Up-Frameshift Factors from Phytopathogenic Fungi Play a Crucial Role in Nonsense-Mediated mRNA Decay
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition of Protein Sequences
2.2. Identification of Homologous Genes and Protein Domains
2.3. Prediction of Protein Phosphorylation Sites
2.4. Prediction of Protein-Protein Interactions
3. Results
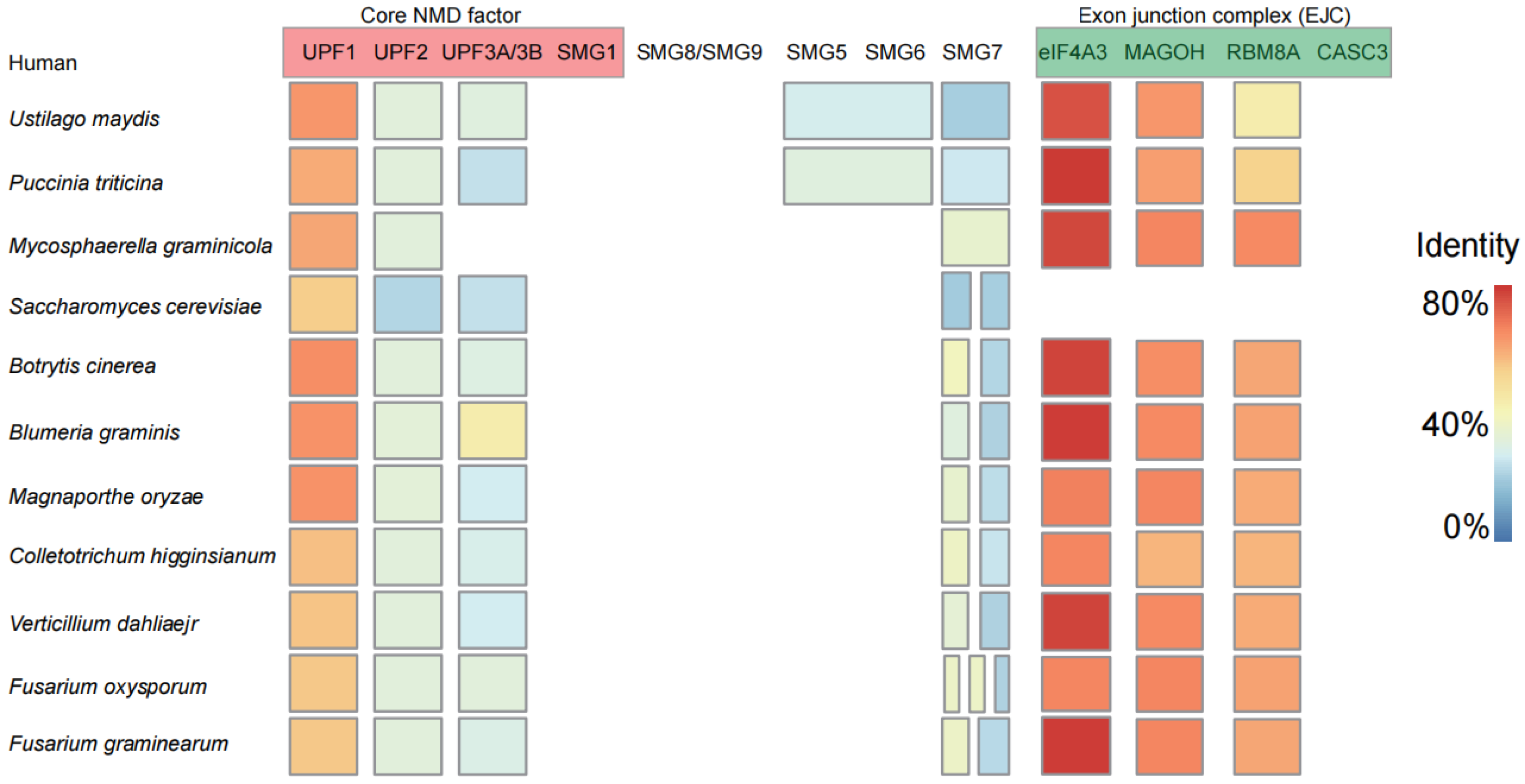
3.1. Core Elements Involved in Nonsense-Mediated Decay (NMD) Are Conserved in Plant Pathogenic Fungi
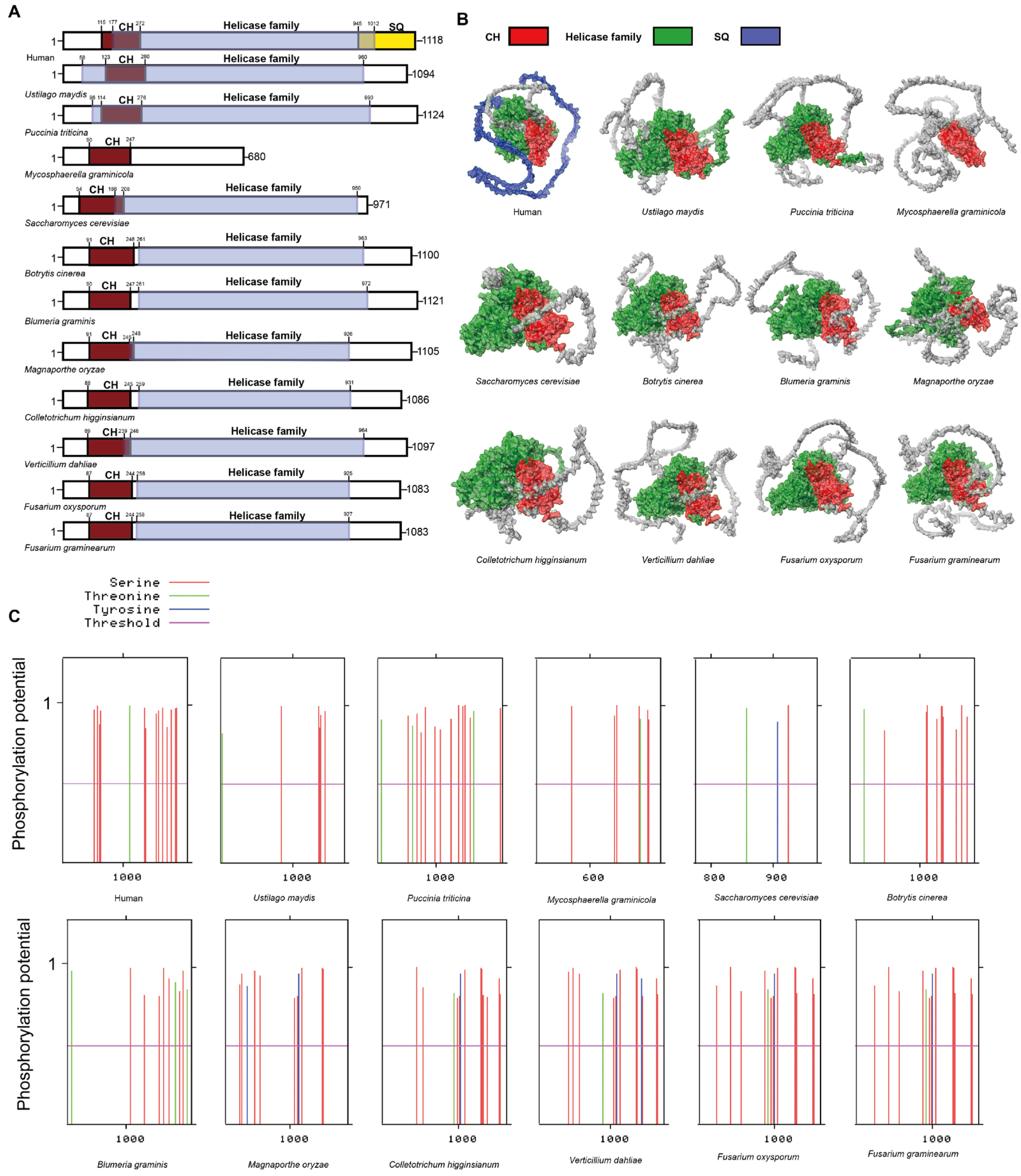
3.2. UPF1 in Plant Pathogenic Fungi
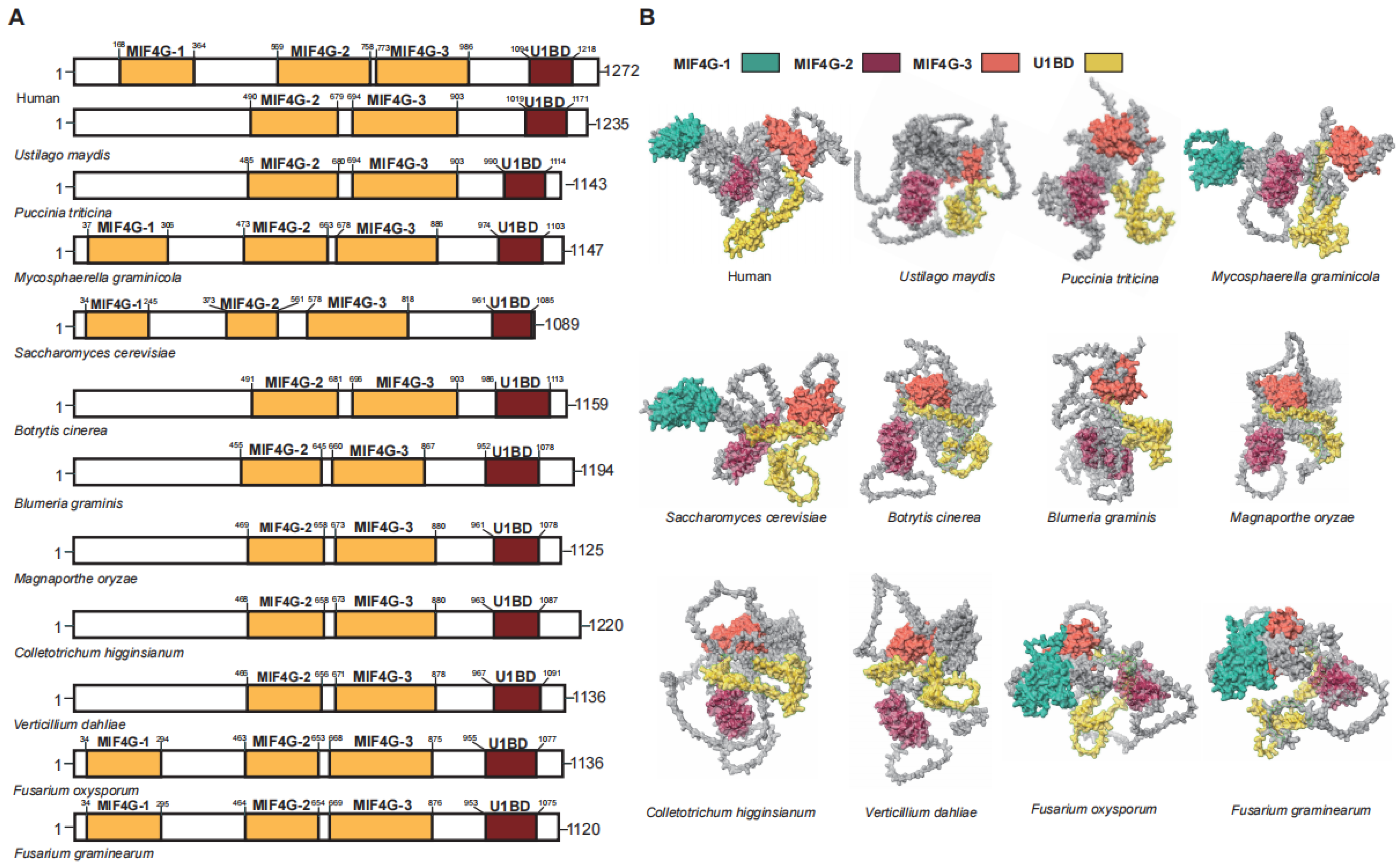
3.3. UPF2 in Plant Pathogenic Fungi
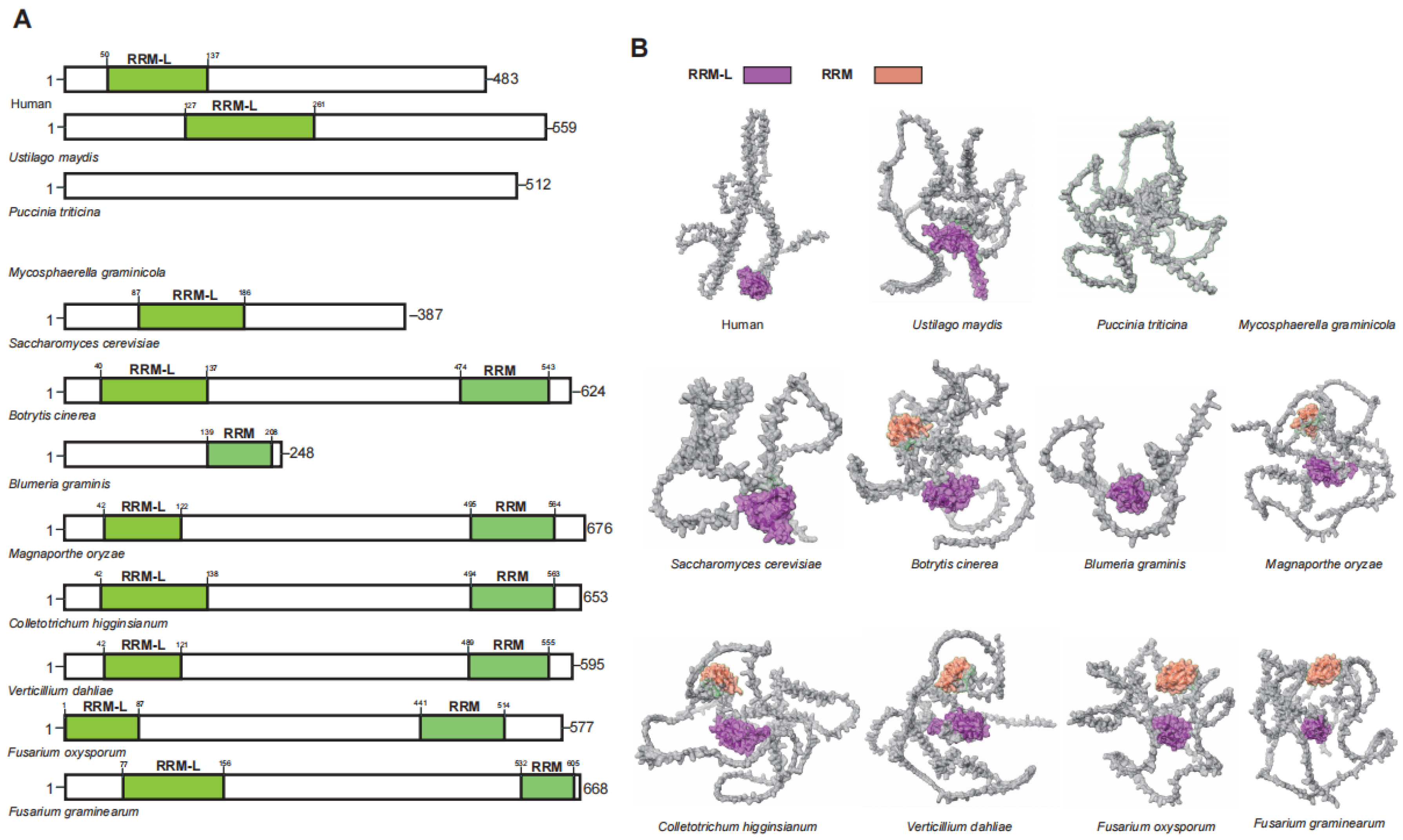
3.4. UPF3 in Plant Pathogenic Fungi
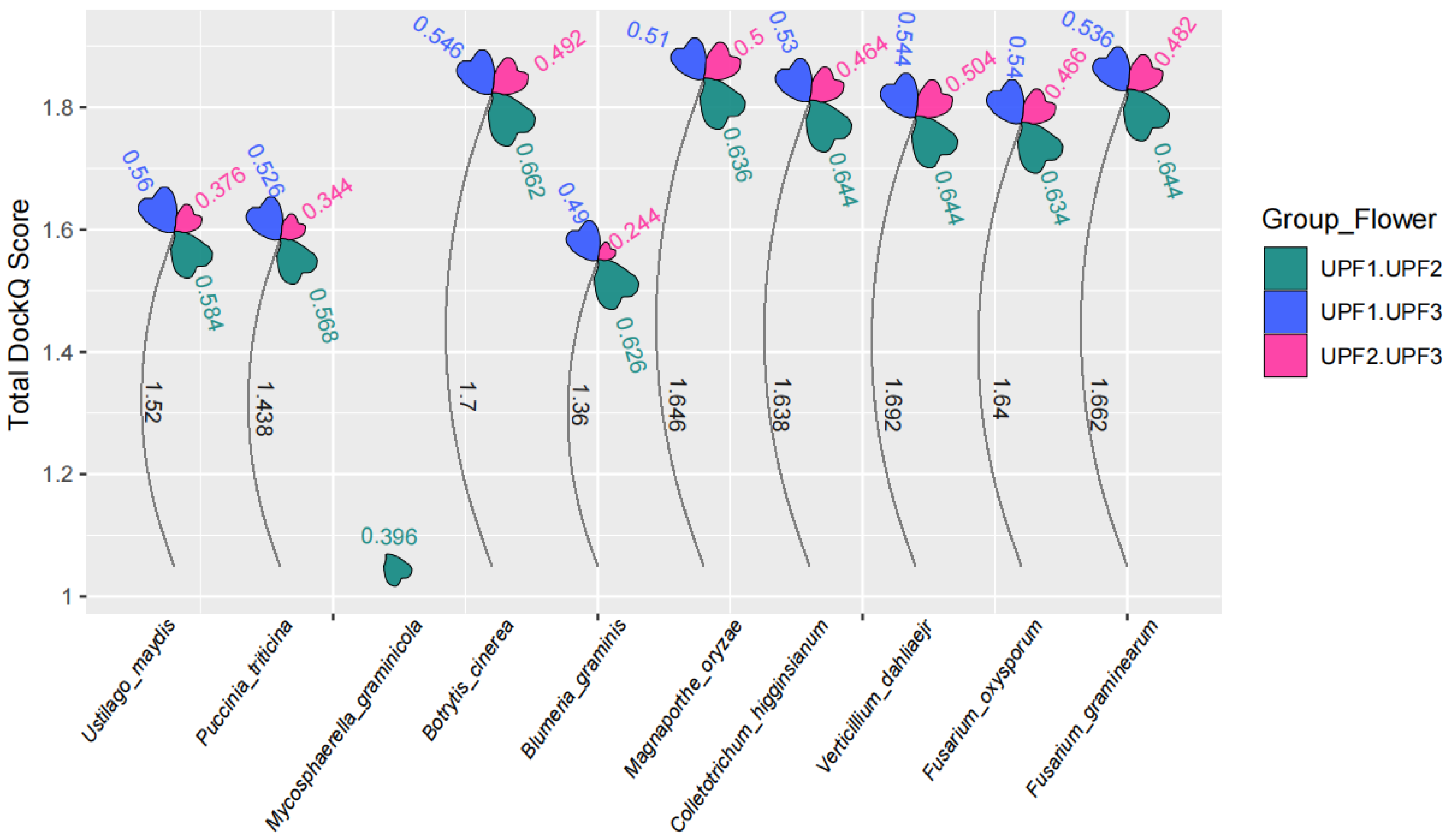
3.5. Interaction of Nonsense-Mediated Decay Core Components in Plant Pathogenic Fungi
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rossi, A.; Moro, A.; Tebaldi, T.; Cornella, N.; Gasperini, L.; Lunelli, L.; Quattrone, A.; Viero, G.; Macchi, P. Identification and dynamic changes of RNAs isolated from RALY-containing ribonucleoprotein complexes. Nucleic Acids Res. 2017, 45, 6775–6792. [Google Scholar] [CrossRef]
- Amir, R.E.; Van den Veyver, I.B.; Wan, M.; Tran, C.Q.; Francke, U.; Zoghbi, H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999, 23, 185–188. [Google Scholar] [CrossRef]
- Zachariah, R.M.; Olson, C.O.; Ezeonwuka, C.; Rastegar, M. Novel MeCP2 isoform-specific antibody reveals the endogenous MeCP2E1 expression in murine brain, primary neurons and astrocytes. PloS ONE 2012, 7, e49763. [Google Scholar] [CrossRef]
- Yasui, D.H.; Gonzales, M.L.; Aflatooni, J.O.; Crary, F.K.; Hu, D.J.; Gavino, B.J.; Golub, M.S.; Vincent, J.B.; Carolyn Schanen, N.; Olson, C.O.; et al. Mice with an isoform-ablating Mecp2 exon 1 mutation recapitulate the neurologic deficits of Rett syndrome. Hum. Mol. Genet. 2014, 23, 2447–2458. [Google Scholar] [CrossRef]
- Dastidar, S.G.; Bardai, F.H.; Ma, C.; Price, V.; Rawat, V.; Verma, P.; Narayanan, V.; D’Mello, S.R. Isoform-specific toxicity of Mecp2 in postmitotic neurons: Suppression of neurotoxicity by FoxG1. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 2846–2855. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; Li, L.F.; Guo, P.; Wang, Y.; Cushman, S.A.; Shang, F.D. Roles and regulatory patterns of protein isoforms in plant adaptation and development. New Phytol. 2025, 245, 1887–1896. [Google Scholar] [CrossRef]
- Wang, B.; Regulski, M.; Tseng, E.; Olson, A.; Goodwin, S.; McCombie, W.R.; Ware, D. A comparative transcriptional landscape of maize and sorghum obtained by single-molecule sequencing. Genome Res. 2018, 28, 921–932. [Google Scholar] [CrossRef]
- Conti, E.; Izaurralde, E. Nonsense-mediated mRNA decay: Molecular insights and mechanistic variations across species. Curr. Opin. Cell Biol. 2005, 17, 316–325. [Google Scholar] [CrossRef]
- Nicholson, P.; Josi, C.; Kurosawa, H.; Yamashita, A.; Mühlemann, O. A novel phosphorylation-independent interaction between SMG6 and UPF1 is essential for human NMD. Nucleic Acids Res. 2014, 42, 9217–9235. [Google Scholar] [CrossRef]
- Losson, R.; Lacroute, F. Interference of nonsense mutations with eukaryotic messenger RNA stability. Proc. Natl. Acad. Sci. USA 1979, 76, 5134–5137. [Google Scholar] [CrossRef]
- Chang, J.C.; Kan, Y.W. beta 0 thalassemia, a nonsense mutation in man. Proc. Natl. Acad. Sci. USA 1979, 76, 2886–2889. [Google Scholar] [CrossRef]
- Pulak, R.; Anderson, P. mRNA surveillance by the Caenorhabditis elegans smg genes. Genes Dev. 1993, 7, 1885–1897. [Google Scholar] [CrossRef]
- Brogna, S. Nonsense mutations in the alcohol dehydrogenase gene of Drosophila melanogaster correlate with an abnormal 3’ end processing of the corresponding pre-mRNA. RNA (New York N. Y.) 1999, 5, 562–573. [Google Scholar] [CrossRef]
- Marchant, A.; Bennett, M.J. The Arabidopsis AUX1 gene: A model system to study mRNA processing in plants. Plant Mol. Biol. 1998, 36, 463–471. [Google Scholar] [CrossRef]
- Mangiarotti, G. Coupling of transcription and translation in Dictyostelium discoideum nuclei. Biochemistry 1999, 38, 3996–4000. [Google Scholar] [CrossRef]
- Akhmedov, N.B.; Piriev, N.I.; Chang, B.; Rapoport, A.L.; Hawes, N.L.; Nishina, P.M.; Nusinowitz, S.; Heckenlively, J.R.; Roderick, T.H.; Kozak, C.A.; et al. A deletion in a photoreceptor-specific nuclear receptor mRNA causes retinal degeneration in the rd7 mouse. Proc. Natl. Acad. Sci. USA 2000, 97, 5551–5556. [Google Scholar] [CrossRef]
- Wittkopp, N.; Huntzinger, E.; Weiler, C.; Saulière, J.; Schmidt, S.; Sonawane, M.; Izaurralde, E. Nonsense-mediated mRNA decay effectors are essential for zebrafish embryonic development and survival. Mol. Cell. Biol. 2009, 29, 3517–3528. [Google Scholar] [CrossRef]
- Zhuravskaya, A.; Yap, K.; Hamid, F.; Makeyev, E.V. Alternative splicing coupled to nonsense-mediated decay coordinates downregulation of non-neuronal genes in developing mouse neurons. Genome Biol. 2024, 25, 162. [Google Scholar] [CrossRef]
- Lai, S.; Shiraishi, H.; Sebastian, W.A.; Shimizu, N.; Umeda, R.; Ikeuchi, M.; Kiyota, K.; Takeno, T.; Miyazaki, S.; Yano, S.; et al. Effect of nonsense-mediated mRNA decay factor SMG9 deficiency on premature aging in zebrafish. Commun. Biol. 2024, 7, 654. [Google Scholar] [CrossRef]
- Chen, Y.; Jia, M.; Ge, L.; Li, Z.; He, H.; Zhou, X.; Li, F. A Negative Feedback Loop Compromises NMD-Mediated Virus Restriction by the Autophagy Pathway in Plants. Adv. Sci. (Weinh. Baden-Wurtt. Ger.) 2024, 11, e2400978. [Google Scholar] [CrossRef]
- Luha, R.; Rana, V.; Vainstein, A.; Kumar, V. Nonsense-mediated mRNA decay pathway in plants under stress: General gene regulatory mechanism and advances. Planta 2024, 259, 51. [Google Scholar] [CrossRef]
- Le Hir, H.; Izaurralde, E.; Maquat, L.E.; Moore, M.J. The spliceosome deposits multiple proteins 20-24 nucleotides upstream of mRNA exon-exon junctions. EMBO J. 2000, 19, 6860–6869. [Google Scholar] [CrossRef]
- Komili, S.; Silver, P.A. Coupling and coordination in gene expression processes: A systems biology view. Nat. Rev. Genet. 2008, 9, 38–48. [Google Scholar] [CrossRef]
- Orphanides, G.; Reinberg, D. A unified theory of gene expression. Cell 2002, 108, 439–451. [Google Scholar] [CrossRef]
- Carrard, J.; Lejeune, F. Nonsense-mediated mRNA decay, a simplified view of a complex mechanism. BMB Rep. 2023, 56, 625–632. [Google Scholar] [CrossRef]
- He, F.; Jacobson, A. Nonsense-Mediated mRNA Decay: Degradation of Defective Transcripts Is Only Part of the Story. Annu. Rev. Genet. 2015, 49, 339–366. [Google Scholar] [CrossRef]
- Lloyd, J.P.B. The evolution and diversity of the nonsense-mediated mRNA decay pathway. F1000Research 2018, 7, 1299. [Google Scholar] [CrossRef]
- Lu, P.; Chen, D.; Qi, Z.; Wang, H.; Chen, Y.; Wang, Q.; Jiang, C.; Xu, J.R.; Liu, H. Landscape and regulation of alternative splicing and alternative polyadenylation in a plant pathogenic fungus. New Phytol. 2022, 235, 674–689. [Google Scholar] [CrossRef]
- Mitchell, A.L.; Attwood, T.K.; Babbitt, P.C.; Blum, M.; Bork, P.; Bridge, A.; Brown, S.D.; Chang, H.Y.; El-Gebali, S.; Fraser, M.I.; et al. InterPro in 2019: Improving coverage, classification and access to protein sequence annotations. Nucleic Acids Res. 2019, 47, D351–D360. [Google Scholar] [CrossRef]
- Homma, F.; Huang, J.; van der Hoorn, R.A.L. AlphaFold-Multimer predicts cross-kingdom interactions at the plant-pathogen interface. Nat. Commun. 2023, 14, 6040. [Google Scholar] [CrossRef]
- Basu, S.; Wallner, B. DockQ: A Quality Measure for Protein-Protein Docking Models. PloS ONE 2016, 11, e0161879. [Google Scholar] [CrossRef]
- Mailliot, J.; Vivoli-Vega, M.; Schaffitzel, C. No-nonsense: Insights into the functional interplay of nonsense-mediated mRNA decay factors. Biochem. J. 2022, 479, 973–993. [Google Scholar] [CrossRef]
- Clerici, M.; Deniaud, A.; Boehm, V.; Gehring, N.H.; Schaffitzel, C.; Cusack, S. Structural and functional analysis of the three MIF4G domains of nonsense-mediated decay factor UPF2. Nucleic Acids Res. 2014, 42, 2673–2686. [Google Scholar] [CrossRef]
- Boisramé, A.; Devillers, H.; Onésime, D.; Brunel, F.; Pouch, J.; Piot, M.; Neuvéglise, C. Exon junction complex components Y14 and Mago still play a role in budding yeast. Sci. Rep. 2019, 9, 849. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Jayachandran, U.; Bonneau, F.; Fiorini, F.; Basquin, C.; Domcke, S.; Le Hir, H.; Conti, E. Molecular mechanisms for the RNA-dependent ATPase activity of Upf1 and its regulation by Upf2. Mol. Cell 2011, 41, 693–703. [Google Scholar] [CrossRef]
- Kadlec, J.; Guilligay, D.; Ravelli, R.B.; Cusack, S. Crystal structure of the UPF2-interacting domain of nonsense-mediated mRNA decay factor UPF1. RNA (New York N. Y.) 2006, 12, 1817–1824. [Google Scholar] [CrossRef]
- Clerici, M.; Mourão, A.; Gutsche, I.; Gehring, N.H.; Hentze, M.W.; Kulozik, A.; Kadlec, J.; Sattler, M.; Cusack, S. Unusual bipartite mode of interaction between the nonsense-mediated decay factors, UPF1 and UPF2. EMBO J. 2009, 28, 2293–2306. [Google Scholar] [CrossRef]
- Gowravaram, M.; Bonneau, F.; Kanaan, J.; Maciej, V.D.; Fiorini, F.; Raj, S.; Croquette, V.; Le Hir, H.; Chakrabarti, S. A conserved structural element in the RNA helicase UPF1 regulates its catalytic activity in an isoform-specific manner. Nucleic Acids Res. 2018, 46, 2648–2659. [Google Scholar] [CrossRef]
- Yamashita, A.; Ohnishi, T.; Kashima, I.; Taya, Y.; Ohno, S. Human SMG-1, a novel phosphatidylinositol 3-kinase-related protein kinase, associates with components of the mRNA surveillance complex and is involved in the regulation of nonsense-mediated mRNA decay. Genes Dev. 2001, 15, 2215–2228. [Google Scholar] [CrossRef]
- Kadlec, J.; Izaurralde, E.; Cusack, S. The structural basis for the interaction between nonsense-mediated mRNA decay factors UPF2 and UPF3. Nat. Struct. Mol. Biol. 2004, 11, 330–337. [Google Scholar] [CrossRef]
- Melero, R.; Buchwald, G.; Castaño, R.; Raabe, M.; Gil, D.; Lázaro, M.; Urlaub, H.; Conti, E.; Llorca, O. The cryo-EM structure of the UPF-EJC complex shows UPF1 poised toward the RNA 3’ end. Nat. Struct. Mol. Biol. 2012, 19, 498–505, s491–s492. [Google Scholar] [CrossRef]
- Buchwald, G.; Ebert, J.; Basquin, C.; Sauliere, J.; Jayachandran, U.; Bono, F.; Le Hir, H.; Conti, E. Insights into the recruitment of the NMD machinery from the crystal structure of a core EJC-UPF3b complex. Proc. Natl. Acad. Sci. USA 2010, 107, 10050–10055. [Google Scholar] [CrossRef]
- Lindeboom, R.G.; Supek, F.; Lehner, B. The rules and impact of nonsense-mediated mRNA decay in human cancers. Nat. Genet. 2016, 48, 1112–1118. [Google Scholar] [CrossRef]
- Bufton, J.C.; Powers, K.T.; Szeto, J.A.; Toelzer, C.; Berger, I.; Schaffitzel, C. Structures of nonsense-mediated mRNA decay factors UPF3B and UPF3A in complex with UPF2 reveal molecular basis for competitive binding and for neurodevelopmental disorder-causing mutation. Nucleic Acids Res. 2022, 50, 5934–5947. [Google Scholar] [CrossRef]
- Chamieh, H.; Ballut, L.; Bonneau, F.; Le Hir, H. NMD factors UPF2 and UPF3 bridge UPF1 to the exon junction complex and stimulate its RNA helicase activity. Nat. Struct. Mol. Biol. 2008, 15, 85–93. [Google Scholar] [CrossRef]
- Kashima, I.; Yamashita, A.; Izumi, N.; Kataoka, N.; Morishita, R.; Hoshino, S.; Ohno, M.; Dreyfuss, G.; Ohno, S. Binding of a novel SMG-1-Upf1-eRF1-eRF3 complex (SURF) to the exon junction complex triggers Upf1 phosphorylation and nonsense-mediated mRNA decay. Genes Dev. 2006, 20, 355–367. [Google Scholar] [CrossRef]
- Yamashita, A.; Izumi, N.; Kashima, I.; Ohnishi, T.; Saari, B.; Katsuhata, Y.; Muramatsu, R.; Morita, T.; Iwamatsu, A.; Hachiya, T.; et al. SMG-8 and SMG-9, two novel subunits of the SMG-1 complex, regulate remodeling of the mRNA surveillance complex during nonsense-mediated mRNA decay. Genes Dev. 2009, 23, 1091–1105. [Google Scholar] [CrossRef]
- Bufton, J.C.; Powers, K.T.; Szeto, J.-Y.A.; Schaffitzel, C.J.b. Molecular basis of neurodevelopmental disorder-causing mutation in nonsense-mediated mRNA decay factor UPF3B. bioRxiv 2022. [Google Scholar] [CrossRef]
- Le Hir, H.; Gatfield, D.; Izaurralde, E.; Moore, M.J. The exon-exon junction complex provides a binding platform for factors involved in mRNA export and nonsense-mediated mRNA decay. EMBO J. 2001, 20, 4987–4997. [Google Scholar] [CrossRef]
- Singh, K.K.; Wachsmuth, L.; Kulozik, A.E.; Gehring, N.H. Two mammalian MAGOH genes contribute to exon junction complex composition and nonsense-mediated decay. RNA Biol. 2013, 10, 1291–1298. [Google Scholar] [CrossRef]
- Neu-Yilik, G.; Raimondeau, E.; Eliseev, B.; Yeramala, L.; Amthor, B.; Deniaud, A.; Huard, K.; Kerschgens, K.; Hentze, M.W.; Schaffitzel, C.; et al. Dual function of UPF3B in early and late translation termination. EMBO J. 2017, 36, 2968–2986. [Google Scholar] [CrossRef]
- Bryant, P.; Pozzati, G.; Elofsson, A. Improved prediction of protein-protein interactions using AlphaFold2. Nat. Commun. 2022, 13, 1265. [Google Scholar] [CrossRef]
- Han, L.; Liu, J.; Zhang, R.; Cheng, Y.; Dong, L.; Wei, L.; Liu, J.; Wang, K.; Yu, J. Insights from Nonsense-Mediated mRNA Decay for Prognosis in Homologous Recombination-Deficient Ovarian Cancer. Cancer Sci. 2025, 116, 1449–1463. [Google Scholar] [CrossRef]
- Cook, A.L.; Sur, S.; Dobbyn, L.; Watson, E.; Cohen, J.D.; Ptak, B.; Lee, B.S.; Paul, S.; Hsiue, E.; Popoli, M.; et al. Identification of nonsense-mediated decay inhibitors that alter the tumor immune landscape. eLife 2025, 13, RP95952. [Google Scholar] [CrossRef]
- Behera, A.; Panigrahi, G.K.; Sahoo, A. Nonsense-Mediated mRNA Decay in Human Health and Diseases: Current Understanding, Regulatory Mechanisms and Future Perspectives. In Molecular Biotechnology; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar] [CrossRef]
- Wang, W.; Cajigas, I.J.; Peltz, S.W.; Wilkinson, M.F.; González, C.I. Role for Upf2p phosphorylation in Saccharomyces cerevisiae nonsense-mediated mRNA decay. Mol. Cell. Biol. 2006, 26, 3390–3400. [Google Scholar] [CrossRef]
- Schirman, D.; Yakhini, Z.; Pilpel, Y.; Dahan, O. A broad analysis of splicing regulation in yeast using a large library of synthetic introns. PLoS Genet. 2021, 17, e1009805. [Google Scholar] [CrossRef]
| Species | Year | Key Findings | Reference |
|---|---|---|---|
| S. cerevisiae | 1979 | NMD first discovered in eukaryotes. | [10] |
| Human | 1979 | The first discovery of thalassemia directly caused by NMD in humans. | [11] |
| C. elegans | 1993 | First demonstration of the importance of smg in NMD in nematodes. | [12] |
| A. thaliana | 1998 | The presence of NMD in plants was verified. | [14] |
| Drosophilidae | 1999 | NMD may affect the 3′ end processing of pre-mRNA. | [13] |
| D. discoideum | 1999 | NMD occurs in the nucleus in D. discoideum. | [15] |
| mice | 2000 | NMD is involved retinal function in mouse. | [16] |
| zebrafish | 2009 | NMD is involved in embryonic development in zebrafish. | [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, P.; Wang, J.; Wang, X.; Wang, D.; Shi, H. Up-Frameshift Factors from Phytopathogenic Fungi Play a Crucial Role in Nonsense-Mediated mRNA Decay. J. Fungi 2025, 11, 404. https://doi.org/10.3390/jof11060404
Lu P, Wang J, Wang X, Wang D, Shi H. Up-Frameshift Factors from Phytopathogenic Fungi Play a Crucial Role in Nonsense-Mediated mRNA Decay. Journal of Fungi. 2025; 11(6):404. https://doi.org/10.3390/jof11060404
Chicago/Turabian StyleLu, Ping, Jiaqi Wang, Xiaoli Wang, Dan Wang, and Haojie Shi. 2025. "Up-Frameshift Factors from Phytopathogenic Fungi Play a Crucial Role in Nonsense-Mediated mRNA Decay" Journal of Fungi 11, no. 6: 404. https://doi.org/10.3390/jof11060404
APA StyleLu, P., Wang, J., Wang, X., Wang, D., & Shi, H. (2025). Up-Frameshift Factors from Phytopathogenic Fungi Play a Crucial Role in Nonsense-Mediated mRNA Decay. Journal of Fungi, 11(6), 404. https://doi.org/10.3390/jof11060404






