Abstract
The EORTC/MSGERC definition lacks sufficient sensitivity for diagnosing invasive pulmonary aspergillosis (IPA) in patients with autoimmune inflammatory rheumatic diseases (AIIRDs). We hypothesized that the partial fulfillment of the EORTC/MSGERC definition can improve its diagnostic sensitivity. This retrospective observational study included patients with AIIRDs on immunosuppressive therapy who underwent serum galactomannan antigen testing for suspected IPA. Patients who fulfilled the clinical features or mycological evidence as per the EORTC/MSGERC definition were considered as having “potential IPA.” We compared the clinical characteristics of 364 patients who were categorized into 3 groups—potential IPA (n = 29), proven/probable IPA (n = 24), and non-IPA (n = 311; not meeting any definition). The potential and proven/probable IPA groups had significantly lower survival rates than the non-IPA group (p < 0.001). The potential IPA (adjusted hazard ratio [aHR], 2.0; 95% confidence interval [CI], 1.1–3.8) and proven/probable IPA (aHR, 2.6; 95% CI, 1.4–4.9) were independent risk factors for mortality. Compared with the EORTC/MSGERC definition, our proposed criteria improved sensitivity based on the diagnosis at the end of observation (50.0%, 100.0%, respectively). The characteristics and mortality rates of patients were similar between the potential and proven/probable IPA groups. Using these criteria for clinical diagnosis may provide high sensitivity.
1. Introduction
The advent of new immunosuppressive and biological agents has improved the prognosis of patients with AIIRDs over the past decade. However, opportunistic infections remain a major challenge [1]. Invasive pulmonary aspergillosis (IPA) is one of the most important opportunistic infections, and is the third most common invasive fungal disease (IFD), following pneumocystis pneumonia and candidemia, with a mortality rate of 25–70% [2,3]. Therefore, timely diagnosis and treatment are of paramount importance.
The European Organization for Research and Treatment of Cancer/Mycosis Study Group Education and Research Consortium (EORTC/MSGERC) consensus definition is the most widely used criteria worldwide for diagnosing IFDs, including IPA [4]. The EORTC/MSGERC definition considers three elements: host factors, clinical features, and mycological evidence. The EORTC/MSGERC definition was originally proposed for use in patients with hematologic malignancies in clinical trials [5]. Nonetheless, it is frequently used in clinical practice to guide management in various patients beyond the original target population, including those with AIIRDs [6]. As the sensitivity and specificity of the EORTC/MSGERC definition depend on the unique characteristics of each patient population, criteria have been proposed for research classification and/or clinical diagnosis in specific populations, such as patients in the intensive care unit [7] and those with chronic obstructive pulmonary disease (COPD) [8], influenza [9].
Considering their unique characteristics, patients with AIIRDs require special attention. Previous studies have repeatedly indicated that the EORTC/MSGERC definition has limited sensitivity as patients with AIIRDs often do not fulfill the host factor requirement. Many cases of IPA have been reported in patients with AIIRD who have only received low-dose corticosteroids or other immunosuppressive therapies [10,11,12]. Moreover, they do not often fulfill the clinical features’ requirement, such as typical radiologic findings [12,13,14,15]. The difficulty in diagnosing IPA among patients with AIIRDs is reflected by the fact that it is frequently first diagnosed postmortem [16]. There is concern that many cases of IPA in AIIRD patients may be overlooked. Therefore, it is essential to establish better criteria to identify patients who might benefit from antifungal treatment in a timely manner within a clinical setting [17,18]. Nonetheless, to the best of our knowledge, no criteria have been developed specifically for patients with AIIRDs.
Therefore, this study proposed criteria for patients with AIIRDs that can be applied in clinical settings and clinical trials by modifying the EORTC/MSGERC definition [19]. To prove the concept, we compared this criteria with the EORTC/MSGERC 2019 definition to explore the differences in clinical characteristics and prognosis for different categories.
2. Materials and Methods
2.1. Study Design and Participants
This single-center, retrospective, observational cohort study was performed at the Institute of Science of Tokyo Hospital (STH), Tokyo, Japan. It is an 813-bed, tertiary care academic hospital. The Department of Rheumatology conducts approximately 5000 outpatient visits and 300 hospitalizations annually. In the current study, we included patients who met the following criteria: visited the rheumatology clinic or required hospitalization in the Department of Rheumatology between June 2007 and July 2022; underwent serum galactomannan antigen (GMA) testing for suspected IPA; and received immunosuppressive therapy for AIIRDs at the time of GMA testing. To focus on IPA occurring in immunosuppressed patients with AIIRDs, the following exclusion criteria were considered: receipt of immunosuppressive therapy for conditions other than AIIRDs within 2 years of enrollment (e.g., antitumor chemotherapy); history of known IPA prior to the initiation of immunosuppressive therapy for AIIRDs; and serum GMA testing performed without a suspicion of new IPA (e.g., screening or follow-up).
Considering the retrospective nature of the study, the requirement for patient consent was waived. Our study complied with the principles of the Declaration of Helsinki, and the protocol was approved by the local ethics committee (13 November 2023, Permission number C2023-041). The reporting of this study complies with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
2.2. Proposed Definition of IPA
Based on the EORTC/MSGERC 2019 definition [4], we developed criteria for diagnosing patients with AIIRDs by adding the category of “potential IPA.” Eligible patients in this study were categorized into proven/probable IPA, potential IPA, and non-IPA groups. The potential IPA group comprised patients who fulfilled the clinical features or mycological evidence of the EORTC/MSGERC 2019 definition and exhibited symptomatic pulmonary lesions that progressed over days to weeks, and other etiologies were dismissed (Table 1). The host factor requirement was also relaxed to include all forms of immunosuppressive therapy. All these criteria were intended to improve the sensitivity of clinical diagnosis by expanding “possible IPA.” Aspergillus PCR, which is included in the original EORTC/MSGERC definition, was not used to collect mycological evidence because it was not approved in Japan or available at STH. A comparison of our proposed criteria with the EORTC/MSGERC 2019 definition [4] and others for specific populations is presented in Table S1.

Table 1.
Definition of potential IPA in this study.
The classifications of potential IPA, proven/probable IPA, and non-IPA were ascertained by two infectious disease (ID) physicians (TK and KO) based on a manual chart review. In cases of disagreement, consensus was reached through discussion with a third ID physician (NS). The classification was performed according to the information available within 2 weeks after conducting initial serum GMA testing for suspected IPA to taking into account the time for a diagnostic work-up. Similarly, the final diagnosis was performed using the information available at the end of the observation period.
2.3. Outcomes and Variables
The primary endpoint was survival calculated from the initial day of serum GMA testing. The observation was censored at the time of death or on 31 March 2024. A manual chart review was performed to collect clinical information. GMA and (1,3)-beta-d-glucan (BDG) levels were measured using the Platelia Aspergillus Ag assay (reference value, <0.5; Bio-Rad, Hercules, CA, USA) and Fungitec G-test MK-II (reference value, <20.0 pg/mL; Shimadzu, Kyoto, Japan), respectively.
2.4. Diagnostic Performance
The diagnostic performances of our proposed criteria and the EORTC/MSGERC 2019 definition were evaluated by estimating their sensitivity, specificity, positive predictive value, and negative predictive value, alongside their respective 95% CIs, with the final diagnosis serving as the gold standard.
2.5. Statistical Analysis
Categorical variables were expressed as n (%), and continuous variables were expressed as the median and interquartile range. Comparisons among each group were performed using one-way ANOVA for continuous variables and the chi-squared test for categorical variables. Survival curves were constructed using the Kaplan–Meier method, and the log-rank test was used to assess significant differences among the three groups. Cox proportional hazard regression analysis was performed to estimate the hazard ratios (HRs) and 95% CIs. Factors such as age and the presence of interstitial lung disease (ILD) were included in the multivariate model owing to their impact on mortality [20,21]. All p-values were two-tailed, and p-values of <0.05 were considered to indicate statistical significance. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Study Population Characteristics
During the study period, 1324 patients underwent serum GMA testing in the Department of Rheumatology at STH (Figure 1). After applying the exclusion criteria and excluding patients without AIIRDs, 364 patients were analyzed. Overall, 24 (6.6%) patients had proven/probable IPA, 29 (7.9%) had potential IPA, and 311 (85.4%) had non-IPA.
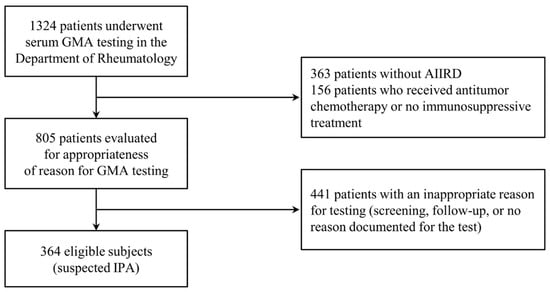
Figure 1.
Flow diagram of patient enrollment in this study. Abbreviations: AIIRDs, autoimmune inflammatory rheumatic diseases; GMA, galactomannan antigen; IPA, invasive pulmonary aspergillosis.
3.2. Differences in Clinical Characteristics
The clinical characteristics of the patients in each group are presented in Table 2. Age (p = 0.023) and the proportion of male patients (p = 0.023) differed among the three groups. Neutrophil counts differed significantly among the three groups (p = 0.044), however few patients had neutrophil counts of <500/µL for >10 days (no patients with proven/probable IPA, one with potential IPA, and two with non-IPA). The incidences of systemic vasculitis as an underlying disease (p < 0.001) and ILD as a pulmonary complication (p = 0.004) differed among the three groups. In the proven/probable and potential IPA groups, the patterns of abnormal radiologic findings of the lungs were characterized by “dense, well-circumscribed lesions,” “air crescent,” and “cavity,” which corresponded to the clinical features described in the EORTC/MSGERC 2019 definition.

Table 2.
Characteristics of each category of patients with AIIRDs.
3.3. Survival Analysis
The survival curves of the proven/probable IPA, potential IPA, and non-IPA groups are presented in Figure 2. Patients with proven/probable IPA and those with potential IPA had significantly lower survival rates than those with non-IPA (p < 0.001). After adjusting for age and the presence of ILD using the Cox proportional hazards model, compared with the non-IPA group, the proven/probable IPA (adjusted hazard ratio [aHR] = 2.6; 95% CI = 1.4–4.9; p < 0.01) and potential IPA (aHR = 2.0; 95% CI = 1.1–3.8; p = 0.03) groups were independently associated with higher mortality (Table 3).
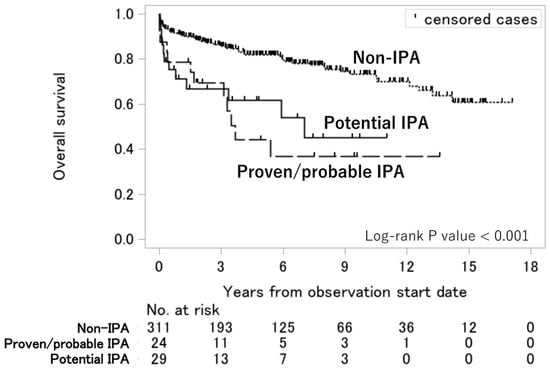
Figure 2.
Overall survival among the proven/probable IPA, potential IPA, and non-IPA groups. Survival curves were constructed using the Kaplan–Meier method, and the log-rank test was used to assess significant differences among the three groups. Abbreviation: IPA, invasive pulmonary aspergillosis.

Table 3.
Factors associated with mortality and their HRs.
3.4. Final Diagnosis of Pulmonary Diseases and Antifungal Treatment
Overall, 4.2% (1/24) of patients with proven/probable IPA and 24.1% (7/29) of those with potential IPA had a final diagnosis other than IPA. Among 311 patients with non-IPA, the final diagnoses included other infections (n = 177, 57.1%), worsening of primary disease (n = 86, 23.4%), malignancy (n = 25, 6.8%), and drug-induced lung disease (n = 12, 3.3%; Table 4). Pulmonary disease remained undiagnosed in eight (2.6%) patients, only two of whom were treated for IPA by a primary rheumatologist. In contrast, 83.0% of patients with either proven/probable or potential IPA were prescribed antifungals by primary rheumatologists (Table S3). The potential IPA group had a significantly higher proportion of culture-negative cases than the proven/probable IPA group (48.2% vs. 0.0%, p = 0.002, Table S3).

Table 4.
Final diagnosis of pulmonary lesions by manual chart review in each category.
3.5. Comparison of Our Proposed Criteria and the EORTC/MSGERC 2019 Definition
Table 5 presents the diagnostic performances of our proposed criteria and the EORTC/MSGERC 2019 definition. The diagnostic sensitivities of potential IPA defined by our proposed criteria, proven/probable IPA, and possible IPA defined by the EORTC/MSGERC 2019 definition were 100%, 50.0%, and 61.7%, respectively. The specificities of potential IPA, proven/probable IPA, and possible IPA were 97.8%, 99.7%, and 99.1%, respectively.

Table 5.
Diagnostic performance of our proposed criteria with the category of “potential IPA” versus the EORTC/MSGERC 2019 definition.
Figure 3 presents the missing factors for patients with potential IPA that would have led to a classification of probable IPA. The most common missing factor was clinical features in 14 patients, followed by host factors in 9 patients and mycological evidence in 7 patients. Among patients lacking mycological evidence, five met the possible IPA criteria in accordance with the EORTC/MSGERC 2019 definition. A schematic diagram of each classification group is presented in Figure S1.
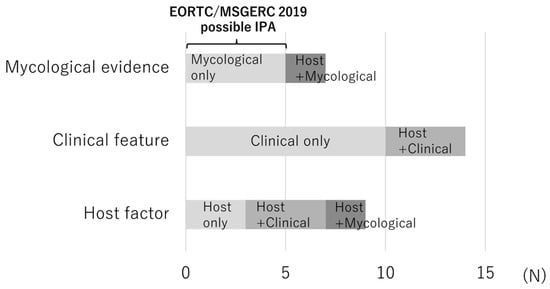
Figure 3.
Reasons for non-classification of potential IPA as probable IPA by the EORTC/MSGERC 2019 definition (missing factors) for each case. Each bar indicates the missing factors among the three components of the EORTC/MSGERC 2019 definition that would have allowed the case of potential IPA to be classified as probable IPA. Abbreviations: EORTC/MSGERC, European Organization for Research and Treatment of Cancer/Mycosis Study Group Education and Research Consortium; IPA, invasive pulmonary aspergillosis.
4. Discussion
Patients with potential IPA, which was based on our newly proposed criteria specifically targeting patients with AIIRDs, had similar survival rates as those with proven/probable IPA but worse survival rates than those with non-IPA. In addition, our proposed criteria had statistically higher sensitivity than the EORTC/MSGERC 2019 definition while maintaining high specificity in diagnosing IPA.
The clinical characteristics of patients were extremely similar between the potential IPA and proven/probable IPA groups. Both groups rarely had neutropenia. The patients generally received high corticosteroid doses and showed high rates of systemic vasculitis and/or ILDs. These findings were consistent with the characteristics of IPA in patients with AIIRDs reported in previous observational studies [12,13,14,15,22]. We speculated that structural changes secondary to ILDs predispose patients to Aspergillus colonization, as observed in patients with COPD [8,23]. In contrast, the potential IPA group had lower rates of hemoptysis, fever, positive cultures, and typical radiographic findings than the proven/probable IPA group. Notably, these differences, excluding those related to clinical symptoms, were pertinent to the definition of potential IPA itself. As the criteria have been expanded with the intention of improving diagnostic sensitivity, we hypothesized that the potential IPA group includes heterogeneous patients, involving those with “true IPA” and “airway colonization” [24]. The differences in the clinical symptoms between these groups might reflect this heterogeneity.
Most patients with potential IPA in this cohort did not meet the criteria for possible IPA, as stipulated by the EORTC/MSGERC 2019 definition. Nonetheless, the survival rate was approximately 50% in the proven/probable and potential IPA groups, in line with previous findings on IPA among patients with AIIRDs [2,3]. The difference remained even after adjusting for age and the presence of ILDs. Furthermore, the causes of mortality were similar between the proven/probable and potential IPA groups. Together with the other clinical characteristics, we hypothesized that patients with potential IPA may benefit from antifungal therapy.
In our cohort, the addition of potential IPA in our criteria greatly increased the diagnostic sensitivity without significantly compromising specificity compared with the EORTC/MSGERC 2019 definition. This increase was attributable to the expansion of host factors and clinical features. Since patients with AIIRDs can develop IPA at lower steroid doses, other risk factors, such as the presence of ILD, might play an important role in the development of IPA. Clinical features in the EORTC/MSGERC 2019 definition, such as halo signs and cavities, reflect angioinvasion by hyphae, which commonly occurs in patients with neutropenia [25,26], but rarely patients with AIIRDs [12,13,14,15]. We speculate that these characteristics contribute to false-negative results when using the EORTC/MSGERC 2019 definition.
It remains unexplored whether early antifungal therapy improves the prognosis of IPA in patients with AIIRDs. In this study, most patients with IPA received antifungal agents, making it difficult to compare patients with and without treatment. Although early antifungal therapy for proven/probable IPA has been reported to improve prognosis in patients with severe neutropenia [27], its efficacy in patients without neutropenia, including those with AIIRDs, remains unknown. One of the major reasons was that enrolling patients with AIIRDs in clinical trials is challenging given the lack of an appropriate early diagnosis [18], in contrast to patients with hematological malignancies who can be enrolled in clinical trials using the EORTC/MSGERC 2019 definition [28,29]. In the future, our proposed criteria are expected to facilitate the conduct of clinical trials, including those aimed at evaluating the efficacy of early antifungal therapies targeting IPA among patients with AIIRDs.
We acknowledge that our study had limitations. First, owing to its single-center retrospective design, our study results had information bias and limited generalizability. Pathological examination was rarely performed, and the final diagnosis relied on the clinical information documented in the medical records. Although the three ID physicians independently reviewed the cases to maximize the certainty, this remains a major limitation. In addition, bronchoalveolar lavage, which is a highly sensitive method for diagnosis [30,31], was not sufficiently performed in this cohort, potentially leading to an underdiagnosis of IPA. Also, the sample size of patients with IPA was insufficient to allow complete adjustment for potential confounders. Therefore, our results do not have sufficient external validity to enable extrapolation to the daily clinical practice among patients with AIIRDs. Meanwhile, the criteria development methodology in our study was not based on a systematic review or consensus within an expert working group, but on the insights from previous reports [17,18]. However, this study might encourage future validation, which would lead to improved outcomes for patients with AIIRDs through early diagnosis and treatment.
In conclusion, patients with potential IPA identified using our proposed criteria had similar clinical characteristics and mortality rates as those with proven/probable IPA identified by the EORTC/MSGERC 2019 definition. Using these criteria for clinical diagnosis may provide higher sensitivity compared with the EORTC/MSGERC definition. Further validation is needed to determine whether the use of these criteria can improve the prognosis of such patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof11060437/s1, Table S1: Comparison of the IPA diagnostic/classification criteria; Table S2: Relationships of duration from the start of observation to death and the causes of mortality; Table S3: Pathogens causing IPA and antifungal therapy in the proven/probable IPA and potential IPA groups; Table S4: Comparison of pathogens and anti-fungal treatment between survivors and deaths among patients with potential IPA; Figure S1: Schematic diagram of each classified group.
Author Contributions
T.K. and K.O. contributed to study conception and design. T.K. collected and analyzed the survey data. T.K., K.O. and N.S. conducted a manual chart review to confirm the diagnosis and classification. K.O., N.S., A.Y., T.H., S.Y. and Y.G. aided in interpreting the results and assisted with writing the manuscript. R.H. and A.H. performed statistical analysis and prepared figures and tables. T.K. wrote the first draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the local ethics committee of Science Tokyo (13 November 2023, Permission number C2023-041).
Informed Consent Statement
Considering the retrospective nature of the study, the requirement for patient consent was waived.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank the medical staff of our institute for their contributions. The parts of the study results were presented at ESCMID Global 2025 (Vienna, Austria, 12 April 2025) [19].
Conflicts of Interest
The authors have no competing interests to disclose.
References
- Mok, C.C.; Kwok, C.L.; Ho, L.Y.; Chan, P.T.; Yip, S.F. Life expectancy, standardized mortality ratios, and causes of death in six rheumatic diseases in Hong Kong, China. Arthritis Rheum. 2011, 63, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.R.; Barber, C.E.; Johnson, A.S.; Barnabe, C. Invasive fungal disease in systemic lupus erythematosus: A systematic review of disease characteristics, risk factors, and prognosis. Semin. Arthritis Rheum. 2014, 44, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Galmiche, S.; Thoreau, B.; Bretagne, S.; Alanio, A.; Paugam, A.; Letscher-Bru, V.; Cassaing, S.; Gangneux, J.-P.; Guegan, H.; Favennec, L.; et al. Invasive fungal diseases in patients with autoimmune diseases: A case series from the French RESSIF network. RMD Open 2023, 9, e003281. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef]
- Ascioglu, S.; Rex, J.H.; De Pauw, B.; Bennett, J.E.; Bille, J.; Crokaert, F.; Denning, D.W.; Donnelly, J.P.; Edwards, J.E.; Erjavec, Z.; et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: An international consensus. Clin. Infect. Dis. 2002, 34, 7–14. [Google Scholar] [CrossRef]
- Vinicki, J.P.; Pellet, S.C.; Pappalardo, C.; Cruzat, V.C.; Spinetto, M.A.; Dubinsky, D.; Tiraboschi, I.N.; Laborde, H.A.; Nasswetter, G. Invasive fungal infections in Argentine patients with systemic lupus erythematosus. Lupus 2013, 22, 892–898. [Google Scholar] [CrossRef]
- Blot, S.I.; Taccone, F.S.; Van den Abeele, A.-M.; Bulpa, P.; Meersseman, W.; Brusselaers, N.; Dimopoulos, G.; Paiva, J.A.; Misset, B.; Rello, J.; et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am. J. Respir. Crit. Care Med. 2012, 186, 56–64. [Google Scholar] [CrossRef]
- Bulpa, P.; Dive, A.; Sibille, Y. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease. Eur. Respir. J. 2007, 30, 782–800. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Rijnders, B.J.A.; Brüggemann, R.J.M.; Azoulay, E.; Bassetti, M.; Blot, S.; Calandra, T.; Clancy, C.J.; Cornely, O.A.; Chiller, T.; et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: An expert opinion. Intensive Care Med. 2020, 46, 1524–1535. [Google Scholar] [CrossRef]
- Şeyhoğlu, E.; Erden, A.; Kılıç, L.; Karadağ, Ö.; Akdağlı, S.A.; Akdoğan, A.; Kalyoncu, U. Pulmonary aspergillosis after treatment with infliximab in Still’s disease and a literature review of Still’s disease and pulmonary aspergillosis. Eur. J. Rheumatol. Inflamm. 2018, 5, 75–78. [Google Scholar] [CrossRef]
- O’Reilly, S.; Hartley, P.; Jeffers, M.; Casey, E.; Clancy, L. Invasive pulmonary aspergillosis associated with low dose methotrexate therapy for rheumatoid arthritis: A case report of treatment with itraconazole. Tuber. Lung Dis. 1994, 75, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Baliga, S.; Yadav, S.; Sagdeo, P.; Balakrishnan, C. Invasive fungal infection in ANCA-associated vasculitis: Between the Devil and Deep blue sea. Case series and review of the literature. Clin. Rheumatol. 2024, 43, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.L.; Liao, H.T.; Chen, W.S.; Chen, M.H.; Lai, C.C.; Tsai, C.Y.; Chang, D.M. Invasive aspergillosis in patients with systemic lupus erythematosus: A retrospective study on clinical characteristics and risk factors for mortality. Lupus 2018, 27, 1944–1952. [Google Scholar] [CrossRef]
- Cornillet, A.; Camus, C.; Nimubona, S.; Gandemer, V.; Tattevin, P.; Belleguic, C.; Chevrier, S.; Meunier, C.; Lebert, C.; Aupée, M.; et al. Comparison of epidemiological, clinical, and biological features of invasive aspergillosis in neutropenic and nonneutropenic patients: A 6-year survey. Clin. Infect. Dis. 2006, 43, 577–584. [Google Scholar] [CrossRef]
- Lewis, R.E.; Kontoyiannis, D.P. Invasive aspergillosis in glucocorticoid-treated patients. Med. Mycol. 2009, 47 (Suppl. 1), S271–S281. [Google Scholar] [CrossRef]
- Mudrakola, H.V.; Tandon, Y.K.; DeMartino, E.; Tosh, P.K.; Yi, E.S.; Ryu, J.H. Autopsy study of fatal invasive pulmonary aspergillosis: Often undiagnosed premortem. Respir. Med. 2022, 199, 106882. [Google Scholar] [CrossRef] [PubMed]
- Tio, S.Y.; Chen, S.C.-A.; Hamilton, K.; Heath, C.H.; Pradhan, A.; Morris, A.J.; Korman, T.M.; Morrissey, O.; Halliday, C.L.; Kidd, S.; et al. Invasive aspergillosis in adult patients in Australia and New Zealand: 2017–2020. Lancet Reg. Health West. Pac. 2023, 40, 100888. [Google Scholar] [CrossRef]
- Tio, S.Y.; Chen, S.C.A.; Heath, C.H.; Pradhan, A.; Morris, A.J.; Korman, T.M.; Morrissey, C.O.; Halliday, C.L.; Kidd, S.; Spelman, T.; et al. Identifying gaps in the international consensus case definitions for invasive aspergillosis: A review of clinical cases not meeting these definitions. Open Forum Infect. Dis. 2024, 11, ofae594. [Google Scholar] [CrossRef]
- Kurita, T.; Okamoto, K.; Sekiya, N.; Hanazawa, R.; Yamamoto, A.; Hosoya, T.; Hirakawa, A.; Yasuda, S.; Gu, Y. A proposal of the refined diagnostic criteria for invasive pulmonary aspergillosis in autoimmune inflammatory rheumatic diseases treated with immunosuppressive agents. In Proceedings of the ESCMID Global 2025, Vienna, Austria, 12 April 2025. [Google Scholar]
- Mathai, S.C.; Danoff, S.K. Management of interstitial lung disease associated with connective tissue disease. BMJ 2016, 352, h6819. [Google Scholar] [CrossRef]
- Fischer, A.; du Bois, R. Interstitial lung disease in connective tissue disorders. Lancet 2012, 380, 689–698. [Google Scholar] [CrossRef]
- Su, T.; Li, H.-C.; Chen, M.; Gao, L.; Zhou, F.-D.; Wang, R.-G.; Zhang, H.; Li, X.-M.; Zhao, M.-H. Invasive pulmonary aspergillosis in patients with antineutrophil cytoplasmic antibody associated vasculitis. J. Clin. Rheumatol. 2009, 15, 380–382. [Google Scholar] [CrossRef]
- Richardson, M.; Bowyer, P.; Sabino, R. The human lung and Aspergillus: You are what you breathe in? Med. Mycol. 2019, 57, S145–S154. [Google Scholar] [CrossRef]
- Latgé, J.-P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, e00140-18. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Nouér, S.A.; Cappone, D.; Anaissie, E. Early diagnosis of invasive pulmonary aspergillosis in hematologic patients: An opportunity to improve the outcome. Haematologica 2013, 98, 1657–1660. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Nouér, S.A.; Grazziutti, M.; Kumar, N.S.; Barlogie, B.; Anaissie, E. Probable invasive aspergillosis without prespecified radiologic findings: Proposal for inclusion of a new category of aspergillosis and implications for studying novel therapies. Clin. Infect. Dis. 2010, 51, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24 (Suppl. 1), e1–e38. [Google Scholar] [CrossRef]
- Tan, B.H.; Low, J.G.H.; Chlebicka, N.L.; Kurup, A.; Cheah, F.K.; Lin, R.T.P.; Goh, Y.T.; Wong, G.C. Galactomannan-guided preemptive vs. empirical antifungals in the persistently febrile neutropenic patient: A prospective randomized study. Int. J. Infect. Dis. 2011, 15, e350–e356. [Google Scholar] [CrossRef][Green Version]
- Maertens, J.; Lodewyck, T.; Donnelly, J.P.; Chantepie, S.; Robin, C.; Blijlevens, N.; Turlure, P.; Selleslag, D.; Baron, F.; Aoun, M. Empiric vs. preemptive antifungal strategy in high-risk neutropenic patients on fluconazole prophylaxis: A randomized trial of the European organization for research and treatment of cancer. Clin. Infect. Dis. 2023, 76, 674–682. [Google Scholar] [CrossRef]
- Prattes, J.; Flick, H.; Prüller, F.; Koidl, C.; Raggam, R.B.; Palfner, M.; Eigl, S.; Buzina, W.; Zollner-Schwetz, I.; Thornton, C.R.; et al. Novel tests for diagnosis of invasive aspergillosis in patients with underlying respiratory diseases. Am. J. Respir. Crit. Care Med. 2014, 190, 922–929. [Google Scholar] [CrossRef]
- Han, Y.; Wu, X.; Jiang, G.; Guo, A.; Jin, Z.; Ying, Y.; Lai, J.; Li, W.; Yan, F. Bronchoalveolar lavage fluid polymerase chain reaction for invasive pulmonary aspergillosis among high-risk patients: A diagnostic meta-analysis. BMC Pulm. Med. 2023, 23, 58. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).