed with Ibrexafungerp. Journal of Fungi. 2025; 11(6):442. https://doi.org/10.3390/jof11060442
Chicago/Turabian StyleNadarevic, Ella, Jack McHugh, and Paschalis Vergidis. 2025. "Candida glabrata Prosthetic Joint Infection Managed with Ibrexafungerp" Journal of Fungi 11, no. 6: 442. https://doi.org/10.3390/jof11060442
APA StyleNadarevic, E., McHugh, J., & Vergidis, P. (2025). Candida glabrata Prosthetic Joint Infection Managed with Ibrexafungerp. Journal of Fungi, 11(6), 442. https://doi.org/10.3390/jof11060442







