Neocotylidia gen. nov. (Hymenochaetales, Basidiomycota) Segregated from Cotylidia Based on Morphological, Phylogenetic, and Ecological Evidence
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Morphological Evaluation
2.3. DNA Extraction and Sequencing
2.4. Phylogenetic Analyses
3. Results
3.1. Phylogeny
3.2. Taxonomy
- Neocotylidia Jing Si and Hai J. Li, gen. nov.

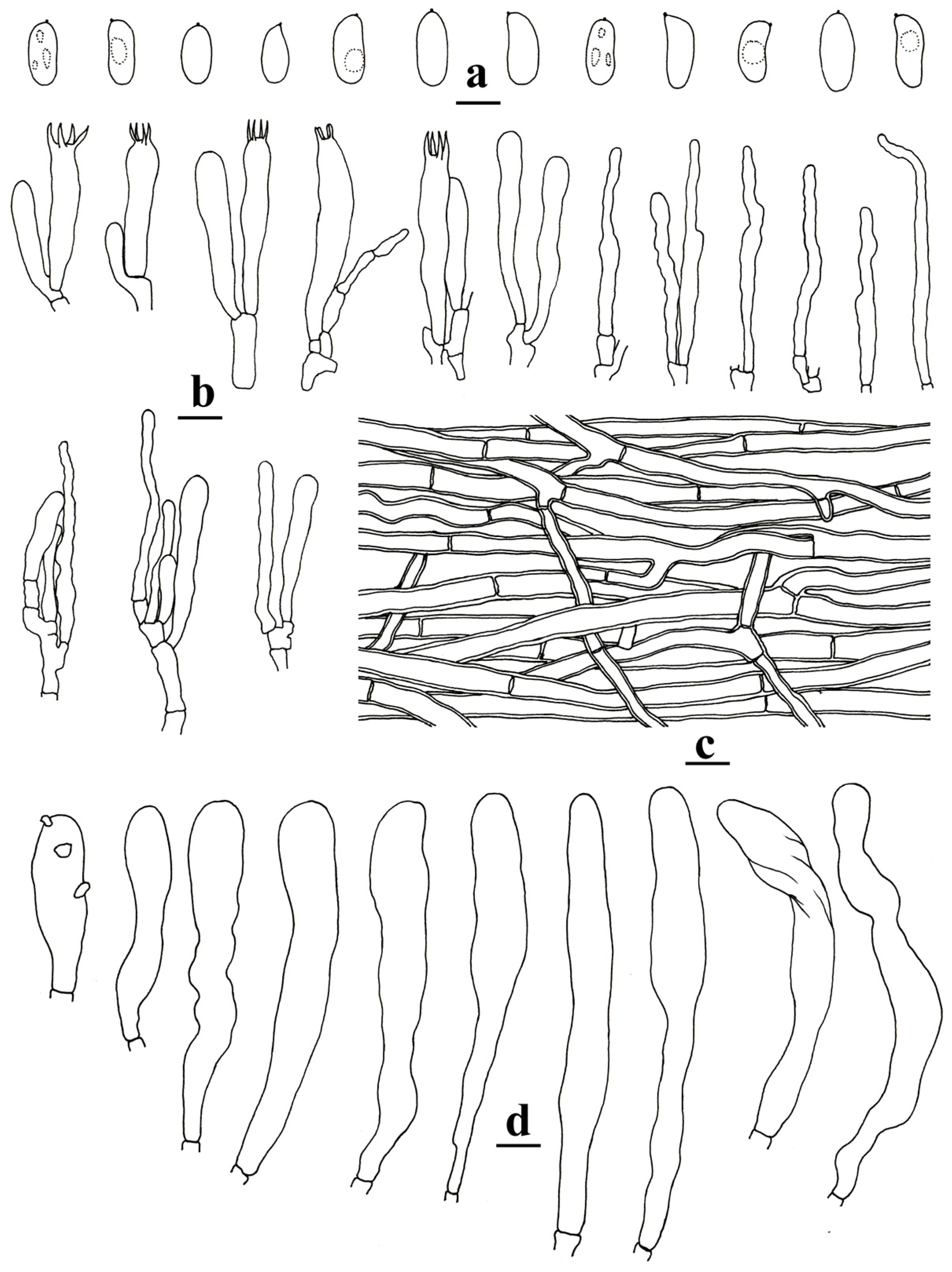
- Neocotylidia diaphana (Cooke) Jing Si and Hai J. Li, comb. nov.
- Neocotylidia fibrae (L. Fan and C. Yang) Jing Si and Hai J. Li, comb. nov.
- Cotylidia aurantiaca (Pat.) A.L. Welden, Lloydia 21: 40. 1958.
- Cotylidia aurantiaca var. alba D.A. Reid, Beih. Nova Hedwig. 18: 67. 1965.
- Notes on other species of Cotylidia recorded without molecular data
- Cotylidia guttulata L. Rémy, Bull. trimest. Soc. mycol. Fr. 80(4): 579. 1965.
- Cotylidia harmandii (Lloyd) D.A. Reid, Beih. Nova Hedwigia. 18: 76. 1965.
- Cotylidia komabensis (Henn.) D.A. Reid, Beih. Nova Hedwigia. 18: 77. 1965.
- Cotylidia marsicana Lonati, Micol. Veg. Medit. 15(1): 3. 2000.
- Cotylidia muscigena L. Rémy, Bull. trimest. Soc. mycol. Fr. 80(4): 579. 1965.
- Cotylidia pannosa (Sowerby) D.A. Reid, Beih. Nova Hedwigia 18: 81. 1965.
4. Discussion
| Key to the worldwide species of Cotylidia s.l. |
| 1. Producing hymenial cystidia, pileocystidia, and caulocystidia, growth on moss············2 |
| 1. Only producing hymenial cystidia, growth on soil or wood··············································5 |
| 2. Basidiospores with two guttules································································Cotylidia guttulata |
| 2. Basidiospores without guttules······························································································3 |
| 3. Basidiospores > 5 µm in length·································································Cotylidia muscigena |
| 3. Basidiospores < 5 µm in length·······························································································4 |
| 4. Pileus pale alutaceous brown; stipe lateral; basidiospores 3–3.75 × 1.2–2.5 µm················· |
| ····························································································································Cotylidia carpatica |
| 4. Pileus buff to brownish, darker in the center; stipe central to eccentric; basidiospores |
| 4–5 × 2–2.5 µm···································································································Cotylidia undulata |
| 5. Basidiomata growing on wood·······························································································6 |
| 5. Basidiomata growing on soil···································································································8 |
| 6. Basidiomata growing on burnt wood, with white basidiomata, small hymenial cystidia |
| (40–60 × 4–6 μm) and basidiospores (4–5 × 3–3.5 µm)································Cotylidia marsicana |
| 6. Basidiomata most frequently growing on woody substrates, with bright yellow or white |
| basidiomata, large hymenial cystidia (up to 122 × 6–26 μm) and basidiospores (6.8–7.5 × |
| 3–3.75 µm)·····································································································································7 |
| 7. Pileus bright yellow··························································Cotylidia aurantiaca var. aurantiaca |
| 7. Pileus white to cream··································································Cotylidia aurantiaca var. alba |
| 8. Hymenial cystidia septate······································································Neocotylidia diaphana |
| 8. Hymenial cystidia nonseptate·································································································9 |
| 9. Basidiomata more or less white to cream·············································································10 |
| 9. Basidiomata pinkish, purplish-red, or at least bright red-orange at the margin·············12 |
| 10. Growing on the ground under Pinus tabuliformis, hymenophore covered with distinct |
| white fibers, hymenial cystidia (70–115 × 12.5–20 µm), basidiospores (5–5.5 × 3–3.5 µm)···· |
| ····························································································································Neocotylidia fibrae |
| 10. Not growing on the ground under Pinus tabuliformis, hymenophore without white |
| fibers··············································································································································11 |
| 11. Hymenial cystidia thick-walled, basidiospores large (7–9.3 × 3.2–4.4 µm)························· |
| ···················································································································Neocotylidia bambusicola |
| 11. Hymenial cystidia thin-walled, basidiospores small (6–8.5 × 2.5–3.5 µm)························· |
| ··························································································································Cotylidia komabensis |
| 12. Pileal surface bright red-orange at the margin (all basidiomata when young), large |
| hymenial cystidia (70–160 × 10–14 μm) and basidiospores (8–10 × 4–4.6 µm)························· |
| ······························································································································Cotylidia pannosa |
| 12. Pileus of dried material pinkish or purplish-red, small hymenial cystidia (up to 78 × |
| 7–9 μm) and basidiospores (6 × 3 µm)··························································Cotylidia harmandii |
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sjökvist, E.; Larsson, E.; Eberhardt, U.; Ryvarden, L.; Larsson, K.H. Stipitate stereoid basidiocarps have evolved multiple times. Mycologia 2012, 104, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Pine, E.M.; Hibbett, D.S.; Donoghue, M.J. Phylogenetic relationships of cantharelloid and clavarioid homobasidiomycetes based on mitochondrial and nuclear rDNA sequences. Mycologia 1999, 91, 944–963. [Google Scholar] [CrossRef][Green Version]
- Moncalvo, J.M.; Vilgalys, R.; Redhead, S.A.; Johnson, J.E.; James, T.Y.; Aime, M.C.; Hofstetter, V.; Verduin, S.J.W.; Larsson, E.; Baroni, T.J.; et al. One hundred and seventeen clades of euagarics. Mol. Phylogenet. Evol. 2002, 23, 357–400. [Google Scholar] [CrossRef] [PubMed]
- Larsson, K.H.; Larsson, E.; Kõljalg, U. High phylogenetic diversity among corticioid homobasidiomycetes. Mycol. Res. 2004, 108, 983–1002. [Google Scholar] [CrossRef]
- Larsson, K.H. Re-thinking the classification of corticioid fungi. Mycol. Res. 2007, 111, 1040–1063. [Google Scholar] [CrossRef]
- Binder, M.; Larsson, K.H.; Matheny, P.B.; Hibbett, D.S. Amylocorticiales ord. nov. and Jaapiales ord. nov.: Early diverging clades of Agaricomycetidae dominated by corticioid forms. Mycologia 2010, 102, 865–880. [Google Scholar] [CrossRef]
- Sjökvist, E.; Pfeil, B.E.; Larsson, E.; Larsson, K.H. Stereopsidales—A new order of mushroom-forming fungi. PLoS ONE 2014, 9, e106204. [Google Scholar] [CrossRef]
- Wu, S.H.; Wei, C.L.; Chen, Y.P.; Chen, C.C.; Chen, S.Z. Schizocorticium gen. nov. (Hymenochaetales, Basidiomycota) with three new species. Mycol. Prog. 2021, 20, 769–779. [Google Scholar] [CrossRef]
- Yang, C.; Xu, Y.Y.; Fan, L. Cotylidia fibrae (Rickenellaceae, Hymenochaetales), a new species from China. Phytotaxa 2021, 487, 139–148. [Google Scholar] [CrossRef]
- Wang, X.W.; Liu, S.L.; Zhou, L.W. An updated taxonomic framework of Hymenochaetales (Agaricomycetes, Basidiomycota). Mycosphere 2023, 14, 452–496. [Google Scholar] [CrossRef]
- Wang, X.W.; Zhou, L.W. Umbellaceae fam. nov. (Hymenochaetales, Basidiomycota) for Umbellus sinensis gen. et sp. nov. and three new combinations. J. Fungi 2024, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Vizzini, A. Segnalazioni di muscinupta laevis (Basidiomycota, Agaricomycetes) per il Nord Italia. Micol. Veg. Mediterr. 2010, 25, 141–148. [Google Scholar]
- Liu, S.L.; Gafforov, Y.; Zhang, X.Y.; Wang, H.L.; Wang, X.W.; Zhou, L.W. Reinstatement of the corticioid genus Leifia (Hymenochaetales, Basidiomycota) with a new species L. brevispora from Hubei, Central China. MycoKeys 2019, 51, 85–96. [Google Scholar] [CrossRef]
- Yu, J.; Wang, X.W.; Liu, S.L.; Shen, S.; Zhou, L.W. Taxonomy and phylogeny of Resinicium sensu lato from Asia-Pacific revealing a new genus and five new species (Hymenochaetales, Basidiomycota). IMA Fungus 2021, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Karsten, P.A. Enumeratio Thelephorearum Fr. et Clavariearum Fr. Fennicarum, systemate novo dispositarum. Rev. Mycol. Toulouse 1881, 3, 21–23. [Google Scholar]
- Liu, Z.B.; Zhou, M.; Yuan, Y.; Dai, Y.C. Global diversity and taxonomy of Sidera (Hymenochaetales, Basidiomycota): Four new species and keys to species of the genus. J. Fungi 2021, 7, 251. [Google Scholar] [CrossRef]
- Korotkin, H.B.; Swenie, R.A.; Miettinen, O.; Budke, J.M.; Chen, K.H.; Lutzoni, F.; Smith, M.E.; Matheny, P.B. Stable isotope analyses reveal previously unknown trophic mode diversity in the Hymenochaetales. Am. J. Bot. 2018, 105, 1869–1887. [Google Scholar] [CrossRef]
- Reid, D.A. A Monograph of the Stipitate Stereoid Fungi; Beihefte zur Nova Hedwigia; Cramer: Weinheim, Germany, 1965; Volume 18, pp. 1–382. [Google Scholar]
- Lonati, G. Una nuova specie: Cotylidia marsicana. Micol. Veg. Mediterranea 2000, 15, 3–5. [Google Scholar]
- Ryvarden, L. Stereoid fungi of America. Fungiflora 2009, 28, 1–235. [Google Scholar]
- Bernicchia, A.; Gorjón, S.P. Corticiaceae s. l., Fungi Europaei; Candusso: Alassio, Italy, 2010; Volume 12, pp. 1–1008. [Google Scholar]
- Kout, J.; Zíbarová, L. Revision of the genus Cotylidia (Basidiomycota, Hymenochaetales) in the Czech Republic. Czech Mycol. 2013, 65, 1–13. [Google Scholar] [CrossRef]
- Huijsman, H.S.C. Cotylidia carpatica (Pilát) comb. nov. Bull. Société Mycol. France 1954, 70, 57–62. [Google Scholar]
- Lentz, P.L. Stereum and Allied Genera of Fungi in the Upper Mississippi Valley; Agriculture Monograph; US Department of Agriculture: Washington, DC, USA, 1955; Volume 24, pp. 1–74.
- Welden, A.L. A contribution toward a monograph of Colytidia. Lloydia 1958, 21, 38–44. [Google Scholar]
- Boidin, J. Hétérobasidiomycèstes saprophytes et Homobasidiomycètes résupinés: VI. Essai sur le genre Stereum sensu lato. Rev. Mycol. 1959, 24, 197–225. [Google Scholar]
- Reid, D.A. Notes on fungi which have been referred to the Thelephoraceae senso lato. Persoonia 1962, 2, 109–170. [Google Scholar]
- Rémy, L. Contribution à ľétude de la flore mycologique briançonnaise (Basidiomycètes et Discomycètes). Bull. Société Mycol. France 1965, 80, 459–585. [Google Scholar]
- Shang, B.; He, W.; Yu, C.J. Cotylidia harmandii−a new corticioid fungus in China. For. Res. 2006, 19, 114–116. [Google Scholar]
- Dai, Y.C. A revised checklist of corticioid and hydnoid fungi in China for 2010. Mycoscience 2011, 52, 69–79. [Google Scholar] [CrossRef]
- Yang, L. Discussion upon the vegetational types and the regularities of their distribution of the Qingling Mountain in Guiyang. J. Guizhou Norm. Univ. 1987, 1, 25–31. [Google Scholar]
- Chen, X.J.; Ren, X.B.; Huang, Y.; Chen, X. Investigation on ecological characteristics of Cornus controversa Hensl in Qianling Mountain. Guizhou Sci. 2006, 24, 75–80. [Google Scholar]
- Petersen, J.H. The Danish Mycological Society’s Colour-Chart; Foreningen til Svampekundskabens Fremme: Greve, Denmark, 1996. [Google Scholar]
- Li, Y.; He, S.H.; Zhang, Y.Z.; Liang, J.Q.; Sheng, W.Y.; Si, J.; Li, H.J. Crinipellis deutziae, Marasmius pinicola spp. nov., and C. rhizomaticola (Agaricales, Basidiomycota) new to China from Beijing. Forests 2023, 14, 1480. [Google Scholar] [CrossRef]
- Si, J.; Zhang, Y.Z.; Liang, J.Q.; Li, H.J. Morphology and phylogeny identify two new species and one new subspecies of Podoscypha from Yunnan Province, Southwest China. Front. Microbiol. 2023, 14, 1151365. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.X.; Zhang, Y.Z.; Liang, J.Q.; Jiang, S.F.; Si, J.; Li, H.J. Pyrofomes lagerstroemiae (Polyporaceae, Basidiomycota), a new species from Hubei Province, Central China. Phytotaxa 2023, 583, 191–198. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Hibbett, D.S. Phylogenetic evidence for horizontal transmission of group I introns in the nuclear ribosomal DNA of mushroomforming fungi. Mol. Biol. Evol. 1996, 13, 903–917. [Google Scholar] [CrossRef]
- Matheny, P.B. Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe; Agaricales). Mol. Phylogenet. Evol. 2005, 35, 1–20. [Google Scholar] [CrossRef]
- Rehner, S.A.; Buckley, E. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Chenna, R.; Sugawara, H.; Koike, T.; Lopez, R.; Gibson, T.J.; Higgins, D.G.; Thompson, J.D. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003, 31, 3497–3500. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Stamatakis, A. RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hőhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Page, R.D.M. TreeView: An application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 1996, 12, 357–358. [Google Scholar] [PubMed]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.A.; Audet, S. Une récolte du champignon Cotylidia carpatica au Québec. Le Nat. Can. 2008, 132, 5–9. [Google Scholar]
- Jülich, W. Die Nichtblätterpilze, Gallertpilze und Bauchpilze. Aphyllophorales, Heterobasidiomycetes, Gastromycetes. In Kleine Kryptogamenflora Band; Gams, H., Ed.; G. Fischer: Stuttgart, Germany, 1984; Volume IIb/1, pp. 1–626. [Google Scholar]
- Moreau, P.A.; Wuilbaut, J.J.; Courtecuisse, R. Cyphellostereum, Cotylidia et autres Podoscyphaceae stipitées d’Europe. Doc. Mycol. 2008, 34, 53–75. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Zhang, Y.; Liang, J.; Li, Y.; Zhao, H.; Shang, Z.; Si, J.; Li, H. Neocotylidia gen. nov. (Hymenochaetales, Basidiomycota) Segregated from Cotylidia Based on Morphological, Phylogenetic, and Ecological Evidence. J. Fungi 2025, 11, 390. https://doi.org/10.3390/jof11050390
Ma J, Zhang Y, Liang J, Li Y, Zhao H, Shang Z, Si J, Li H. Neocotylidia gen. nov. (Hymenochaetales, Basidiomycota) Segregated from Cotylidia Based on Morphological, Phylogenetic, and Ecological Evidence. Journal of Fungi. 2025; 11(5):390. https://doi.org/10.3390/jof11050390
Chicago/Turabian StyleMa, Jinxin, Yizhe Zhang, Jiaqi Liang, Yue Li, Heng Zhao, Zhirui Shang, Jing Si, and Haijiao Li. 2025. "Neocotylidia gen. nov. (Hymenochaetales, Basidiomycota) Segregated from Cotylidia Based on Morphological, Phylogenetic, and Ecological Evidence" Journal of Fungi 11, no. 5: 390. https://doi.org/10.3390/jof11050390
APA StyleMa, J., Zhang, Y., Liang, J., Li, Y., Zhao, H., Shang, Z., Si, J., & Li, H. (2025). Neocotylidia gen. nov. (Hymenochaetales, Basidiomycota) Segregated from Cotylidia Based on Morphological, Phylogenetic, and Ecological Evidence. Journal of Fungi, 11(5), 390. https://doi.org/10.3390/jof11050390





