Myricetin Exerts Antibiofilm Effects on Candida albicans by Targeting the RAS1/cAMP/EFG1 Pathway and Disruption of the Hyphal Network
Abstract
1. Introduction
2. Materials and Methods
2.1. Candida Strains and Chemicals
2.2. Antifungal Susceptibility
2.3. Biofilm Formation and Inhibition Assay
2.3.1. Biofilm Biomass Determination by Crystal Violet Staining
2.3.2. Investigation of the Effect of MYR on Candida Strain Biofilms by CCK-8 Test
2.4. RNA Isolation, cDNA Synthesis, and qRT-PCR Expression Analysis
2.5. FESEM Analysis
2.6. In Vivo Relative Toxicity Evaluation with Galleria mellonella Larvae Model
2.7. Statistical Analysis
3. Results
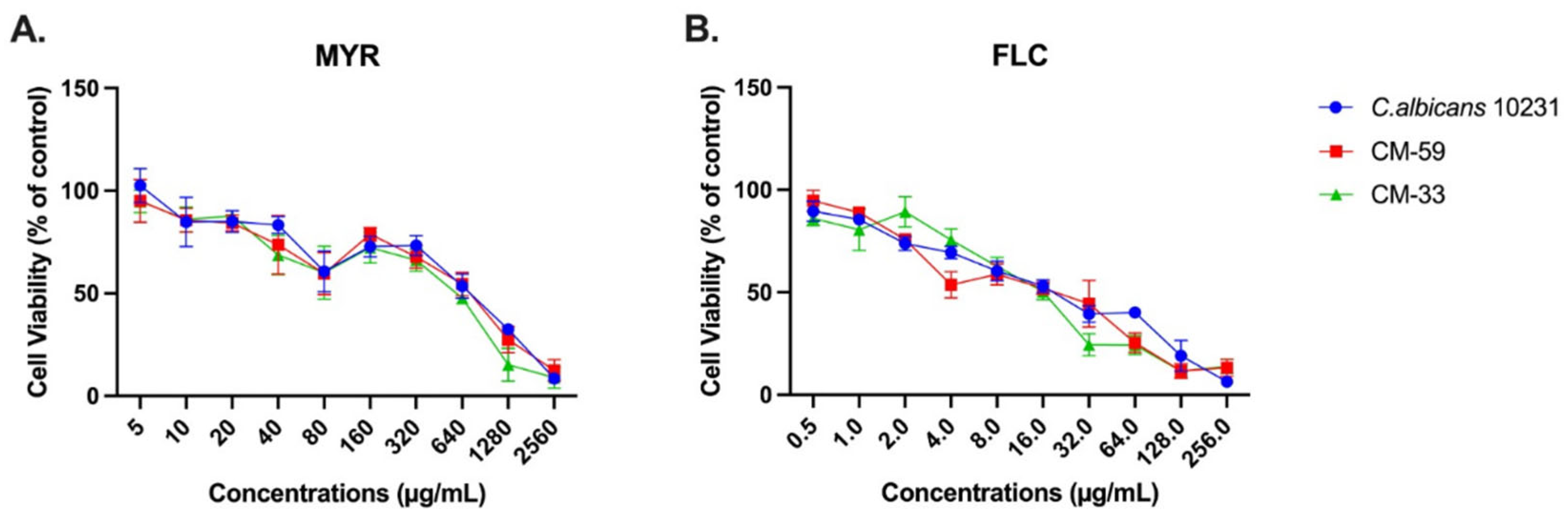
3.1. Antifungal Activity of Myricetin Against Candida Species
3.2. Biofilm-Forming Ability of Candida albicans Strains
3.3. Effect of Myricetin on Biofilm Formation Using the CCK-8 Assay
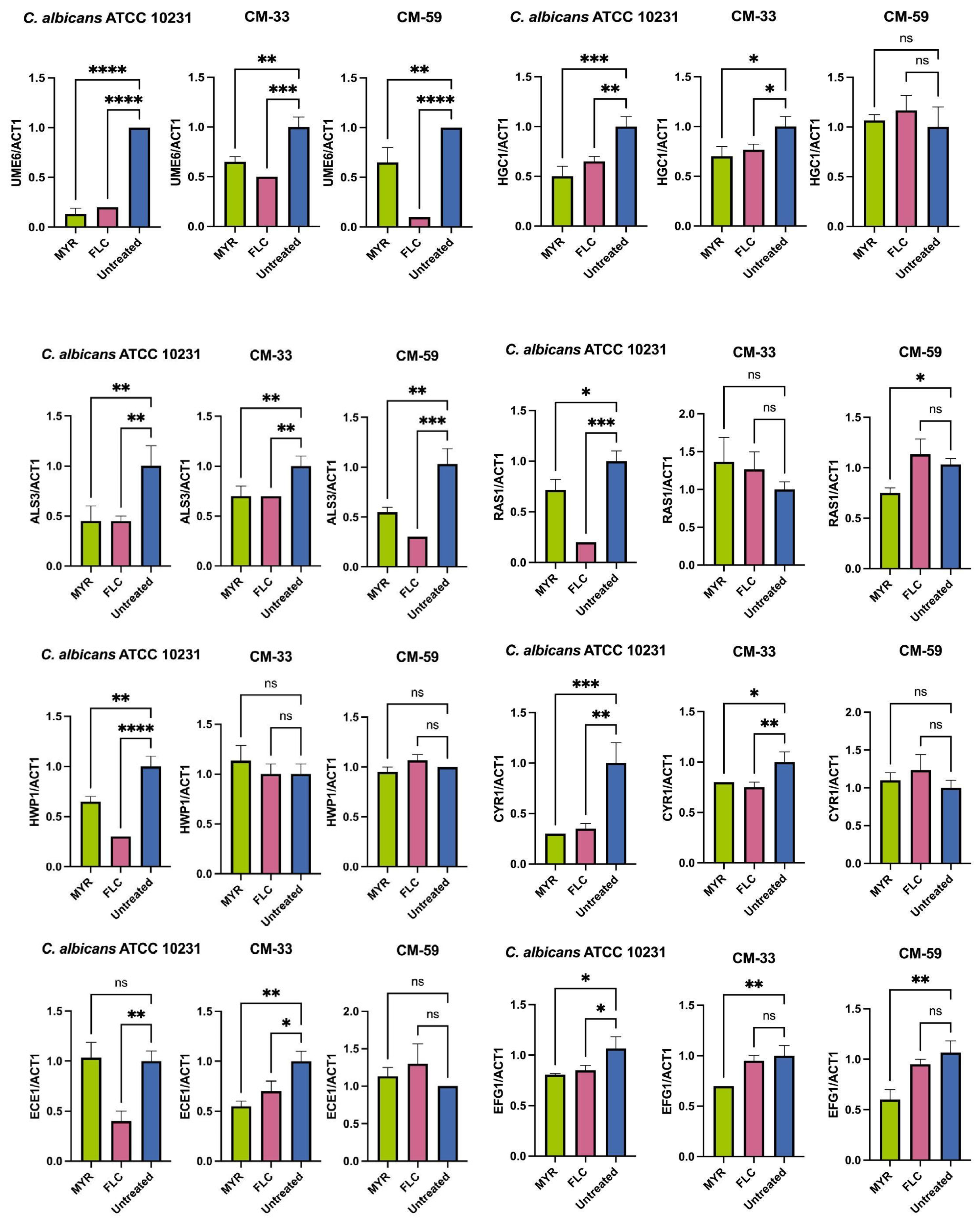
3.4. Effect of Myricetin on Biofilm-Related Gene Expression in C. albicans
3.5. Effect of Myricetin on C. albicans Biofilms by FESEM Analysis
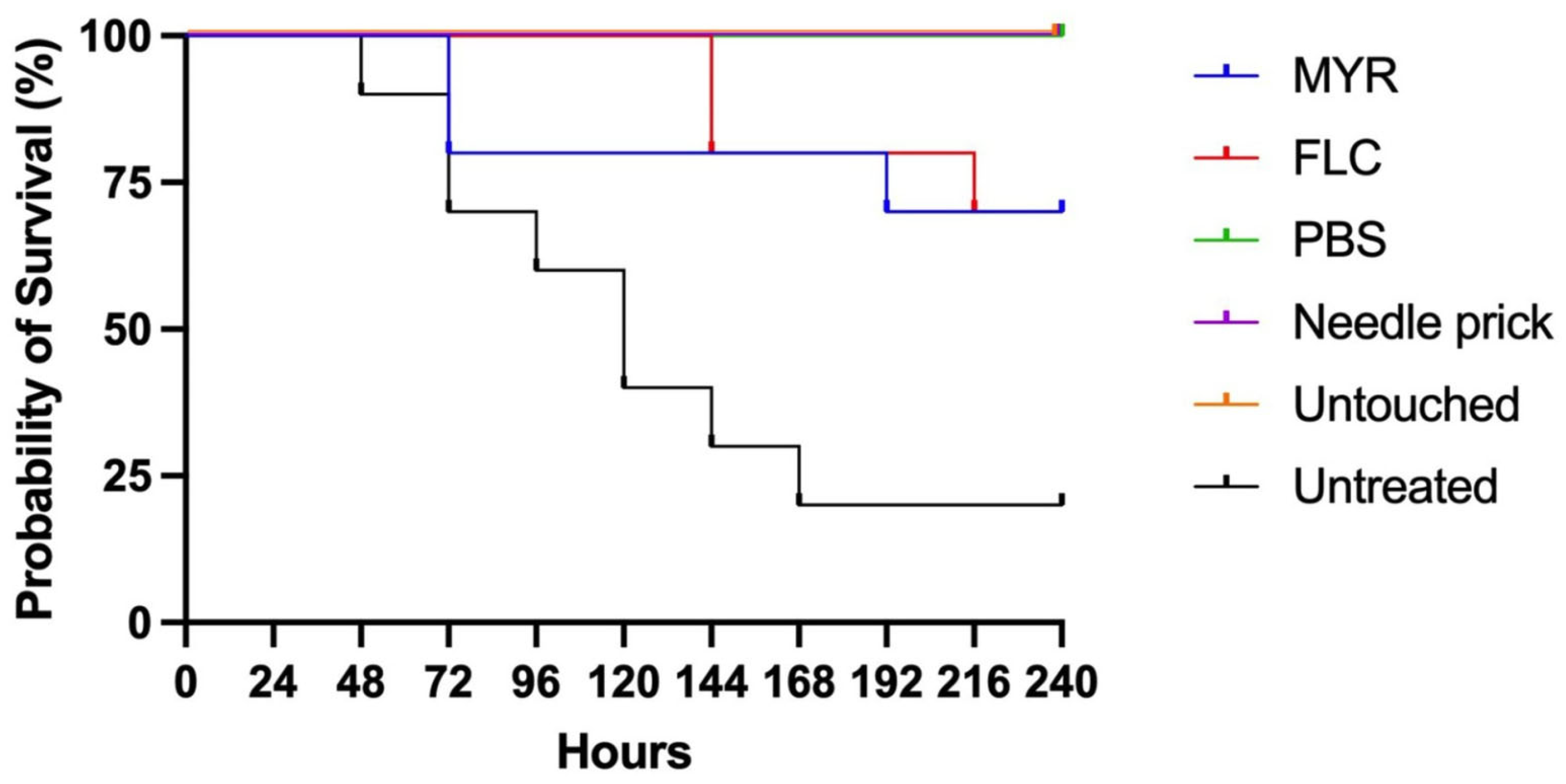
3.6. In Vivo Toxicity and Efficacy of Myricetin Using the Galleria Mellonella Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, D.J.; Lee, M.W.; Choi, J.S.; Lee, S.G.; Park, J.Y.; Kim, S.W. Inhibitory activity of hinokitiol against biofilm formation in fluconazole-resistant Candida species. PLoS ONE 2017, 12, e0171244. [Google Scholar] [CrossRef] [PubMed]
- Tsui, C.; Kong, E.F.; Jabra-Rizk, M.A. Pathogenesis of Candida albicans biofilm. Pathog. Dis. 2016, 74, ftw018. [Google Scholar] [CrossRef] [PubMed]
- Q-Malinovská, Z.; Čonková, E.; Váczi, P. Biofilm formation in medically important Candida species. J. Fungi 2023, 9, 955. [Google Scholar] [CrossRef] [PubMed]
- McCarty, T.P.; Pappas, P.G. Invasive Candidiasis. Infect. Dis. Clin. N. Am. 2016, 30, 103–124. [Google Scholar] [CrossRef]
- de Almeida, R.F.M.; Santos, F.C.; Marycz, K.; Alicka, M.; Krasowska, A.; Suchodolski, J.; Panek, J.J.; Jezierska, A.; Starosta, R. New diphenylphosphane derivatives of ketoconazole are promising antifungal agents. Sci. Rep. 2019, 9, 16214. [Google Scholar] [CrossRef]
- Ng, K.P.; Kuan, C.S.; Kaur, H.; Na, S.L.; Atiya, N.; Velayuthan, R.D. Candida species epidemiology 2000–2013: A laboratory-based report. Trop. Med. Int. Health 2015, 20, 1447–1453. [Google Scholar] [CrossRef]
- Rodríguez-Cerdeira, C.; Martínez-Herrera, E.; Carnero-Gregorio, M.; López-Barcenas, A.; Fabbrocini, G.; Fida, M.; El-Samahy, M.; González-Cespón, J.L. Pathogenesis and clinical relevance of Candida biofilms in vulvovaginal candidiasis. Front. Microbiol. 2020, 11, 544480. [Google Scholar] [CrossRef]
- Araújo, D.; Henriques, M.; Silva, S. Portrait of Candida species biofilm regulatory network genes. Trends Microbiol. 2017, 25, 62–75. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, A. Molecular Determinants Involved in Candida albicans Biofilm Formation and Regulation. Mol. Biotechnol. 2024, 66, 1640–1659. [Google Scholar] [CrossRef]
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef]
- Glazier, V.E. EFG1, everyone’s favorite gene in Candida albicans: A comprehensive literature review. Front. Cell. Infect. Microbiol. 2022, 12, 855229. [Google Scholar] [CrossRef] [PubMed]
- Zeidler, U.; Lettner, T.; Lassnig, C.; Müller, M.; Lajko, R.; Hintner, H.; Breitenbach, M.; Bito, A. UME6 is a crucial downstream target of other transcriptional regulators of true hyphal development in Candida albicans. FEMS Yeast Res. 2009, 9, 126–142. [Google Scholar] [CrossRef]
- Sumlu, E.; Aydin, M.; Korucu, E.N.; Alyar, S.; Nsangou, A.M. Artemisinin May Disrupt Hyphae Formation by Suppressing Biofilm-Related Genes of Candida albicans: In Vitro and In Silico Approaches. Antibiotics 2024, 13, 310. [Google Scholar] [CrossRef]
- Ajetunmobi, O.H.; Badali, H.; Romo, J.A.; Ramage, G.; Lopez-Ribot, J.L. Antifungal therapy of Candida biofilms: Past, present and future. Biofilm 2023, 5, 100126. [Google Scholar] [CrossRef]
- Pierce, C.G.; Srinivasan, A.; Uppuluri, P.; Ramasubramanian, A.K.; López-Ribot, J.L. Antifungal therapy with an emphasis on biofilms. Curr. Opin. Pharmacol. 2013, 13, 726–730. [Google Scholar] [CrossRef]
- Roscetto, E.; Contursi, P.; Vollaro, A.; Fusco, S.; Notomista, E.; Catania, M.R. Antifungal and anti-biofilm activity of the first cryptic antimicrobial peptide from an archaeal protein against Candida spp. clinical isolates. Sci. Rep. 2018, 8, 17570. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, Y. Myricetin Disturbs the Cell Wall Integrity and Increases the Membrane Permeability of Candida albicans. J. Microbiol. Biotechnol. 2022, 32, 37–45. [Google Scholar] [CrossRef]
- Salazar-Aranda, R.; Granados-Guzmán, G.; Pérez-Meseguer, J.; González, G.M.; de Torres, N.W. Activity of Polyphenolic Compounds against Candida glabrata. Molecules 2015, 20, 17903–17912. [Google Scholar] [CrossRef]
- Rocha, G.R.; Florez Salamanca, E.J.; de Barros, A.L.; Lobo, C.I.V.; Klein, M.I. Effect of tt-farnesol and myricetin on in vitro biofilm formation by Streptococcus mutans and Candida albicans. BMC Complement. Altern. Med. 2018, 18, 61. [Google Scholar] [CrossRef]
- Mo, F.; Ma, J.; Yang, X.; Zhang, P.; Li, Q.; Zhang, J. In vitro and in vivo effects of the combination of myricetin and miconazole nitrate incorporated into thermosensitive hydrogels on C. albicans biofilms. Phytomedicine 2020, 71, 153223. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Siddiqui, M.A.; Khan, M.M.; Ajmal, M.; Ahsan, R.; Rahaman, M.A.; Ahmad, M.A.; Arshad, M.; Khushtar, M. Current Pharmacological Trends on Myricetin. Drug Res. 2020, 70, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Fourth Informational Supplement; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- Aydin, M.; Unusan, N.; Sumlu, E.; Korucu, E.N. Rosmarinic Acid Exhibits Antifungal and Antibiofilm Activities Against Candida albicans: Insights into Gene Expression and Morphological Changes. J. Fungi 2024, 10, 751. [Google Scholar] [CrossRef]
- Stepanovic, S.; Vuković, D.; Hola, V.; Bonaventura, G.D.; Djukić, S.; Ćirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Holcombe, L.J.; McAlester, G.; Munro, C.A.; Enjalbert, B.; Brown, A.J.P.; Gow, N.A.R.; Ding, C.; Butler, G.; O’Gara, F.; Morrissey, J.P. Pseudomonas aeruginosa secreted factors impair biofilm development in Candida albicans. Microbiology 2010, 156, 1476–1486. [Google Scholar] [CrossRef]
- Willems, H.M.E.; Lowes, D.J.; Barker, K.S.; Palmer, G.E.; Peters, B.M. Comparative Analysis of the Capacity of the Candida Species to Elicit Vaginal Immunopathology. Infect. Immun. 2018, 86, e00527-18. [Google Scholar] [CrossRef]
- Hsu, C.C.; Lai, W.L.; Chuang, K.C.; Lee, M.H.; Tsai, Y.C. The inhibitory activity of linalool against the filamentous growth and biofilm formation in Candida albicans. Med. Mycol. 2013, 51, 473–482. [Google Scholar] [CrossRef]
- Uppuluri, P.; Dinakaran, H.; Thomas, D.P.; Chaturvedi, A.K.; Lopez-Ribot, J.L. Characteristics of Candida albicans biofilms grown in a synthetic urine medium. J. Clin. Microbiol. 2009, 47, 4078–4083. [Google Scholar] [CrossRef]
- O’Connor, L.; Caplice, N.; Coleman, D.C.; Sullivan, D.J.; Moran, G.P. Differential filamentation of Candida albicans and Candida dubliniensis is governed by nutrient regulation of UME6 expression. Eukaryot. Cell 2010, 9, 1383–1397. [Google Scholar] [CrossRef]
- Song, X.; Tan, L.; Wang, M.; Ren, C.; Guo, C.; Yang, B.; Ren, Y.; Cao, Z.; Li, Y.; Pei, J. Myricetin: A review of the most recent research. Biomed. Pharmacother. 2021, 134, 111017. [Google Scholar] [CrossRef]
- Imran, M.; Saeed, F.; Hussain, G.; Imran, A.; Mehmood, Z.; Gondal, T.A.; El-Ghorab, A.; Ahmad, I.; Pezzani, R.; Arshad, M.U.; et al. Myricetin: A comprehensive review on its biological potentials. Food Sci. Nutr. 2021, 9, 5854–5868. [Google Scholar] [CrossRef] [PubMed]
- Aboody, M.S.A.; Mickymaray, S. Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef]
- Kadosh, D. Regulatory mechanisms controlling morphology and pathogenesis in Candida albicans. Curr. Opin. Microbiol. 2019, 52, 27–34. [Google Scholar] [CrossRef]
- Huang, G.; Huang, Q.; Wei, Y.; Wang, Y.; Du, H. Multiple roles and diverse regulation of the Ras/cAMP/protein kinase A pathway in Candida albicans. Mol. Microbiol. 2019, 111, 6–16. [Google Scholar] [CrossRef]
- Chow, E.W.L.; Pang, L.M.; Wang, Y. From Jekyll to Hyde: The Yeast-Hyphal Transition of Candida albicans. Pathogens 2021, 10, 859. [Google Scholar] [CrossRef]
- Lu, Y.; Su, C.; Liu, H. Candida albicans hyphal initiation and elongation. Trends Microbiol. 2014, 22, 707–714. [Google Scholar] [CrossRef]
- Marena, G.D.; Thomaz, L.; Nosanchuk, J.D.; Taborda, C.P. Galleria mellonella as an Invertebrate Model for Studying Fungal Infections. J. Fungi 2025, 11, 157. [Google Scholar] [CrossRef]
- Silva, L.N.; Da Hora, G.C.A.; Soares, T.A.; Bojer, M.S.; Ingmer, H.; Macedo, A.J.; Trentin, D.S. Myricetin protects Galleria mellonella against Staphylococcus aureus infection and inhibits multiple virulence factors. Sci. Rep. 2017, 7, 2823. [Google Scholar] [CrossRef]


| Genes | Primer | Sequence (5′ ⟶ 3′) | Reference |
|---|---|---|---|
| ALS3 | Forward | TCGTCCTCATTACACCAACCA | Sumlu et al., 2024 [13]. |
| Reverse | TGAAGTTGCAGATGGGGCTT | ||
| HWP1 | Forward | GCTCCTGCTCCTGAAATGAC | Holcombe et al., 2010 [26] |
| Reverse | CTGGAGCAATTGGTGAGGTT | ||
| ECE1 | Forward | TTGCTAATGCCGTCGTCAGA | Willems et al., 2018 [27] |
| Reverse | GAACGACCATCTCTCTTGGCAT | ||
| RAS1 | Forward | TGGATGTTGTGTTATTGTTTGAGC | Sumlu et al., 2024 [13] |
| Reverse | GTCTTGAATTGTTCATCTTCTCCCA | ||
| CYR1 | Forward | CCAACAAACGACCAAAAGGT | Hsu et al., 2013 [28] |
| Reverse | TCTTGAACTGCCAGACGATG | ||
| EFG1 | Forward | GCCTCGAGCACTTCCACTGT | Uppuluri et al., 2009 [29] |
| Reverse | TTTTTTCATCTTCCCACATGGTAGT | ||
| UME6 | Forward | ACCACCACTACCACCACCAC | O’Connor et al., 2010 [30] |
| Reverse | TATCCCCATTTCCAAGTCCA | ||
| HGC1 | Forward | GCTTCCTGCACCTCATCAAT | Hsu et al., 2013 [28] |
| Reverse | AGCACGAGAACCAGCGATAC | ||
| ACT1 | Forward | TTTCATCTTCTGTATCAGAGGAACTTATTT | Sumlu et al., 2024 [13] |
| Reverse | ATGGGATGAATCATCAAACAAGAG |
| Species (Number) | MIC50 Range of FLC (µg/mL) | MIC50 Range of MYR (µg/mL) |
|---|---|---|
| C. albicans ATCC 10231 | 0.5 | 320 |
| C. krusei ATCC 6258 | 32 | 80 |
| C. parapsilosis ATCC 22019 | 16 | 40 |
| C. glabrata ATCC 90030 | 16 | 40 |
| C. albicans (10) | 0.5–16 | 40–640 |
| C. glabrata (4) | 2–16 | 40 |
| C. tropicalis (4) | 2–16 | 40–640 |
| C. kefyr (4) | 1 | 320 |
| C. spherica (2) | 2 | 320 |
| C. krusei (2) | 32–128 | 320 |
| C. lusitaniae (2) | 0.5 | 320 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meral Ocal, M.; Aydin, M.; Sumlu, E.; Korucu, E.N.; Ozturk, A. Myricetin Exerts Antibiofilm Effects on Candida albicans by Targeting the RAS1/cAMP/EFG1 Pathway and Disruption of the Hyphal Network. J. Fungi 2025, 11, 398. https://doi.org/10.3390/jof11050398
Meral Ocal M, Aydin M, Sumlu E, Korucu EN, Ozturk A. Myricetin Exerts Antibiofilm Effects on Candida albicans by Targeting the RAS1/cAMP/EFG1 Pathway and Disruption of the Hyphal Network. Journal of Fungi. 2025; 11(5):398. https://doi.org/10.3390/jof11050398
Chicago/Turabian StyleMeral Ocal, Melda, Merve Aydin, Esra Sumlu, Emine Nedime Korucu, and Ali Ozturk. 2025. "Myricetin Exerts Antibiofilm Effects on Candida albicans by Targeting the RAS1/cAMP/EFG1 Pathway and Disruption of the Hyphal Network" Journal of Fungi 11, no. 5: 398. https://doi.org/10.3390/jof11050398
APA StyleMeral Ocal, M., Aydin, M., Sumlu, E., Korucu, E. N., & Ozturk, A. (2025). Myricetin Exerts Antibiofilm Effects on Candida albicans by Targeting the RAS1/cAMP/EFG1 Pathway and Disruption of the Hyphal Network. Journal of Fungi, 11(5), 398. https://doi.org/10.3390/jof11050398






