Toxicity Assessment and Antifungal Potential of Copper(II) and Silver(I) Complexes with 1,10-Phenanthroline-5,6-dione Against Drug-Resistant Clinical Isolates of Cryptococcus gattii and Cryptococcus neoformans
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungi and Growth Conditions
2.2. Test Compounds
2.3. Antifungal Susceptibility Profile of Clinically Available Antifungal Agents
2.4. Assessment of the Potential Antifungal Activity of Test Compounds
2.5. Chemical Stability of the Test Compounds
2.6. Prediction of Drug-Likeness and Pharmacokinetics of the Test Compounds
2.7. Hemolysis Assay
2.8. In Vivo Toxicity of the Test Compounds in Galleria mellonella
2.8.1. Single Treatment (Acute Toxicity)
2.8.2. Multiple Treatment (Chronic Toxicity)
2.9. Hemocyte Density Analysis
2.10. Statistical Analyses
3. Results
3.1. Susceptibility Profile of Cryptococcus Clinical Isolates to Conventional Antifungal Agents
3.2. Impact of Test Compounds on Fungal Viability
3.3. Assessment of the Stability of Antifungal Activity in the Evaluated Complexes
3.4. Prediction of Drug-Likeness and Pharmacokinetics of Test Complexes
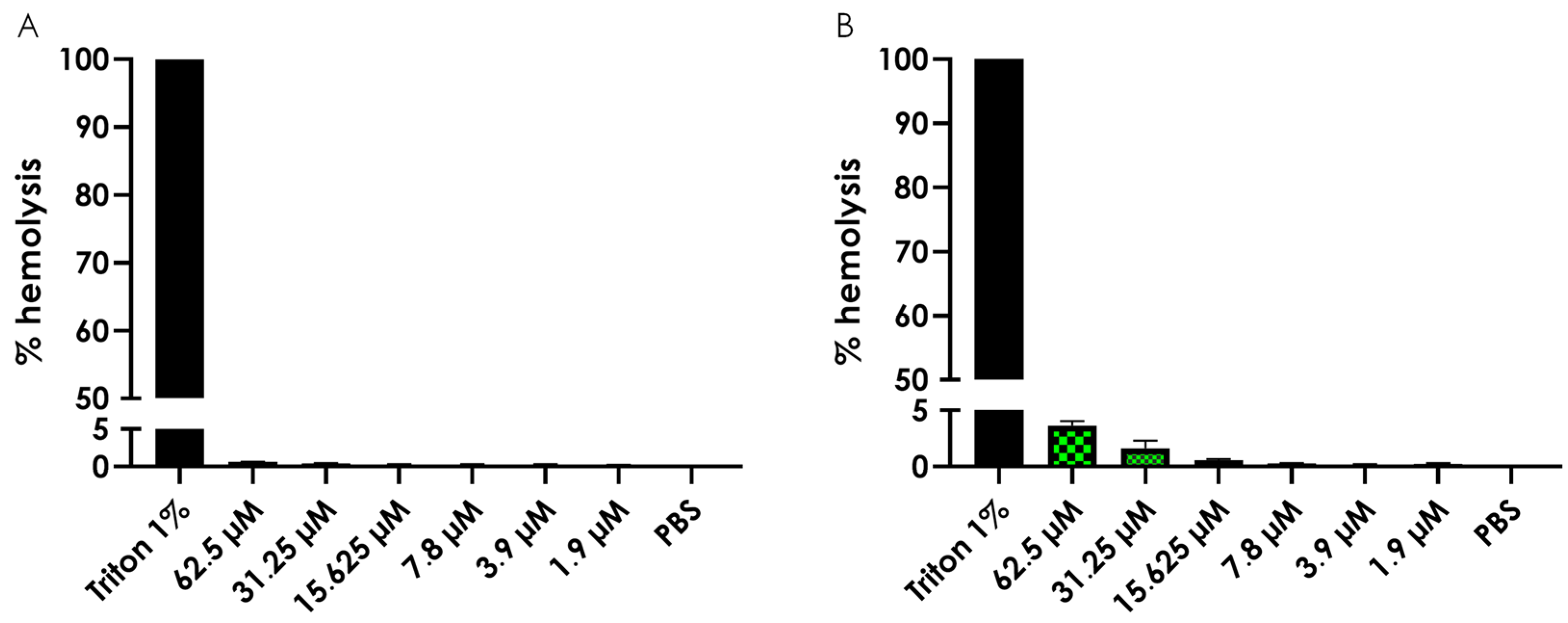
3.5. Hemolysis Assay
3.6. In Vivo Toxicity of Test Complexes in G. mellonella
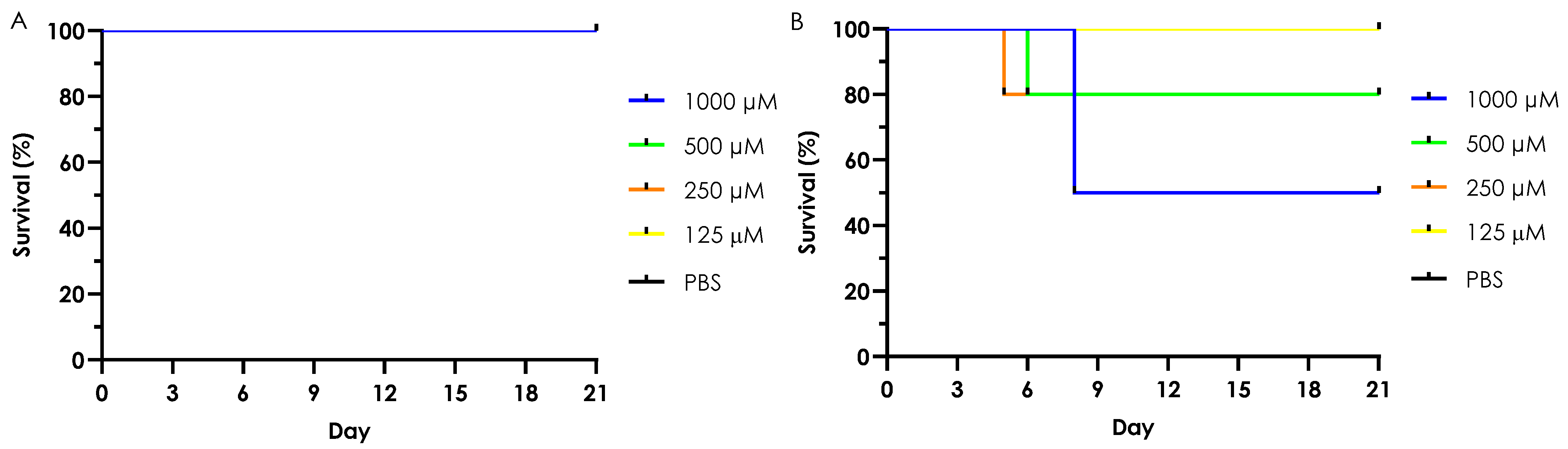
3.6.1. Acute Toxicity (Single Dose Treatment)
3.6.2. Chronic Toxicity (Multiple Dose Treatment)
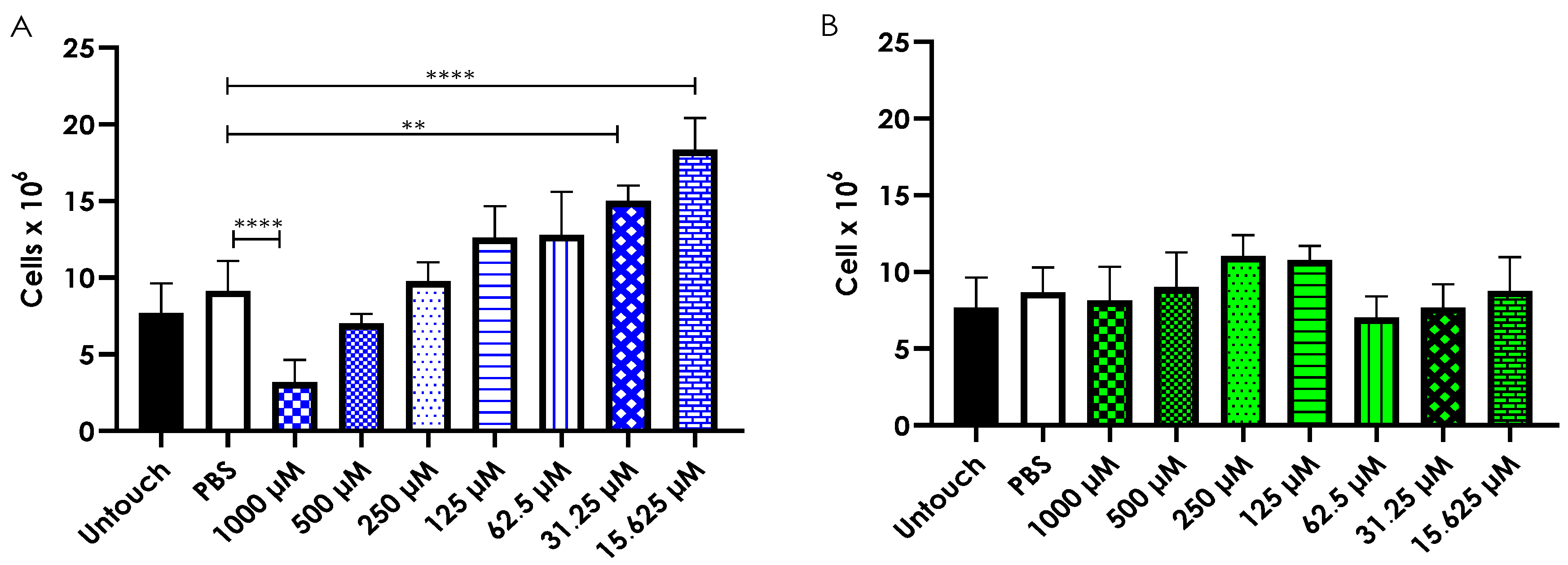
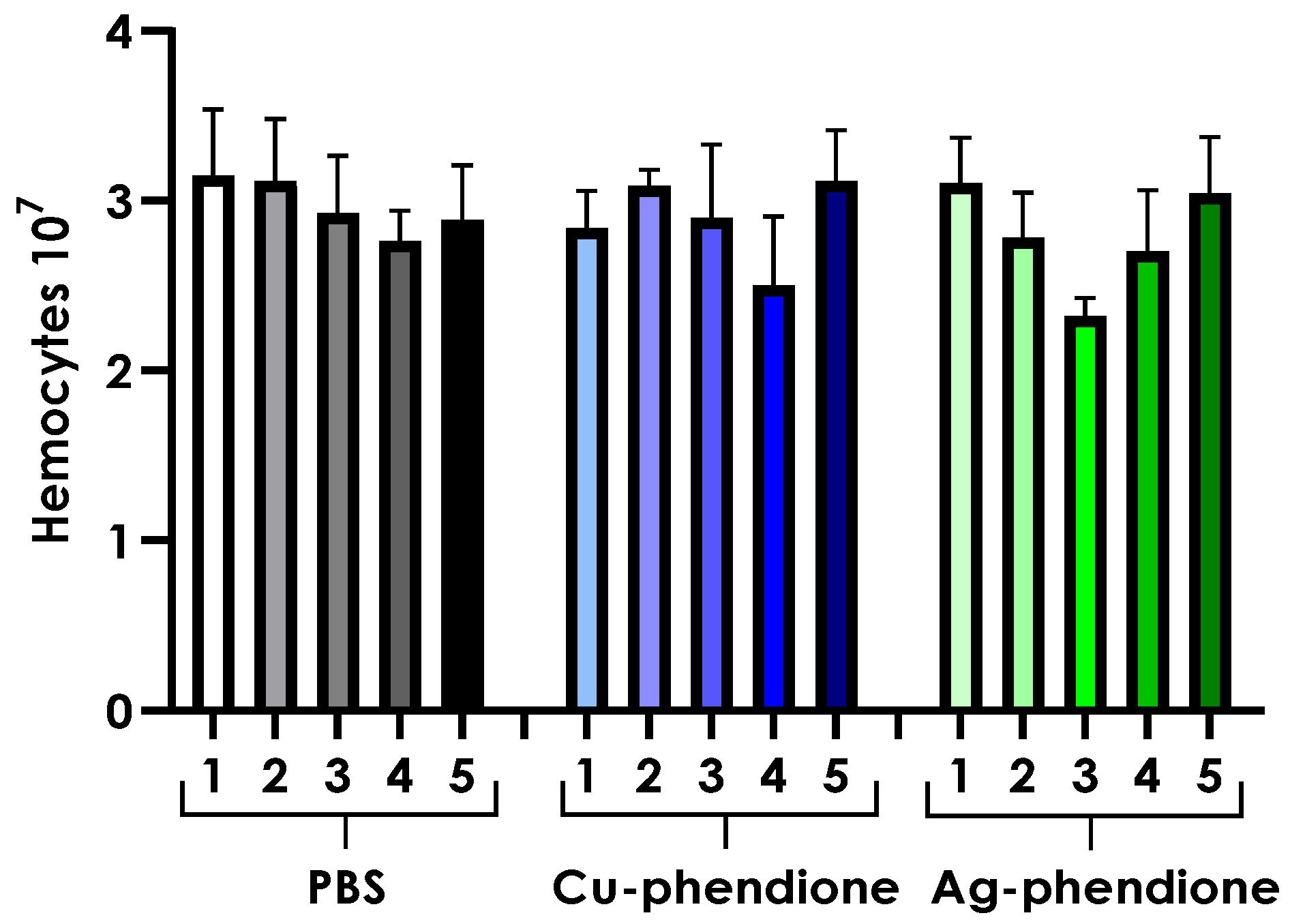
3.7. Hemocyte Density Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denning, D.W. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 2024, 24, E428–E438. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.L.; Toda, M.; Chiller, T.; Brunkard, J.M.; Litvintseva, A.P. Effects of climate change on fungal infections. PLoS Pathog. 2024, 20, e1012219. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ye, L.; Zhao, F.; Zhang, L.; Lu, Z.; Chu, T.; Wang, S.; Liu, Z.; Sun, Y.; Chen, M.; et al. Cryptococcus neoformans, a global threat to human health. Infect. Dis. Poverty 2023, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Gbadamosi, H.; Afriyie-Mensah, J.S.; Ansah, E.N.; Dadzie, S.K.; Puplampu, P. A rare case of disseminated pulmonary cryptococcosis in an immunocompetent patient. BMC Pulm. Med. 2024, 24, 484. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Liu, Y.M.; Chen, T.C. Disseminated Cryptococcus neoformans infection involving multiple bones and lung in an immunocompetent patient: A case report. BMC Infect. Dis. 2024, 24, 397. [Google Scholar] [CrossRef]
- Maccora, K.A.; Tang, Y.F.; Lee, J.H.; Chong, E.W.; Chan, H.H.L. Cryptococcal meningitis with immune-reconstitution inflammatory syndrome causing papilledema and visual field defects in an immunocompetent patient. J. Neuroophthalmol. 2024, 44, e376–e378. [Google Scholar] [CrossRef]
- Tarisawa, M.; Kano, T.; Ishimaru, T.; Nomura, T.; Mizushima, K.; Horiuchi, K.; Iwata, I.; Ura, S.; Minami, N.; Hozen, H.; et al. Clinical characteristics of patients with cryptococcal meningitis in Hokkaido: A case series. Intern. Med. 2024, 63, 1281–1287. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for Diagnosing, Preventing and Managing Cryptococcal Disease Among Adults, Adolescents and Children Living with HIV; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240052178 (accessed on 1 April 2025).
- Aguiar, T.K.; Costa, A.C.; Neto, N.A.; Brito, D.M.; Freitas, C.D.; Neto, J.M.; Mesquita, F.P.; Souza, P.F. Rise and fall of caspofungin: The current status of caspofungin as a treatment for Cryptococcus neoformans infection. Future Microbiol. 2024, 19, 621–630. [Google Scholar] [CrossRef]
- Riera, F.; Caeiro, J.P.; Cornely, O.A.; Salmanton-García, J.; Argentinian IFI Diagnostic and Treatment Capacity Group. The Argentinian landscape of mycological diagnostic capacity and treatment accessibility. Med. Mycol. 2023, 61, myad058. [Google Scholar] [CrossRef]
- Abdel-Hafez, Y.; Siaj, H.; Janajri, M.; Abu-Baker, Y.; Nazzal, Z.; Hamdan, Z.; Adwan, R.; Aiesh, B.M.; Anaya, A.I. Tolerability and epidemiology of nephrotoxicity associated with conventional amphotericin B therapy: A retrospective study in tertiary care centers in Palestine. BMC Nephrol. 2022, 23, 132. [Google Scholar] [CrossRef]
- Rakhshan, A.; Rahmati Kamel, B.; Saffaei, A.; Tavakoli-Ardakani, M. Hepatotoxicity induced by azole antifungal agents: A review study. Iran. J. Pharm. Res. 2023, 22, e130336. [Google Scholar] [CrossRef] [PubMed]
- Drakulovski, P.; Krasteva, D.; Bellet, V.; Randazzo, S.; Roger, F.; Pottier, C.; Bertout, S. Exposure of Cryptococcus neoformans to seven commonly used agricultural azole fungicides induces resistance to fluconazole as well as cross-resistance to voriconazole, posaconazole, itraconazole and isavuconazole. Pathogens 2023, 12, 662. [Google Scholar] [CrossRef] [PubMed]
- Melhem, M.S.C.; Leite Júnior, D.P.; Takahashi, J.P.F.; Macioni, M.B.; Oliveira, L.; de Araújo, L.S.; Fava, W.S.; Bonfietti, L.X.; Paniago, A.M.M.; Venturini, J.; et al. Antifungal resistance in cryptococcal infections. Pathogens 2024, 13, 128. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240060241 (accessed on 1 April 2025).
- Dennis, E.K.; Kim, J.H.; Parkin, S.; Awuah, S.G.; Garneau-Tsodikova, S. Distorted gold(I)-phosphine complexes as antifungal agents. J. Med. Chem. 2020, 63, 2455–2469. [Google Scholar] [CrossRef]
- Gandra, R.M.; McCarron, P.; Viganor, L.; Fernandes, M.F.; Kavanagh, K.; McCann, M.; Branquinha, M.H.; Santos, A.L.S.; Howe, O.; Devereux, M. In vivo activity of copper(II), manganese(II), and silver(I) 1,10-phenanthroline chelates against Candida haemulonii using the Galleria mellonella model. Front. Microbiol. 2020, 11, 470. [Google Scholar] [CrossRef]
- Mello, T.P.; Aor, A.C.; Barcellos, I.C.; Pereira, M.M.; McCann, M.; Devereux, M.; Branquinha, M.H.; Santos, A.L.S. Active Cu(II), Mn(II) and Ag(I) 1,10-phenanthroline/1,10-phenanthroline-5,6-dione/dicarboxylate chelates: Effects on Scedosporium. Future Microbiol. 2023, 18, 1049–1059. [Google Scholar] [CrossRef]
- Frota, H.F.; Lorentino, C.M.A.; Barbosa, P.F.; Ramos, L.S.; Barcellos, I.C.; Giovanini, L.; Souza, L.O.P.; Oliveira, S.S.C.; Abosede, O.O.; Ogunlaja, A.S.; et al. Antifungal potential of the new copper(II)-theophylline/1,10-phenanthroline complex against drug-resistant Candida species. Biometals 2024, 37, 321–336. [Google Scholar] [CrossRef]
- Eshwika, A.; Coyle, B.; Devereux, M.; McCann, M.; Kavanagh, K. Metal complexes of 1,10-phenanthroline-5,6-dione alter the susceptibility of the yeast Candida albicans to amphotericin B and miconazole. Biometals 2004, 17, 415–422. [Google Scholar] [CrossRef]
- McCann, M.; Coyle, B.; McKay, S.; McCormack, P.; Kavanagh, K.; Devereux, M.; McKee, V.; Kinsella, P.; O’Connor, R.; Clynes, M. Synthesis and X-ray crystal structure of [Ag(phendio)2]ClO4 (phendio = 1,10-phenanthroline-5,6-dione) and its effects on fungal and mammalian cells. Biometals 2004, 17, 635–645. [Google Scholar] [CrossRef]
- Gandra, R.M.; McCarron, P.; Fernandes, M.F.; Ramos, L.S.; Mello, T.P.; Aor, A.C.; Branquinha, M.H.; McCann, M.; Devereux, M.; Santos, A.L.S. Antifungal potential of copper(II), manganese(II) and silver(I) 1,10-phenanthroline chelates against multidrug-resistant fungal species forming the Candida haemulonii complex: Impact on the planktonic and biofilm lifestyles. Front. Microbiol. 2017, 8, 1257. [Google Scholar] [CrossRef]
- Gandra, R.M.; Pacheco, C.A.; Sangenito, L.S.; Ramos, L.S.; Souza, L.O.; McCarron, P.; McCann, M.; Devereux, M.; Branquinha, M.H.; Santos, A.L.S. Manganese(II), copper(II) and silver(I) complexes containing 1,10-phenanthroline/1,10-phenanthroline-5,6-dione against Candida species. Future Microbiol. 2024, 19, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Granato, M.Q.; Gonçalves, D.S.; Seabra, S.H.; McCann, M.; Devereux, M.; Santos, A.L.S.; Kneipp, L.F. 1,10-phenanthroline-5,6-dione-based compounds are effective in disturbing crucial physiological events of Phialophora verrucosa. Front. Microbiol. 2017, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Granato, M.Q.; Mello, T.P.; Nascimento, R.S.; Pereira, M.D.; Rosa, T.L.S.A.; Pessolani, M.C.V.; McCann, M.; Devereux, M.; Branquinha, M.H.; Santos, A.L.S.; et al. Silver(I) and copper(II) complexes of 1,10-phenanthroline-5,6-dione against Phialophora verrucosa: A focus on the interaction with human macrophages and Galleria mellonella larvae. Front. Microbiol. 2021, 12, 641258. [Google Scholar] [CrossRef] [PubMed]
- Mello, T.P.; Silva, B.A.; Lione, V.; Devereux, M.; McCann, M.; Branquinha, M.H.; Santos, A.L.S. Impact of copper(II) and silver(I) complexes containing 1,10-phenanthroline-5,6-dione on cellular and virulence aspects of Scedosporium apiospermum. Curr. Top. Med. Chem. 2025, 25, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.; Santos, A.L.S.; Silva, B.A.; Romanos, M.T.V.; Pyrrho, A.S.; Devereux, M.; Kavanagh, K.; Fichtner, I.; Kellett, A. In vitro and in vivo studies into the biological activities of 1,10-phenanthroline, 1,10-phenanthroline-5,6-dione and its copper(II) and silver(I) complexes. Toxicol. Res. 2012, 1, 47–54. [Google Scholar] [CrossRef]
- Santos, A.L.S.; Lima, A.K.C.; Oliveira, S.S.C.; dos Santos, R.F.; Devereux, M.; McCann, M.; Branquinha, M.H.; Dutra, P.M.L. Decoding the anti-Leishmania braziliensis activity of 1,10-phenanthroline-5,6-dione and its silver- and copper-based complexes: In vitro and in vivo approaches. Eur. J. Med. Chem. Rep. 2022, 6, 100093. [Google Scholar] [CrossRef]
- Reis, F.C.G.; Borges, B.S.; Jozefowicz, L.J.; Sena, B.A.G.; Garcia, A.W.A.; Medeiros, L.C.; Martins, S.T.; Honorato, L.; Schrank, A.; Vainstein, M.H.; et al. A novel protocol for the isolation of fungal extracellular vesicles reveals the participation of a putative scramblase in polysaccharide export and capsule construction in Cryptococcus gattii. mSphere 2019, 4, e00080-19. [Google Scholar] [CrossRef]
- Cirino, M.E.; Teixeira, T.R.; da Silva, A.M.H.; Borges, A.C.C.; Fukui-Silva, L.; Wagner, L.G.; Fernandes, C.; McCann, M.; Santos, A.L.S.; de Moraes, J. Anthelmintic activity of 1,10-phenanthroline-5,6-dione-based metallodrugs. Sci. Rep. 2025, 15, 4699. [Google Scholar] [CrossRef]
- CLSI Standard M27; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 4th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017.
- CLSI Supplement M59; Epidemiological Cutoff Values for Antifungal Susceptibility Testing, 3rd ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020.
- Frota, H.F.; Barbosa, P.F.; Lorentino, C.M.A.; Affonso, L.R.F.; Ramos, L.S.; Oliveira, S.S.C.; Souza, L.O.P.; Abosede, O.O.; Ogunlaja, A.S.; Branquinha, M.H.; et al. Unveiling the antifungal mechanisms of CTP, a new copper(II)-theophylline/1,10-phenanthroline complex, on drug-resistant non-albicans Candida species. Biometals 2024, 37, 1237–1253. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M., Jr.; Baldwin, J.J. Prediction of drug absorption using multivariate statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple selection criteria for drug-like chemical matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef]
- Evans, B.C.; Nelson, C.E.; Yu, S.S.; Beavers, K.R.; Kim, A.J.; Li, H.; Nelson, H.M.; Giorgio, T.D.; Duvall, C.L. Ex vivo red blood cell hemolysis assay for the evaluation of pH-responsive endosomolytic agents for cytosolic delivery of biomacromolecular drugs. J. Vis. Exp. 2013, 73, e50166. [Google Scholar] [CrossRef]
- Silva, L.N.; Campos-Silva, R.; Ramos, L.S.; Trentin, D.S.; Macedo, A.J.; Branquinha, M.H.; Santos, A.L.S. Virulence of Candida haemulonii complex in Galleria mellonella and efficacy of classical antifungal drugs: A comparative study with other clinically relevant non-albicans Candida species. FEMS Yeast Res. 2018, 18, foy082. [Google Scholar] [CrossRef]
- Guengerich, F.P. Cytochrome P450s and other enzymes in drug metabolism and toxicity. AAPS J. 2006, 8, E101–E111. [Google Scholar] [CrossRef]
- Serrano, I.; Verdial, C.; Tavares, L.; Oliveira, M. The virtuous Galleria mellonella model for scientific experimentation. Antibiotics 2023, 12, 505. [Google Scholar] [CrossRef]
- Ikuta, K.S.; Meštrović, T.; Naghavi, M. Global incidence and mortality of severe fungal disease. Lancet Infect Dis. 2024, 24, e268. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.W. History of the medical use of silver. Surg. Infect 2009, 10, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Barbasz, A.; Oćwieja, M.; Walas, S. Toxicological effects of three types of silver nanoparticles and their salt precursors acting on human U-937 and HL-60 cells. Toxicol. Mech. Methods 2017, 27, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Żyro, D.; Sikora, J.; Szynkowska-Jóźwik, M.I.; Ochocki, J. Silver, its salts and application in medicine and pharmacy. Int. J. Mol. Sci. 2023, 24, 15723. [Google Scholar] [CrossRef]
- Padhye, L.P.; Jasemizad, T.; Bolan, S.; Tsyusko, O.V.; Unrine, J.M.; Biswal, B.K.; Balasubramanian, R.; Zhang, Y.; Zhang, T.; Zhao, J.; et al. Silver contamination and its toxicity and risk management in terrestrial and aquatic ecosystems. Sci. Total Environ. 2023, 871, 161926. [Google Scholar] [CrossRef]
- Ciardulli, M.C.; Mariconda, A.; Sirignano, M.; Lamparelli, E.P.; Longo, R.; Scala, P.; D’Auria, R.; Santoro, A.; Guadagno, L.; Della Porta, G.; et al. Activity and selectivity of novel chemical metallic complexes with potential anticancer effects on melanoma cells. Molecules 2023, 28, 4851. [Google Scholar] [CrossRef]
- Fabijańska, M.; Rybarczyk-Pirek, A.J.; Dominikowska, J.; Stryjska, K.; Żyro, D.; Markowicz-Piasecka, M.; Szynkowska-Jóźwik, M.I.; Ochocki, J.; Sikora, J. Silver complexes of miconazole and metronidazole: Potential candidates for melanoma treatment. Int. J. Mol. Sci. 2024, 25, 5081. [Google Scholar] [CrossRef]
- Mahmoud, A.G.; Sousa, S.A.; Guedes da Silva, M.F.C.; Martins, L.M.D.R.S.; Leitão, J.H. Antimicrobial activity of water-soluble silver complexes bearing c-scorpionate ligands. Antibiotics 2024, 13, 647. [Google Scholar] [CrossRef]
- Kongot, M.; Reddy, D.S.; Singh, V.; Patel, R.; Singhal, N.K.; Kumar, A. Physicochemical, in vitro therapeutic activity and biomolecular interaction studies of Mn(II), Ni(II) and Cu(II) complexes tethered with O2N2 ligand backbone. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 241, 118613. [Google Scholar] [CrossRef]
- Sinha, A.; Chaudhary, R.; Reddy, D.S.; Kongot, M.; Kurjogi, M.M.; Kumar, A. ON donor tethered copper (II) and vanadium (V) complexes as efficacious anti-TB and antifungal agents with spectroscopic approached HSA interactions. Heliyon 2022, 8, e10125. [Google Scholar] [CrossRef]
- Frei, A.; King, A.P.; Lowe, G.J.; Cain, A.K.; Short, F.L.; Dinh, H.; Elliott, A.G.; Zuegg, J.; Wilson, J.J.; Blaskovich, M.A.T. Nontoxic cobalt(III) schiff base complexes with broad-spectrum antifungal activity. Chem. Eur. J. 2021, 27, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.M.; Radacki, K. Antimicrobial properties of half-sandwich Ir(III) cyclopentadienyl complexes with pyridylbenzimidazole ligands. Dalton. Trans. 2020, 49, 4491–4501. [Google Scholar] [CrossRef] [PubMed]
- Mansour, A.M.; Radacki, K. Experimental and DFT studies of sulfadiazine ‘piano-stool’ Ru(II) and Rh(III) complexes. RSC Adv. 2020, 10, 10673–10680. [Google Scholar] [CrossRef]
- Donnici, C.L.; Nogueira, L.J.; Araujo, M.H.; Oliveira, S.R.; Magalhães, T.F.; Lopes, M.T.; Araújo e Silva, A.C.; Ferreira, A.M.; Martins, C.V.; de Resende Stoianoff, M.A. In vitro studies of the activity of Dithiocarbamate organoruthenium complexes against clinically relevant fungal pathogens. Molecules 2014, 19, 5402–5420. [Google Scholar] [CrossRef]
- Agh-Atabay, N.M.; Dulger, B.; Gucin, F. Structural characterization and antimicrobial activity of 1,3-bis(2-benzimidazyl)-2-thiapropane ligand and its Pd(II) and Zn(II) halide complexes. Eur. J. Med. Chem. 2005, 40, 1096–1102. [Google Scholar] [CrossRef]
- Fondjo, E.S.; Siéwé, D.A.; Tamokou, J.D.; Ekom, S.E.; Djeukoua, S.K.D.; Doungmo, G.; Walters, M.E.; Tsopmo, A.; Simon, P.F.W.; Kuiate, J.R. Room temperature synthesis and characterization of novel Bi(III) complex with 2-amino-3-carbomethoxy-4,5,6,7-tetrahydrobenzo[B]thiophene as potential antimicrobial agent. Acta Chim. Slov. 2020, 67, 203–211. [Google Scholar] [CrossRef]
- Kuchar, J.; Rust, J.; Lehmann, C.W.; Mohr, F. Silver(I) complexes with camphorsulfonato and phosphine ligands: Structural diversity and antibacterial activity. Inorg. Chem. 2020, 59, 10557–10568. [Google Scholar] [CrossRef]
- Quiles-Melero, I.; García-Rodríguez, J. Antifúngicos de uso sistémico [Systemic antifungal drugs]. Rev. Iberoam. Micol. 2021, 38, 42–46. [Google Scholar] [CrossRef]
- Di, L.; Kerns, E.H.; Carter, G.T. Drug-like property concepts in pharmaceutical design. Curr. Pharm. Des. 2009, 15, 2184–2194. [Google Scholar] [CrossRef]
- Silva, V.; Gil-Martins, E.; Silva, B.; Rocha-Pereira, C.; Sousa, M.E.; Remião, F.; Silva, R. Xanthones as P-glycoprotein modulators and their impact on drug bioavailability. Expert Opin. Drug Metab. Toxicol. 2021, 17, 441–482. [Google Scholar] [CrossRef]
- Rossato, L.; Loreto, É.S.; Zanette, R.A.; Chassot, F.; Santurio, J.M.; Alves, S.H. In vitro synergistic effects of chlorpromazine and sertraline in combination with amphotericin B against Cryptococcus neoformans var. grubii. Folia Microbiol. 2016, 61, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Iyer, K.R.; Revie, N.M.; Fu, C.; Robbins, N.; Cowen, L.E. Treatment strategies for cryptococcal infection: Challenges, advances and future outlook. Nat. Rev. Microbiol 2021, 19, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.Y.; Zhu, H.M.; Wu, J.H.; Wen, H.; Liu, C.J. Increased permeability of blood-brain barrier is mediated by serine protease during Cryptococcus meningitis. J. Int. Med. Res. 2014, 42, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.H.; Martinez, L.R. Cryptococcus neoformans-astrocyte interactions: Effect on fungal blood brain barrier disruption, brain invasion, and meningitis progression. Crit. Rev. Microbiol. 2021, 47, 206–223. [Google Scholar] [CrossRef]
- Martignoni, M.; Groothuis, G.M.; de Kanter, R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin. Drug Metab. Toxicol. 2006, 2, 875–894. [Google Scholar] [CrossRef]
- Albadry, M.; Küttner, J.; Grzegorzewski, J.; Dirsch, O.; Kindler, E.; Klopfleisch, R.; Liska, V.; Moulisova, V.; Nickel, S.; Palek, R.; et al. Cross-species variability in lobular geometry and cytochrome P450 hepatic zonation: Insights into CYP1A2, CYP2D6, CYP2E1 and CYP3A4. Front. Pharmacol. 2024, 15, 1404938. [Google Scholar] [CrossRef]
- Qian, J.; Guo, Y.; Xu, Y.; Wang, X.; Chen, J.; Wu, X. Combination of micelles and liposomes as a promising drug delivery system: A review. Drug Deliv. Transl. Res. 2023, 13, 2767–2789. [Google Scholar] [CrossRef]
- Lubran, M.M. Hematologic side effects of drugs. Ann. Clin. Lab. Sci. 1989, 19, 114–121. [Google Scholar]
- Singh, U.; Dar, M.M.; Anayutullah, S.; Alam, H.; Manzoor, N.; Al-Thabaiti, S.A.; Hashmi, A.A. Design and synthesis of Co(II) and Cu(II) complexes of a dendrimeric chelate: Promising anticandidal potential of chelotherapeutic agents. J. Coord. Chem. 2015, 68, 2096–2106. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M. Erythrocytes as a biological model for screening of xenobiotics toxicity. Chem. Biol. Interact. 2018, 279, 73–83. [Google Scholar] [CrossRef]
- Marin, M.; Fernández, A.; Sánchez-Yagüe, J.; Cabezas, J.; Llanillo, M. Changes in the phospholipid and fatty acid composition in normal erythrocytes from sheep of different ages. Aminophospholipid organization in the membrane bilayer. Biochimie 1990, 72, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.; Williams, P.; Moore, G. An evaluation of the dorset sheep as a predictive animal model for the response of glucose-6-phosphate dehydrogenase-deficient human erythrocytes to a proposed systemic toxic ozone intermediate, methyl oleate ozonide. Ecotoxicol. Environ. Saf. 1983, 7, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Mikulak, E.; Gliniewicz, A.; Przygodzka, M.; Solecka, J. Galleria mellonella L. as model organism used in biomedical and other studies. Przegl. Epidemiol. 2018, 72, 57–73. [Google Scholar] [PubMed]
- Giammarino, A.; Bellucci, N.; Angiolella, L. Galleria mellonella as a model for the study of fungal pathogens: Advantages and disadvantages. Pathogens 2024, 13, 233. [Google Scholar] [CrossRef]
- Gallorini, M.; Marinacci, B.; Pellegrini, B.; Cataldi, A.; Dindo, M.L.; Carradori, S.; Grande, R. Immunophenotyping of hemocytes from infected Galleria mellonella larvae as an innovative tool for immune profiling, infection studies and drug screening. Sci. Rep. 2024, 14, 759. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Scorzoni, L.; Zaragoza, O. It only takes one to do many jobs: Amphotericin B as antifungal and immunomodulatory drug. Front. Microbiol. 2012, 3, 286. [Google Scholar] [CrossRef]
- Kelly, J.; Kavanagh, K. Caspofungin primes the immune response of the larvae of Galleria mellonella and induces a non-specific antimicrobial response. J. Med. Microbiol. 2011, 60, 189–196. [Google Scholar] [CrossRef]
- Smith, D.F.Q.; Casadevall, A. Fungal immunity and pathogenesis in mammals versus the invertebrate model organism Galleria mellonella. Pathog. Dis. 2021, 79, ftab013. [Google Scholar] [CrossRef]
- Hurlow, J.; Bowler, P.G. Acute and chronic wound infections: Microbiological, immunological, clinical and therapeutic distinctions. J. Wound Care 2022, 31, 436–445. [Google Scholar] [CrossRef]
- Stielow, M.; Witczyńska, A.; Kubryń, N.; Fijałkowski, Ł.; Nowaczyk, J.; Nowaczyk, A. The bioavailability of drugs—The current state of knowledge. Molecules 2023, 28, 8038. [Google Scholar] [CrossRef]
- Louis-Maerten, E.; Rodriguez Perez, C.; Cajiga, R.M.; Persson, K.; Elger, B.S. Conceptual foundations for a clarified meaning of the 3Rs principles in animal experimentation. Anim. Welf. 2024, 33, e37. [Google Scholar] [CrossRef]
| Cryptococcus Species | Isolate | MIC, [µg/mL] | ||||
|---|---|---|---|---|---|---|
| Amphotericin B | Caspofungin | Fluconazole | Flucytosine | Voriconazole | ||
| C. neoformans | 213 | 0.03 (0.03 µM) | >8 (>7.30 µM) | 4 (13 µM) | 4 (30 µM) | 0.5 (1.4 µM) * |
| 223 | >16 (>17.30 µM) * | >8 (>7.30 µM) | >64 (>208 µM) * | 64 (495.76 µM) * | 16 (45.80 µM) * | |
| 350 | 0.03 (0.03 µM) | >8 (>7.30 µM) | 8 (26.12 µM) | 16 (123.94 µM) * | 2 (5.72 µM) * | |
| C. gattii | 27 | 0.03 (0.03 µM) | >8 (>7.30 µM) | 16 (52.24 µM) | 4 (30 µM) | 4 (11.45 µM) * |
| 25 | 0.03 (0.03 µM) | >8 (>7.30 µM) | 16 (52.24 µM) | 4 (30 µM) | 2 (5.72 µM) * | |
| 23 | 0.03 (0.03 µM) | >8 (>7.30 µM) | 32 (104.48 µM) * | 0.125 (0.96 µM) | 4 (11.45 µM) * | |
| Cryptococcus Species | Isolate | MIC/MFC, [µM] | ||||
|---|---|---|---|---|---|---|
| Phendione | Cu-Phendione | Ag-Phendione | AgClO4 | Cu(ClO4)2.6H2O | ||
| C. neoformans | 213 | 12.5/>100 | 3.125/50 | 1.56/25 | 1.56/>100 | >100/ND |
| 223 | 12.5/100 | 3.125/50 | 1.56/25 | 1.56/>100 | >100/ND | |
| 350 | 12.5/100 | 3.125/50 | 1.56/25 | 1.56/>100 | >100/ND | |
| C. gattii | 27 | 12.5/>100 | 6.25/12.5 | 1.56/12.5 | 1.56/12.5 | >100/ND |
| 25 | 12.5/100 | 6.25/12.5 | 1.56/12.5 | 1.56/12.5 | >100/ND | |
| 23 | 12.5/>100 | 6.25/12.5 | 1.56/12.5 | 1.56/12.5 | >100/ND | |
| Cryptococcus Species | Isolate | MIC, [µM] | |
|---|---|---|---|
| Cu-Phendione | Ag-Phendione | ||
| C. neoformans | ATCC 28957 | 1.56 | 0.19 |
| H99 | 0.78 | 0.78 | |
| T1444 | 0.78 | 0.78 | |
| C. gattii | R265 | 0.19 | 0.19 |
| Filters | Number of Violations | |||
|---|---|---|---|---|
| Ag-Phendione | Cu-Phendione | Amphotericin B | Fluconazole | |
| Lipinski | 1 | 2 | 3 | 0 |
| Ghose | 1 | 2 | 3 | 0 |
| Veber | 0 | 0 | 1 | 0 |
| Egan | 0 | 1 | 1 | 0 |
| Muegge | 1 | 2 | 4 | 0 |
| Pharmacokinetic Parameters | Test Compounds | |||
|---|---|---|---|---|
| Ag-Phendione | Cu-Phendione | Amphotericin B | Fluconazole | |
| Inhibition of CYP1A2 | Yes | No | No | No |
| Inhibition of CYP2C19 | Yes | No | No | Yes |
| Inhibition of CYP2C9 | Yes | No | No | No |
| Inhibition of CYP2D6 | No | No | No | No |
| Inhibition of CYP3A4 | No | No | No | No |
| Gastrointestinal absorption | High | High | Low | High |
| Blood-brain barrier permeability | No | No | No | No |
| P-glycoprotein substrate | Yes | Yes | Yes | Yes |
| Log Kp (cm/s) | −7.97 | −8.21 | −11.94 | −7.92 |
| Cryptococcus Species | Cu-Phendione | Ag-Phendione | ||
|---|---|---|---|---|
| Erythrocytes | G. mellonella Larvae | Erythrocytes | G. mellonella Larvae | |
| C. gattii | >10 | >160 | >40 | >641 |
| C. neoformans | >20 | >320 | >40 | >641 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovanini, L.; Casemiro, A.L.; Corrêa, L.S.; Mendes, M.; Mello, T.P.; Souza, L.O.P.; Wagner, L.G.; Fernandes, C.; Pereira, M.M.; de Souza, L.C.S.V.; et al. Toxicity Assessment and Antifungal Potential of Copper(II) and Silver(I) Complexes with 1,10-Phenanthroline-5,6-dione Against Drug-Resistant Clinical Isolates of Cryptococcus gattii and Cryptococcus neoformans. J. Fungi 2025, 11, 436. https://doi.org/10.3390/jof11060436
Giovanini L, Casemiro AL, Corrêa LS, Mendes M, Mello TP, Souza LOP, Wagner LG, Fernandes C, Pereira MM, de Souza LCSV, et al. Toxicity Assessment and Antifungal Potential of Copper(II) and Silver(I) Complexes with 1,10-Phenanthroline-5,6-dione Against Drug-Resistant Clinical Isolates of Cryptococcus gattii and Cryptococcus neoformans. Journal of Fungi. 2025; 11(6):436. https://doi.org/10.3390/jof11060436
Chicago/Turabian StyleGiovanini, Lucas, Ana Lucia Casemiro, Larissa S. Corrêa, Matheus Mendes, Thaís P. Mello, Lucieri O. P. Souza, Luis Gabriel Wagner, Christiane Fernandes, Matheus M. Pereira, Lais C. S. V. de Souza, and et al. 2025. "Toxicity Assessment and Antifungal Potential of Copper(II) and Silver(I) Complexes with 1,10-Phenanthroline-5,6-dione Against Drug-Resistant Clinical Isolates of Cryptococcus gattii and Cryptococcus neoformans" Journal of Fungi 11, no. 6: 436. https://doi.org/10.3390/jof11060436
APA StyleGiovanini, L., Casemiro, A. L., Corrêa, L. S., Mendes, M., Mello, T. P., Souza, L. O. P., Wagner, L. G., Fernandes, C., Pereira, M. M., de Souza, L. C. S. V., Baptista, A. R. S., de Moraes, J., McCann, M., Branquinha, M. H., & Santos, A. L. S. (2025). Toxicity Assessment and Antifungal Potential of Copper(II) and Silver(I) Complexes with 1,10-Phenanthroline-5,6-dione Against Drug-Resistant Clinical Isolates of Cryptococcus gattii and Cryptococcus neoformans. Journal of Fungi, 11(6), 436. https://doi.org/10.3390/jof11060436










