Trichoderma harzianum in Biocontrol of Maize Fungal Diseases and Relevant Mycotoxins: From the Laboratory to the Field
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Material
2.2. Bioreactor Experiment
2.3. In Vitro Experiment
2.4. The Field Trial
2.5. Mycotoxins Detection
2.6. Data Analysis
3. Results
3.1. In Vitro Biocontrol Experiment
3.2. Field Experiment
3.2.1. Disease Severity
3.2.2. Grain Yield
3.3. Mycotoxins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahzad, R.; Khan, A.L.; Bilal, S.; Asaf, S.; Lee, I.-J. What Is There in Seeds? Vertically Transmitted Endophytic Resources for Sustainable Improvement in Plant Growth. Front. Plant Sci. 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, R.A.; Rosentrater, K.A.; Bern, C.J. Effects of deterioration parameters on storage of maize. In Proceedings of the Agricultural and Biological Engineers, Kansas City, MO, USA, 21–24 July 2013; p. 1. [Google Scholar]
- Logrieco, A.; Mule, G.; Moretti, A.; Bottalico, A. Toxigenic Fusarium species and mycotoxins associated with maize ear rot in Europe. Eur. J. Plant Pathol. 2002, 108, 597–609. [Google Scholar] [CrossRef]
- Thompson, M.E.; Raizada, M.N. Fungal pathogens of maize gaining free passage along the silk road. Pathogens 2018, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Szabo, B.; Toth, B.; Toth Toldine, E.; Varga, M.; Kovacs, N.; Varga, J.; Kocsube, S.; Palagyi, A.; Bagi, F.; Budakov, D.; et al. A New Concept to Secure Food Safety Standards against Fusarium Species and Aspergillus flavus and Their Toxins in Maize. Toxins 2018, 10, 372. [Google Scholar] [CrossRef]
- Masiello, M.; Somma, S.; Ghionna, V.; Logrieco, A.F.; Moretti, A. In Vitro and in Field Response of Different Fungicides against Aspergillus flavus and Fusarium Species Causing Ear Rot Disease of Maize. Toxins 2019, 11, 11. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Z.; Lu, P.; Li, R.; Ma, P.; Wu, J.; Li, T.; Zhang, H. Increasing Fusarium verticillioides resistance in maize by genomics-assisted breeding: Methods, progress, and prospects. Crop J. 2023, 11, 1626–1641. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on the Risks for Public Health Related to the Presence of Fumonisins in Food. EFSA J. 2013, 11, 3443. [Google Scholar]
- Sabaly, S.; Tine, Y.; Diallo, A.; Faye, A.; Cisse, M.; Ndiaye, A.; Sambou, C.; Gaye, C.; Wele, A.; Paolini, J.; et al. Antifungal Activity of Cyperus articulatus, Cyperus rotundus and Lippia alba Essential Oils against Aspergillus flavus Isolated from Peanut Seeds. J. Fungi 2024, 10, 591. [Google Scholar] [CrossRef]
- Baranyi, N.; Kocsubé, S.; Vágvölgyi, C.; Varga, J. Current trends in aflatoxin research. Acta Biol. Szeged. 2013, 57, 95–107. [Google Scholar]
- Soares, C.; Rodrigues, P.; Peterson, S.W.; Lima, N.; Venacio, A. Three new species of Aspergillus section Flavi isolated from almonds and maize in Portugal. Mycologia 2012, 104, 682–697. [Google Scholar] [CrossRef]
- Stanković, S.; Lević, J.; Nikolić, M.; Krnjaja, V.; Jauković, M. Prvi Nalaz Aspergillus parasiticus u Proizvodnji Kukuruza u Srbiji. In Proceedings of the XIII Savetovanje o Zaštiti Bilja, Zlatibor, Serbia, 23–27 October 2015; pp. 32–33. [Google Scholar]
- Nikolić, M.; Savić, I.; Nikolić, A.; Stanković, G.; Delić, N.; Mladenović-Drinić, S.; Stanković, S. Maize resistance to ear rot caused by Aspergillus parasiticus. Genetika 2019, 51, 357–363. [Google Scholar] [CrossRef]
- Lević, J.; Gošić-Dondo, S.; Ivanović, D.; Stanković, S.; Krnjaja, V.; Bočarov-Stančić, A.; Stepanić, A. An Outbreak of Aspergillus Species in Response to Environmental Conditions in Serbia. Pestic. Phytomed. 2013, 28, 167–179. [Google Scholar] [CrossRef]
- Calvo, A.M.; Díez, A.J. Aspergillus species and their aflatoxins in maize production. Eur. J. Plant Pathol. 2009, 123, 343–353. [Google Scholar]
- European Food Safety Authority (EFSA). Scientific Opinion on the risks for public health related to the presence of aflatoxins in food. EFSA J. 2016, 14, 4595. [Google Scholar]
- Mielniczuk, E.; Skwaryło-Bednarz, B. Fusarium Head Blight, Mycotoxins and Strategies for Their Reduction. Agronomy 2020, 10, 509. [Google Scholar] [CrossRef]
- Ji, F.; He, D.; Olaniran, A.O.; Mokoena, M.P.; Xu, J.; Shi, J. Occurrence, toxicity, production and detection of Fusarium mycotoxin: A review. Food Prod. Process. Nutr. 2019, 1, 6. [Google Scholar] [CrossRef]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A Global Concern for Food Safety, Human Health and Their Management. Front. Microbiol. 2017, 7, 2170. [Google Scholar] [CrossRef]
- Akinola, S.A.; Ateba, C.N.; Mwanza, M. Behaviour of Aspergillus parasiticus in aflatoxin production as influenced by storage parameters using response surface methodology approach. Int. J. Food Microbiol. 2021, 357, 109369. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Liu, F.; Wang, Q.; Selvaraj, J.N.; Xing, F.; Zhao, Y.; Liu, Y. Ochratoxin A Producing Fungi, Biosynthetic Pathway and Regulatory Mechanisms. Toxins 2016, 8, 83. [Google Scholar] [CrossRef]
- Bily, A.C.; Reid, L.M.; Savard, M.E.; Reddy, R.; Blackwell, B.A.; Campbell, C.M.; Krantis, A.; Durst, T.; Philogene, B.J.R.; Arnason, J.T.; et al. Analysis of Fusarium graminearum mycotoxins in different biological matrices by LC/MS. Mycopathologia 2004, 157, 117–126. [Google Scholar] [CrossRef]
- Rocha, O.; Ansari, K.; Doohan, F.M. Effects of trichothecene mycotoxins on eukaryotic cells: A review. Food Addit. Contam. 2005, 22, 369–378. [Google Scholar] [CrossRef]
- Kamle, M.; Mahato, D.K.; Devi, S.; Eun Lee, K.; Kang, S.G.; Kumar, P. Fumonisins: Impact on Agriculture, Food, and Human Health and their Management Strategies. Toxins 2019, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- Stanković, S.; Lević, J.; Krnjaja, V. Fumonisin b1 in maize, wheat and barley grain in Serbia. Biotechnol. Anim. Husb. 2011, 27, 631–641. [Google Scholar] [CrossRef]
- Terciolo, C.; Bracarense, A.P.; Souto, P.C.M.C.; Cossalter, A.M.; Dopavogui, L.; Loiseau, N.; Oliveira, C.A.F.; Pinton, P.; Oswald, I.P. Fumonisins at Doses below EU Regulatory Limits Induce Histological Alterations in Piglets. Toxins 2019, 11, 548. [Google Scholar] [CrossRef]
- Kimanya, M.E.; Routledge, M.N.; Mpolya, E.; Ezekiel, C.N.; Shirima, C.P.; Gong, Y.Y. Estimating the risk of aflatoxin-induced liver cancer in Tanzania based on biomarker data. PLoS ONE 2021, 16, e0247281. [Google Scholar] [CrossRef] [PubMed]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological Properties and Their Involvement in Cancer Development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef]
- Mahato, D.K.; Lee, K.E.; Kamle, M.; Devi, S.; Dewangan, K.N.; Kumar, P.; Kang, S.G. Aflatoxins in Food and Feed: An Overview on Prevalence, Detection and Control Strategies. Front. Microbiol. 2019, 10, 2266. [Google Scholar] [CrossRef]
- Battilani, P.; Toscano, P.; Van der Fels-Klerx, H.J.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef]
- Mitrović, I.; Grahovac, J.; Hrustić, J.; Jokić, A.; Dodić, J.; Mihajlović, M.; Grahovac, M. Utilization of waste glycerol for the production of biocontrol agents nigericin and niphimycin by Streptomyces hygroscopicus: Bioprocess development. Environ. Technol. 2021, 43, 3000–3013. [Google Scholar] [CrossRef]
- Silva, R.N.; Neves Monteiro, V.; Stecca Steindorff, A.; Vieira Gomes, E.; Ferreira Noronha, E.; Ulhoa, C.J. Trichoderma/pathogen/plant interaction in pre-harvest food security. Fungal Biol. 2019, 123, 565–583. [Google Scholar] [CrossRef]
- Harman, G.E. Myths and dogmas of biocontrol. Changes in perceptions derived from research on Trichoderma harzianum T-22. Plant Dis. 2000, 84, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Shoresh, M.; Mastouri, F.; Harman, G.E. Induced systemic resistance and plant responses to fungal biocontrol agents. Annu. Rev. Phytopathol. 2010, 48, 21–43. [Google Scholar] [CrossRef] [PubMed]
- Harman, G.E. Trichoderma–not just for biocontrol anymore. Phytoparasitica 2011, 39, 103–108. [Google Scholar] [CrossRef]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Al-Ani, L.K.T. Bioactive Secondary Metabolites of Trichoderma spp. for Efficient Management of Phytopathogens. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms; Bahadur Singh, H., Keswani, C., Reddy, M.S., Sansinenea, E., García-Estrada, C., Eds.; Springer Nature, Singapore Pte Ltd.: Singapore, 2019; pp. 125–143. [Google Scholar]
- Tančić Živanov, S.; Jevtić, R.; Lalošević, M.; Živanov, D.; Medić Pap, S.; Župunski, V. Efficacy of Trichoderma spp. against common fungal pathogens. Rat i Povrt. 2017, 54, 104–109. [Google Scholar] [CrossRef]
- Liu, J.B.; Gilardi, G.; Gullino, M.L.; Garibaldi, A. Effectiveness of Trichoderma spp. obtained from re-used soilless substrates against Pythium ultimum on cucumber seedlings. J. Plant Dis. Prot. 2009, 116, 156–163. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma–plant–pathogen interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Tucci, M.; Ruocco, M.; de Masi, L.; de Palma, M.; Lorito, M. The beneficial effect of Trichoderma spp. on tomato is modulated by the plant genotype. Mol. Plant Pathol. 2011, 12, 341–354. [Google Scholar] [CrossRef]
- Shukla, N.; Awasthi, R.P.; Rawat, L.; Kumar, J. Seed biopriming with drought tolerant isolates of Trichoderma harzianum promote growth and drought tolerance in Triticum aestivum. Ann. Appl. Biol. 2015, 166, 171–182. [Google Scholar] [CrossRef]
- Maisuria, K.M.; Patel, S.T. Seed germinability, root and shoot length and vigour index of soybean as influenced by rhizosphere fungi. Karnataka J. Agric. Sci. 2009, 22, 1120–1122. [Google Scholar]
- Asaduzzaman, M.; Alam, M.; Islam, M.M. Effect of Trichoderma on seed germination and seedling parameters of chili. J. Sci. Found. 2010, 8, 141–150. [Google Scholar] [CrossRef]
- Fungicide Resistance Action Committee (FRAC). 2016. Resistance Tables Benz. Available online: https://www.academia.edu/25340522/FRAC_Code_List_2016_Fungicides_sorted_by_mode_of_action_including_FRAC_Code_numbering (accessed on 25 May 2025).
- Lamichhane, J.; You, M.; Laudinot, V.; Barbetti, M.; Aubertot, J. Revisiting Sustainability of Fungicide Seed Treatments for Field Crops. Plant Dis. 2020, 104, 610–623. [Google Scholar] [CrossRef] [PubMed]
- Leslie, J.F.; Summerell, B.A. Fusarium laboratory workshops—A recent history. Mycotoxin Res. 2006, 22, 73–74. [Google Scholar] [CrossRef]
- Samuels, G.J.; Hebbar, P.K. Trichoderma: Identification and Agricultural Application; APS, USDA: St. Paul, MI, USA, 2015.
- Mitrović, I.; Vucurović, D.; Khalil Tawfeeq Al-Ani, L.; Mitrović, B.; Bajić, B.; Dodić, S.; Tančić Živanov, S. Production of Trichoderma harzianum K179 bioagent for maize diseases control: Complete laboratory stage bioprocess development. J. Appl. Microbiol. 2023, 134, lxad115. [Google Scholar] [CrossRef] [PubMed]
- Klich, M.A. Identification of Common Aspergillus Species; Centraalbureau voor Schimmelcultures (CBS) Fungal Biodiversity Centre, Royal Netherlands Academy of Arts and Sciences, Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2002; pp. 426–432. [Google Scholar]
- Grahovac, J.; Mitrović, I.; Dodić, J.; Grahovac, M.; Rončević, Z.; Dodić, S.; Jokić, A. Biocontrol agent for apple Fusarium rot: Optimization of production by Streptomyces hygroscopicus. Zemdirbyste 2020, 107, 263–270. [Google Scholar] [CrossRef]
- Reid, L.M.; Hamilton, R.I.; Mather, D.E. Screening Maize for Resistance to Gibberella Ear Rot; Research Branch Agriculture and Agri-Food Canada: Ottawa, ON, Canada, 1996; Technical Bulleten 5E. [Google Scholar]
- López-Valenzuela, B.E.; Armenta-Bojórquez, A.D.; Hernández-Verdugo, S.; Apodaca-Sánchez, M.A.; Samaniego-Gaxiola, J.A.; Valdez-Ortiz, A. Trichoderma spp. and Bacillus spp. as growth promoters in maize (Zea mays L.). Int. J. Exp. Bot. 2019, 88, 37–46. [Google Scholar]
- Milovac, Ž.; Zorić, M.; Franeta, F.; Terzić, S.; Petrović Obradović, O.; Marjanović Jeromela, A. Analysis of oilseed rape stem weevil chemical control using a damage rating scale. Pest Manag. Sci. 2017, 73, 1962–1971. [Google Scholar] [CrossRef]
- Sangeetha, G. Biological control potential of Trichoderma harzianum Rifai and Trichoderma viride Pers. ex S. F. Gray for the management of wilt of maize caused by Fusarium verticillioides (Nirenberg). J. Biol. Control. 2009, 23, 25–30. [Google Scholar]
- Degani, O.; Dor, S. Trichoderma Biological Control to Protect Sensitive Maize Hybrids against Late Wilt Disease in the Field. J. Fungi 2021, 7, 315. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Rasera, R.; Causin, R. Trichoderma harzianum seed treatment controls Fusarium verticillioides colonization and fumonisin contamination in maize under field conditions. Crop Prot. 2014, 65, 51–56. [Google Scholar] [CrossRef]
- Mitrović, B.; Drašković, B.; Stanisavljević, D.; Perišić, M.; Čanak, P.; Mitrović, I.; Tančić-Živanov, S. Environmental modeling of interaction variance for grain yield of medium early maturity maize hybrids. Genetika 2020, 52, 367–378. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, J.; Li, J.; Jia, Y.; Wang, W.; Li, J.; Lei, Z.; Gao, M. The impact of climate change on maize production: Empirical findings and implications for sustainable agricultural development. Front. Environ. Sci. 2022, 10, 954940. [Google Scholar] [CrossRef]
- Sabillón, L.; Alvarado, J.; Leiva, A.; Mendoza, R.; Espinal, R.; Leslie, J.F.; Bianchini, A. Presence, Co-Occurrence, and Daily Intake Estimates of Aflatoxins and Fumonisins in Maize Consumed in Food-Insecure Regions of Western Honduras. Toxins 2023, 15, 559. [Google Scholar] [CrossRef]
- Yates, I.E.; Meredith, F.; Smart, W.; Bacon, C.W.; Jaworski, A.J. Trichoderma viride Suppresses Fumonisin B1 Production by Fusarium moniliforme. J. Food Prot. 1999, 62, 1326–1332. [Google Scholar] [CrossRef]
- Rojo, F.; Ferez, M.; Reynoso, M.; Torres, A.; Chulze, S. Effect of Trichoderma species on growth of Fusarium proliferatum and production of fumonisins, fusaproliferin and beauvericin. Mycotoxin Res. 2007, 23, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Branà, M.T.; Haidukowski, M.; Gallo, A.; Zhang, Q.; Logrieco, A.F.; Li, P.; Zhao, S.; Altomare, C. Potential of Trichoderma spp. for Biocontrol of Aflatoxin-Producing Aspergillus flavus. Toxins 2022, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; Alborino, V.; Lanzuise, S.; Lombardi, N.; Marra, R.; Balestrieri, A.; Ritieni, A.; Woo, S.L.; Vinale, F. Trichoderma Enzymes for Degradation of Aflatoxin B1 and Ochratoxin A. Molecules 2022, 27, 3959. [Google Scholar] [CrossRef]
- Siri-anusornsak, W.; Kolawole, O.; Mahakarnchanakul, W.; Greer, B.; Petchkongkaew, A.; Meneely, J.; Elliott, C.; Vangnai, K. The Occurrence and Co-Occurrence of Regulated, Emerging, and Masked Mycotoxins in Rice Bran and Maize from Southeast Asia. Toxins 2022, 14, 567. [Google Scholar] [CrossRef]
- Tadijan, I.; Grahovac, J.; Dodić, J.; Grahovac, M.; Dodić, S. Effect of Cultivation Time on Production of Antifungal Metabolite(s) by Streptomyces hygroscopicus in Laboratory-Scale Bioreactor. J. Phytopathol. 2016, 164, 310–317. [Google Scholar] [CrossRef]
- Chiuraise, N.; Yobo, K.S.; Laing, M.D. Seed treatment with Trichoderma harzianum strain kd formulation reduced aflatoxin contamination in groundnuts. J. Plant Dis. Prot. 2015, 122, 74–80. [Google Scholar] [CrossRef]
- Kifle, M.H.; Yobo, K.S.; Laing, M.D. Biocontrol of Aspergillus flavus in groundnut using Trichoderma harzianum stain kd. J. Plant Dis. Prot. 2017, 124, 51–56. [Google Scholar] [CrossRef]
- Tian, Y.; Tan, Y.; Yan, Z.; Liao, Y.; Chen, J.; De Boevre, M.; De Saeger, S.; Wu, A. Antagonistic and Detoxification Potentials of Trichoderma Isolates for Control of Zearalenone (ZEN) Producing Fusarium graminearum. Front. Microbiol. 2018, 8, 2710. [Google Scholar] [CrossRef]
- Rawal, R.; Scheerens, J.C.; Fenstemaker, S.M.; Francis, D.M.; Miller, S.A.; Benitez, M.-S. Novel Trichoderma Isolates Alleviate Water Deficit Stress in Susceptible Tomato Genotypes. Front. Plant Sci. 2022, 13, 869090. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Li, Y.; Yu, C.; Wang, Q.-q.; Wang, M.; Sun, J.; Gao, J.-X.; Chen, J. Effect of Trichoderma harzianum on maize rhizosphere microbiome and biocontrol of Fusarium Stalk rot. Sci. Rep. 2017, 7, 1771. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “Secrets” of a Multitalented Biocontrol Agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Republic Hydrometeorological Service of Serbia (RHMS). Available online: https://www.hidmet.gov.rs/index_eng.php (accessed on 12 July 2024).
- Kashyap, P.L.; Rai, P.; Srivastava, A.K.; Kumar, S. Trichoderma for climate resilient agriculture. World J. Microbiol. Biotechnol. 2017, 33, 155. [Google Scholar] [CrossRef]
- Chepsergon, J.; Mwamburi, L.; Kipkemboi Kassim, M. Mechanism of Drought Tolerance in Plants Using Trichoderma spp. Int. J. Sci. Res. 2012, 3, 1592–1595. [Google Scholar]
- Scudeletti, D.; Crusciol, C.A.C.; Bossolani, J.W.; Moretti, L.G.; Momesso, L.; Servaz Tubaña, B.; de Castro, S.G.Q.; De Oliveira, E.F.; Hungria, M. Trichoderma asperellum Inoculation as a Tool for Attenuating Drought Stress in Sugarcane. Front. Plant Sci. 2021, 12, 645542. [Google Scholar] [CrossRef]
- Zaidi, N.W.; Dar, M.H.; Singh, S.; Singh, U.S. Trichoderma Species as Abiotic Stress Relievers in Plants. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 515–525. [Google Scholar] [CrossRef]
- Cornejo-Ríos, K.; Osorno-Suárez, M.d.P.; Hernández-León, S.; Reyes-Santamaría, M.I.; Juárez-Díaz, J.A.; Pérez-España, V.H.; Peláez-Acero, A.; Madariaga-Navarrete, A.; Saucedo-García, M. Impact of Trichoderma asperellum on Chilling and Drought Stress in Tomato (Solanum lycopersicum). Horticulturae 2021, 7, 385. [Google Scholar] [CrossRef]
- Tyskiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Sciseł, J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef]
- Jambhulkar, P.P.; Raja, M.; Singh, B.; Katoch, S.; Kumar, S.; Sharma, P. Potential native Trichoderma strains against Fusarium verticillioides causing post flowering stalk rot in winter maize. Crop Prot. 2022, 152, 105838. [Google Scholar] [CrossRef]


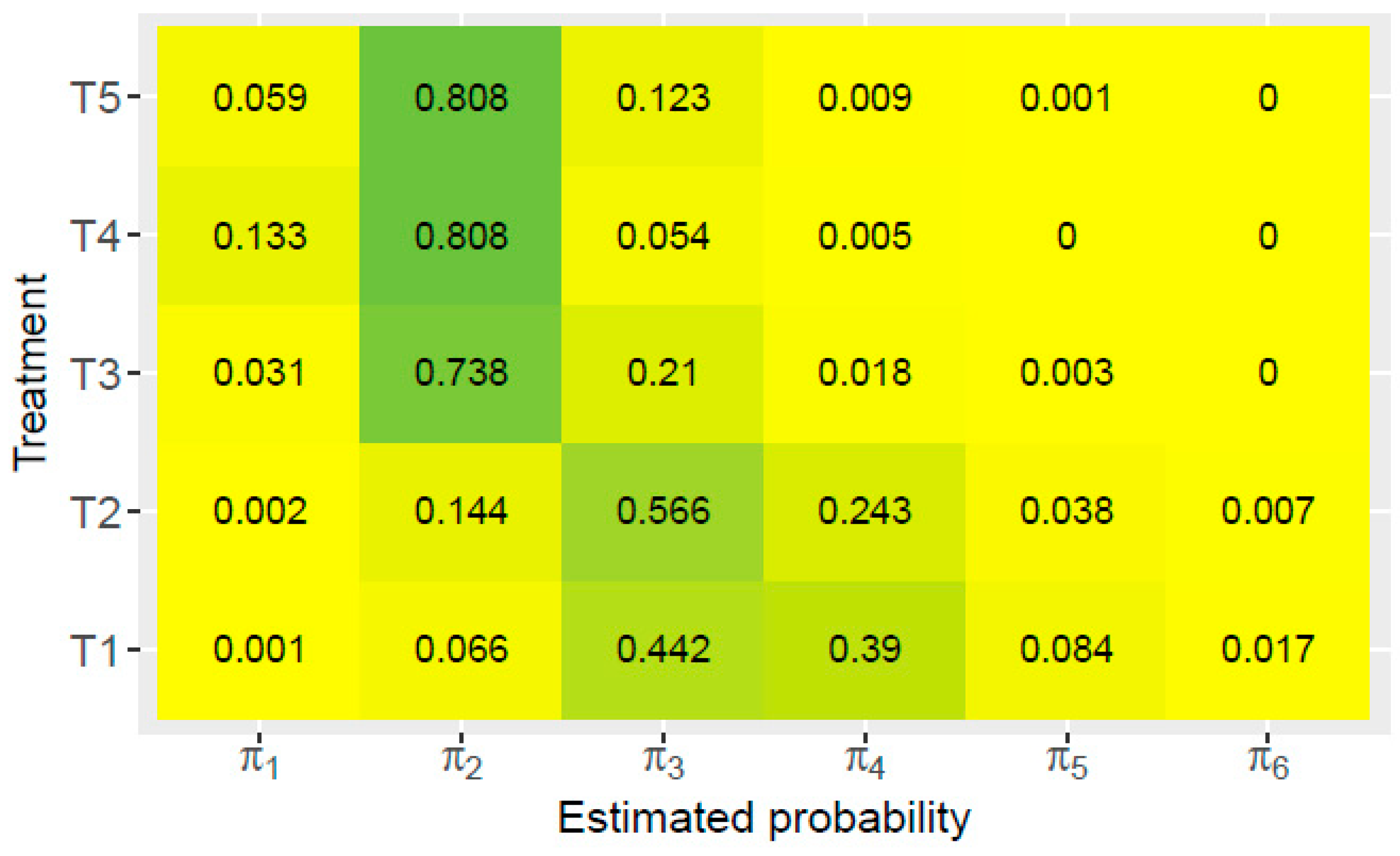
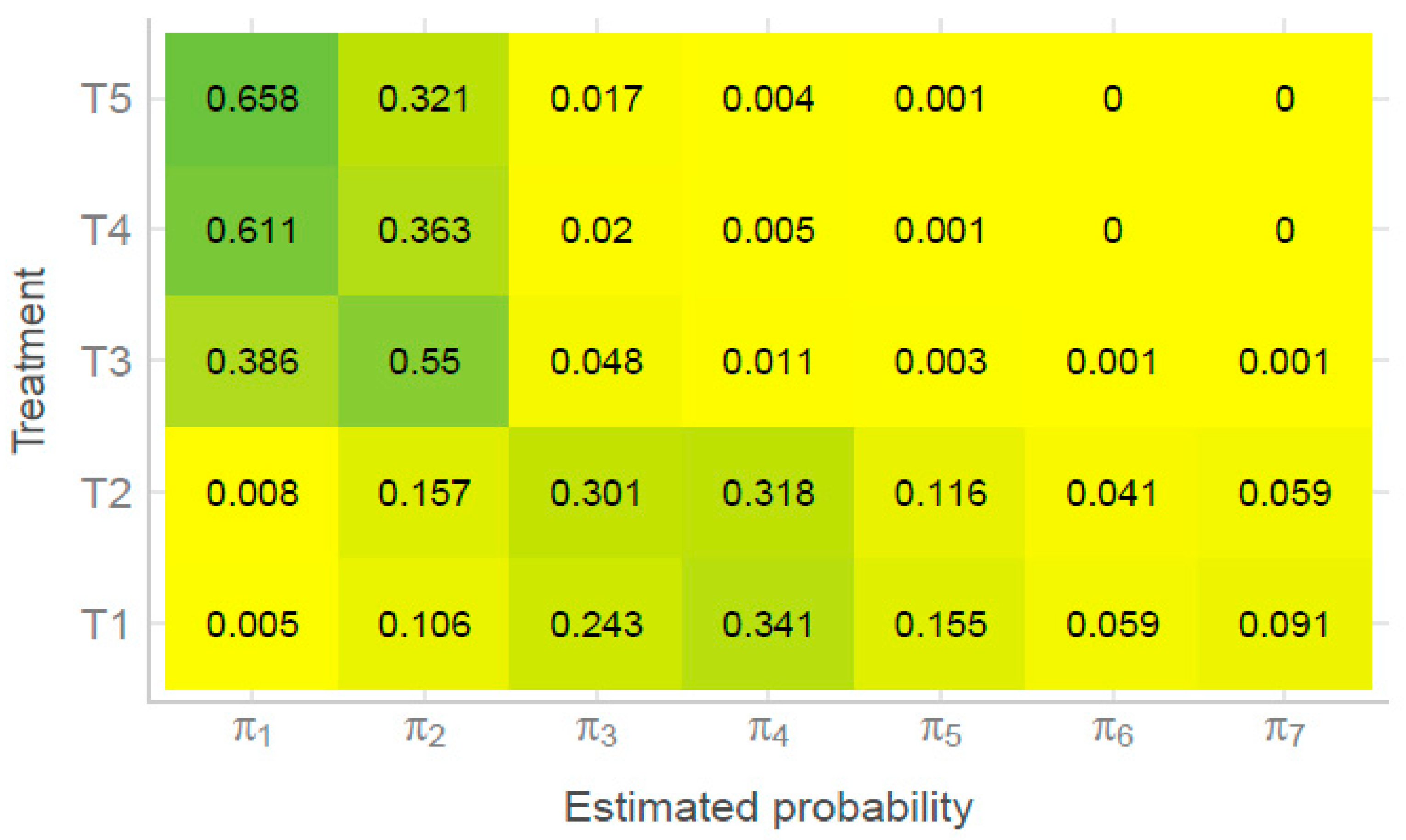

| Treatment | β | Lower CI | Upper CI | p |
|---|---|---|---|---|
| T2 | −0.8710 | −1.7423 | −0.0246 | 0.046 |
| T3 | −3.8406 | −4.9900 | −2.7824 | 0.000 |
| T4 | −5.4154 | −6.8135 | −4.1615 | 0.000 |
| T5 | −4.5105 | −5.7601 | −3.3726 | 0.000 |
| Treatment | β | Lower CI | Upper CI | p |
|---|---|---|---|---|
| T2 | −0.559 | −1.404 | 0.274 | 0.201 |
| T3 | −1.908 | −2.870 | −0.993 | 0.000 |
| T4 | −3.402 | −4.609 | −2.302 | 0.000 |
| T5 | −2.543 | −3.612 | −1.550 | 0.000 |
| μg/kg | Aflatoxins | Ochratoxin A | ZEN | DON | Fumonisins | HT-2 | T-2 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 | B2 | G1 | G2 | B1 | B2 | ||||||
| MI T1 | <0.4 | <0.4 | <0.4 | <0.4 | <1.6 | <16 | <64 | 11,419 | 4384 | <9.6 | <9.6 |
| MII T1 | <0.4 | <0.4 | <0.4 | <0.4 | <1.6 | <16 | <64 | 9037 | 3895 | <9.6 | <9.6 |
| MI T2 | <0.4 | <0.4 | <0.4 | <0.4 | <1.6 | <16 | <64 | 10,848 | 3698 | <9.6 | <9.6 |
| MII T2 | <0.4 | <0.4 | <0.4 | <0.4 | <1.6 | <16 | <64 | 3505 | 924 | 1478 | 692 |
| MI T3 | <0.4 | <0.4 | <0.4 | <0.4 | <1.6 | <16 | <64 | 3822 | 1405 | <9.6 | <9.6 |
| MII T3 | <0.4 | <0.4 | <0.4 | <0.4 | <1.6 | <16 | <64 | 2126 | 660 | <9.6 | <9.6 |
| MI T4 | <0.4 | <0.4 | <0.4 | <0.4 | <1.6 | <16 | <64 | 2813 | 981 | <9.6 | <9.6 |
| MII T4 | <0.4 | <0.4 | <0.4 | <0.4 | <1.6 | <16 | <64 | 2164 | 896 | <9.6 | <9.6 |
| MI T5 | <0.4 | <0.4 | <0.4 | <0.4 | <1.6 | <16 | <64 | 1982 | 709 | <9.6 | <9.6 |
| MII T5 | <0.4 | <0.4 | <0.4 | <0.4 | <1.6 | <16 | <64 | 1491 | 368 | <9.6 | <9.6 |
| μg/kg | Aflatoxins | Ochratoxin A | ZEN | DON | Fumonisins | HT-2 | T-2 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 | B2 | G1 | G2 | B1 | B2 | ||||||
| MI T1 | 2.9 | 0.6 | <0.4 | <0.4 | <1.6 | 19 | <64 | 6043 | 2704 | <9.6 | <9.6 |
| MII T1 | 3.2 | 0.8 | <0.4 | <0.4 | <1.6 | 23 | <64 | 25,867 | 12,569 | <9.6 | <9.6 |
| MI T2 | 0.8 | 0.5 | <0.4 | <0.4 | <1.6 | <16 | <64 | 10,643 | 4552 | <9.6 | <9.6 |
| MII T2 | 0.6 | 0.4 | <0.4 | <0.4 | <1.6 | <16 | <64 | 3664 | 1582 | <9.6 | <9.6 |
| MI T3 | <0.4 | <0.4 | <0.4 | <0.4 | <1.6 | <16 | <64 | 7659 | 2780 | <9.6 | <9.6 |
| MII T3 | <0.4 | <0.4 | <0.4 | <0.4 | <1.6 | <16 | <64 | 14,128 | 5790 | <9.6 | <9.6 |
| MI T4 | <0.4 | <0.4 | <0.4 | <0.4 | <1.6 | <16 | <64 | 6526 | 2347 | <9.6 | <9.6 |
| MII T4 | <0.4 | <0.4 | <0.4 | <0.4 | <1.6 | <16 | <64 | 6223 | 3028 | <9.6 | <9.6 |
| MI T5 | <0.4 | <0.4 | <0.4 | <0.4 | <1.6 | <16 | <64 | 8765 | 4455 | <9.6 | <9.6 |
| MII T5 | <0.4 | <0.4 | <0.4 | <0.4 | <1.6 | <16 | <64 | 5939 | 2423 | <9.6 | <9.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitrović, I.; Čanak, P.; Tančić Živanov, S.; Farkaš, H.; Vasiljević, M.; Ćujić, S.; Zorić, M.; Mitrović, B. Trichoderma harzianum in Biocontrol of Maize Fungal Diseases and Relevant Mycotoxins: From the Laboratory to the Field. J. Fungi 2025, 11, 416. https://doi.org/10.3390/jof11060416
Mitrović I, Čanak P, Tančić Živanov S, Farkaš H, Vasiljević M, Ćujić S, Zorić M, Mitrović B. Trichoderma harzianum in Biocontrol of Maize Fungal Diseases and Relevant Mycotoxins: From the Laboratory to the Field. Journal of Fungi. 2025; 11(6):416. https://doi.org/10.3390/jof11060416
Chicago/Turabian StyleMitrović, Ivana, Petar Čanak, Sonja Tančić Živanov, Hunor Farkaš, Marko Vasiljević, Svetlana Ćujić, Miroslav Zorić, and Bojan Mitrović. 2025. "Trichoderma harzianum in Biocontrol of Maize Fungal Diseases and Relevant Mycotoxins: From the Laboratory to the Field" Journal of Fungi 11, no. 6: 416. https://doi.org/10.3390/jof11060416
APA StyleMitrović, I., Čanak, P., Tančić Živanov, S., Farkaš, H., Vasiljević, M., Ćujić, S., Zorić, M., & Mitrović, B. (2025). Trichoderma harzianum in Biocontrol of Maize Fungal Diseases and Relevant Mycotoxins: From the Laboratory to the Field. Journal of Fungi, 11(6), 416. https://doi.org/10.3390/jof11060416








