Feasibility of Bamboo Sawdust as Sustainable Alternative Substrate for Auricularia heimuer Cultivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains
2.2. Media Formulation
2.3. Transcriptome Sequencing and Analysis
2.4. Oxidizing and Reducing Substances Determination
2.5. Cultivation and Management
2.6. Agronomic and Quality Trait Measurement
2.7. Statistical Analysis
3. Results
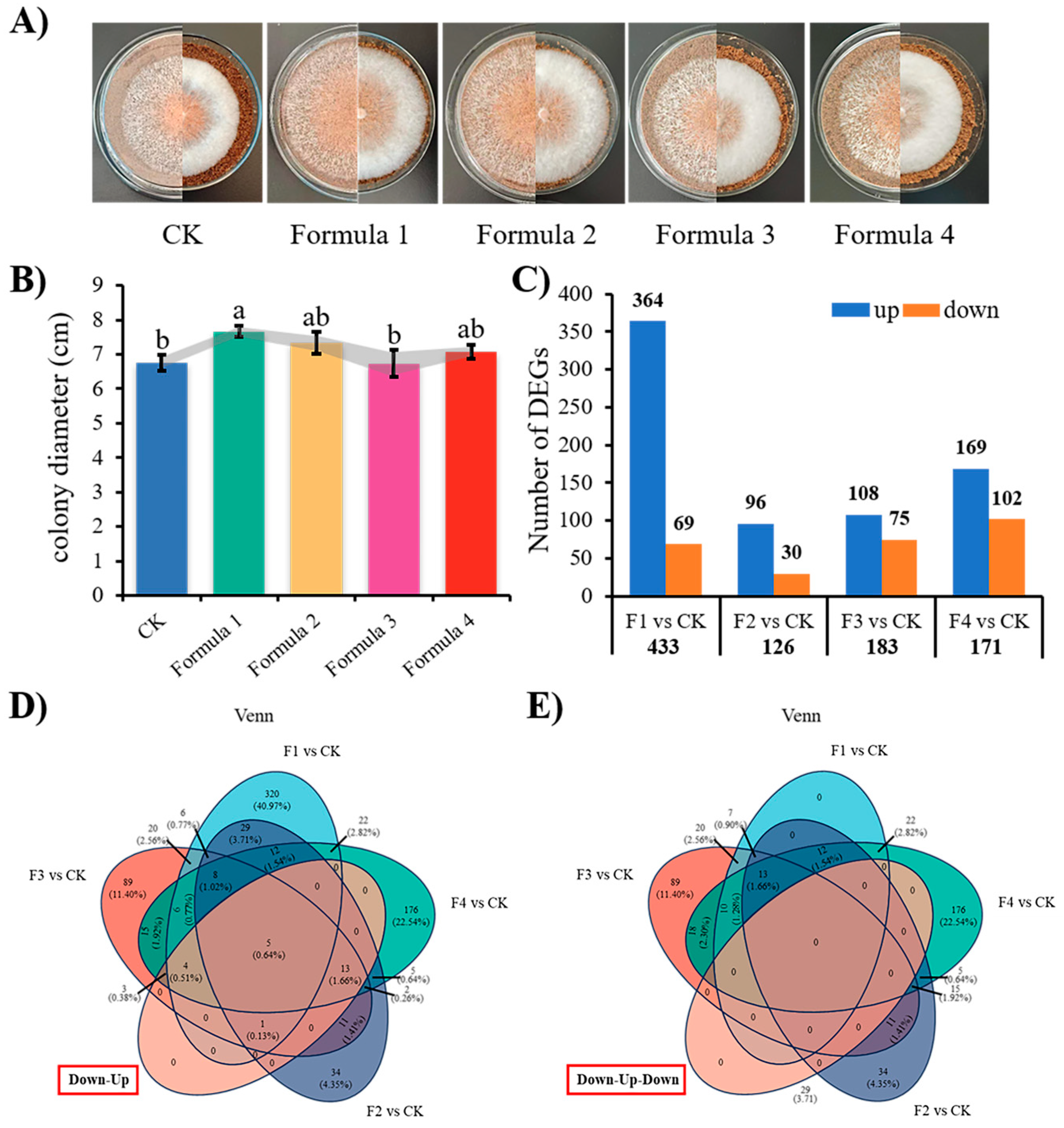
3.1. Mycelial Growth and Transcriptome Analysis
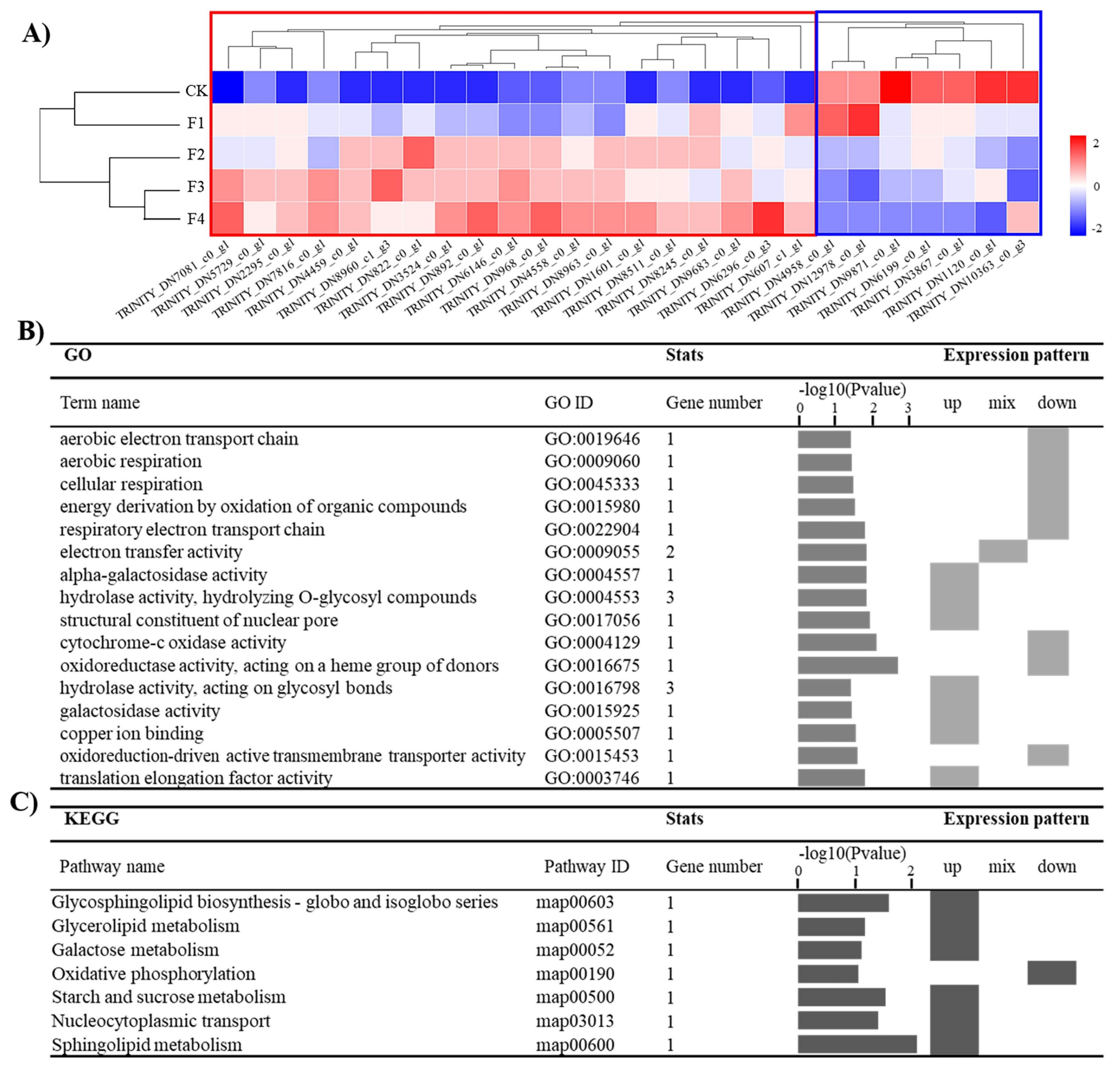
3.2. Down–Up–Down Gene Set Analysis
3.3. Down–Up Gene Set Analysis
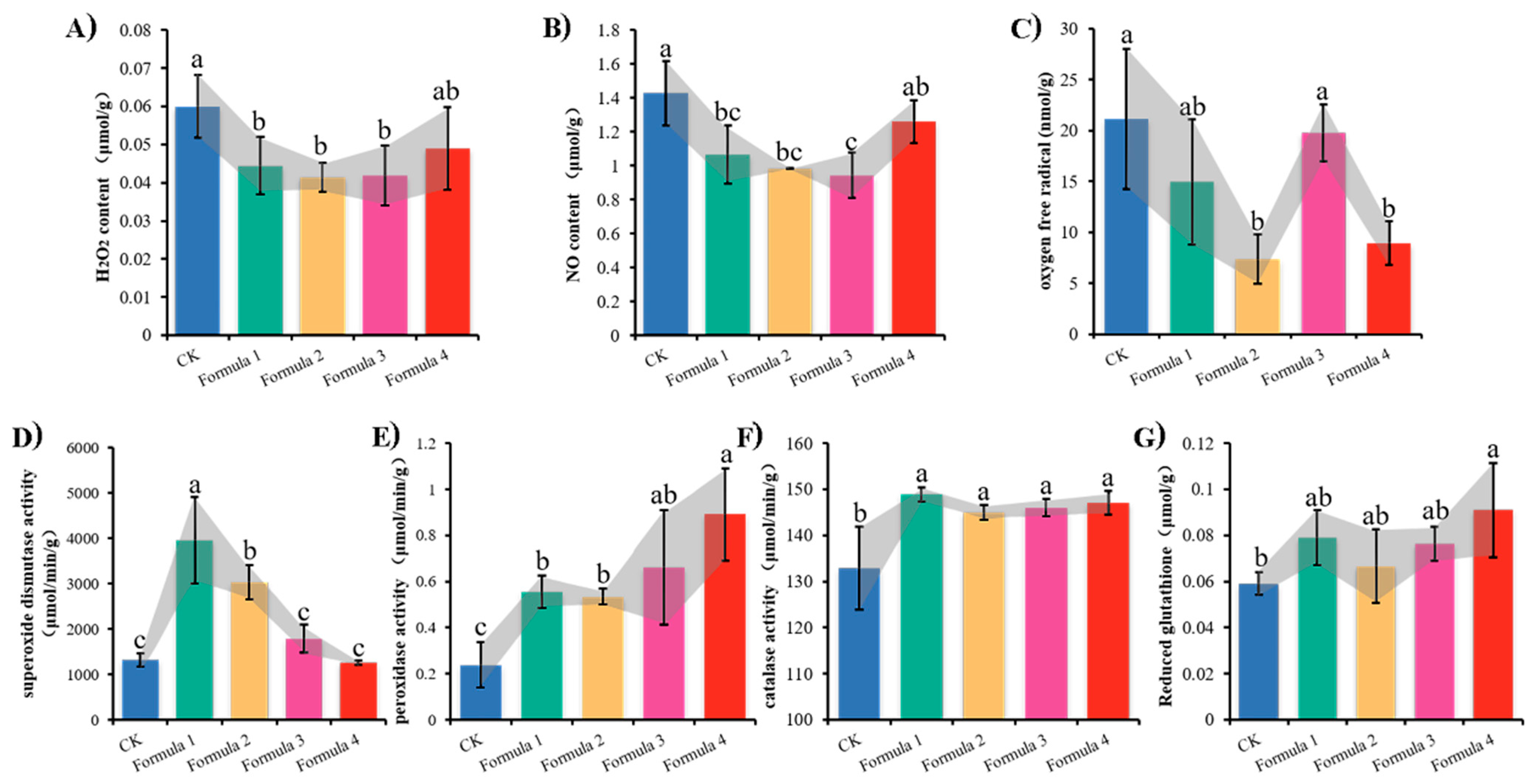
3.4. Mycelial Redox State Analysis
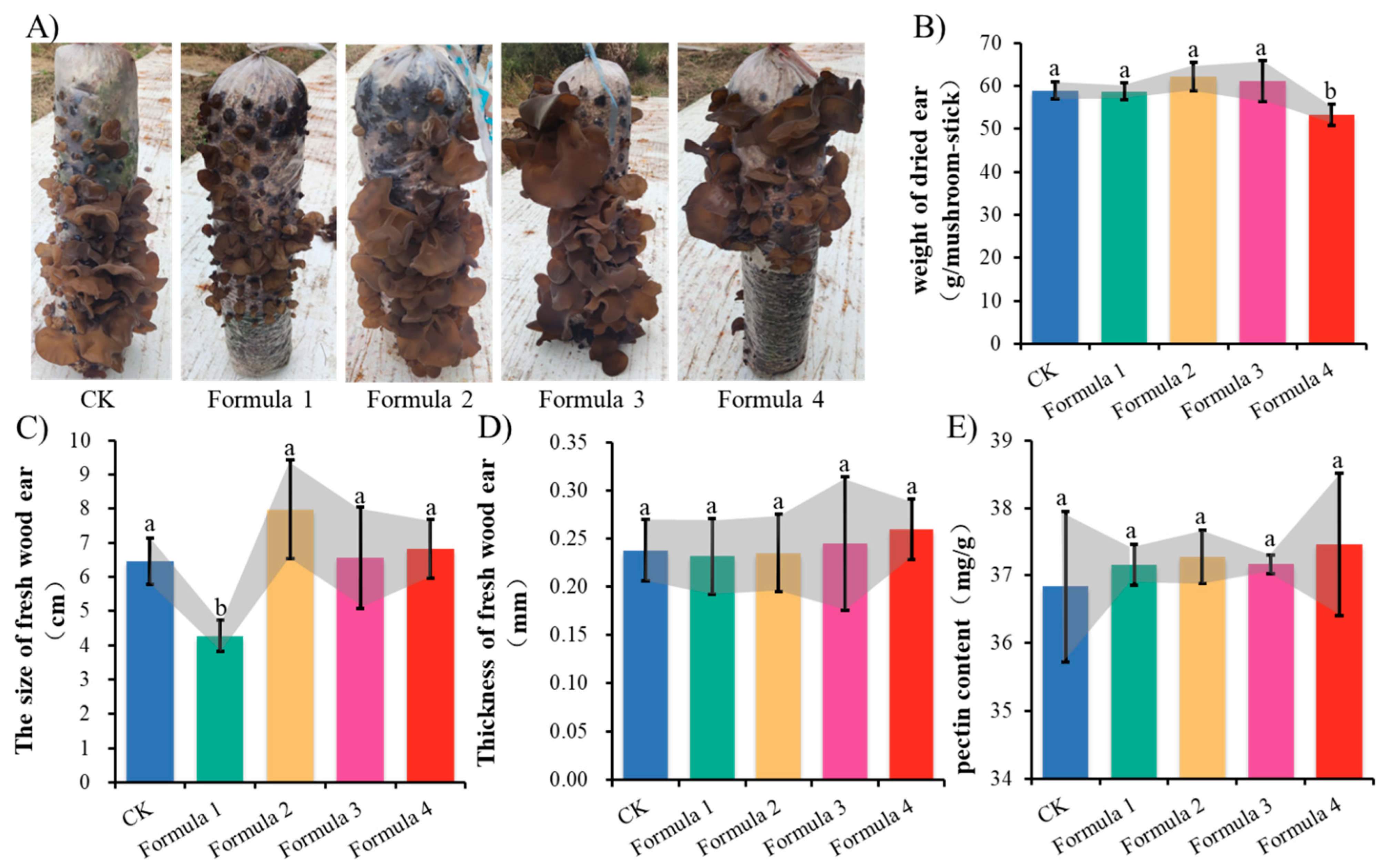
3.5. Bamboo Sawdust Cultivated Wood Ear
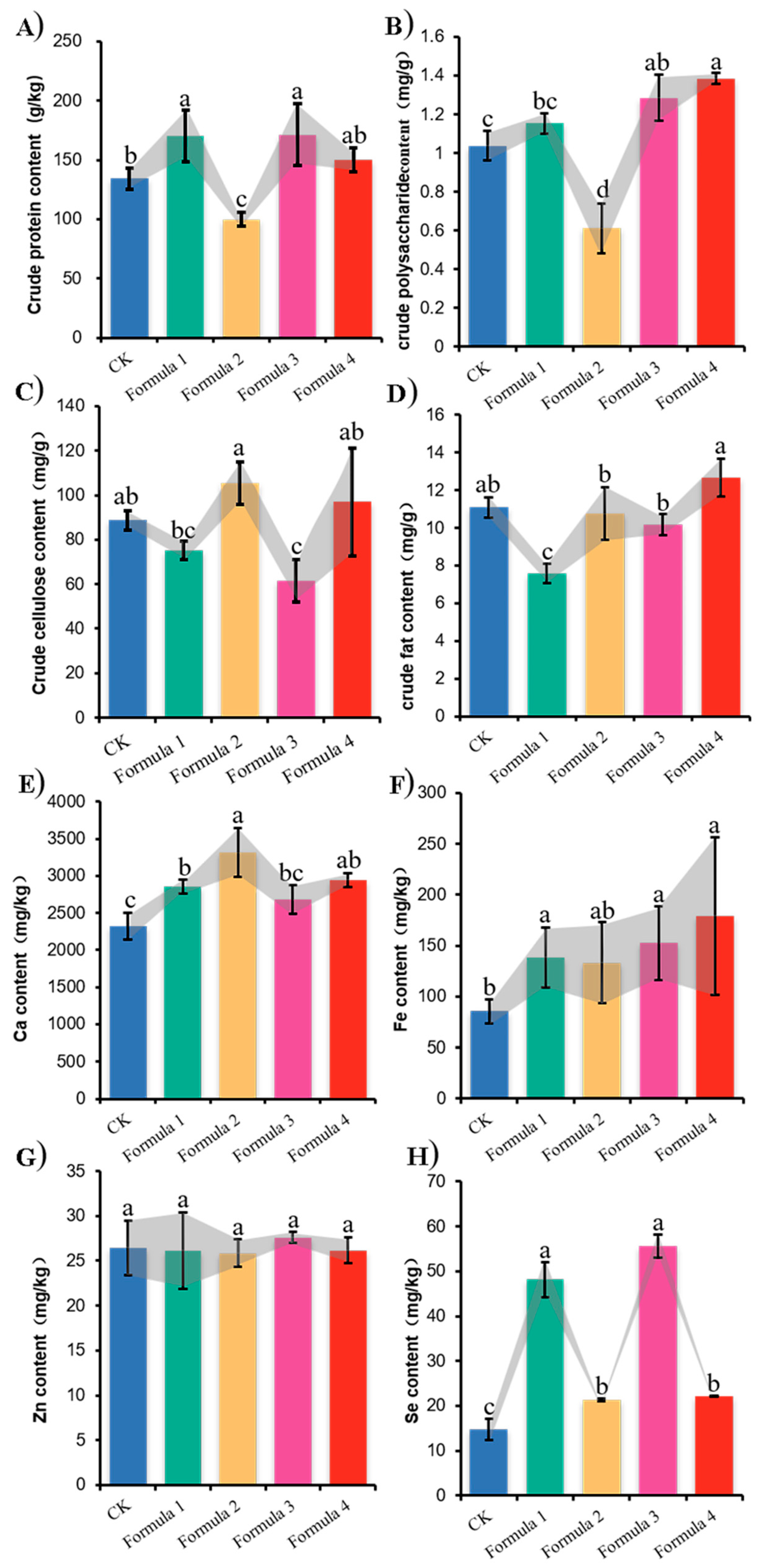
3.6. Nutritional Analysis of Wood Ear
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Xu, S. Edible mushroom industry in China: Current state and perspectives. Appl. Microbiol. Biotechnol. 2022, 106, 3949–3955. [Google Scholar] [CrossRef] [PubMed]
- Balan, V.; Zhu, W.; Krishnamoorthy, H.; Benhaddou, D.; Mowrer, J.; Husain, H.; Eskandari, A. Challenges and opportunities in producing high-quality edible mushrooms from lignocellulosic biomass in a small scale. Appl. Microbiol. Biotechnol. 2022, 106, 1355–1374. [Google Scholar] [CrossRef] [PubMed]
- Rytioja, J.; Hilden, K.; Yuzon, J.; Hatakka, A.; de Vries, R.P.; Makela, M.R. Plant-polysaccharide-degrading enzymes from basidiomycetes. Microbiol. Mol. Biol. Rev. 2014, 78, 614–649. [Google Scholar] [CrossRef]
- Xu, L.; Yang, W.; Qiu, T.; Gao, X.; Zhang, H.; Zhang, S.; Cui, H.; Guo, L.; Yu, H.; Yu, H. Complete genome sequences and comparative secretomic analysis for the industrially cultivated edible mushroom Lyophyllum decastes reveals insights on evolution and lignocellulose degradation potential. Front. Microbiol. 2023, 14, 1137162. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Liang, C.-H.; Wu, K.-J.; Shih, H.-D.; Liang, Z.-C. Effect of different proportions of agrowaste on cultivation yield and nutritional composition of the culinary-medicinal jelly mushroom Auricularia polytricha (Higher Basidiomycetes). Int. J. Med. Mushrooms 2017, 19, 377–385. [Google Scholar] [CrossRef]
- Zhang, Y.; Geng, W.; Shen, Y.; Wang, Y.; Dai, Y.-C. Edible mushroom cultivation for food security and rural development in China: Bio-innovation, technological dissemination and marketing. Sustainability 2014, 6, 2961–2973. [Google Scholar] [CrossRef]
- Xu, A.; Yang, D.; Jacob, M.S.; Qian, K.; Yang, X.; Zhang, B.; Li, X. Comprehensive evaluation of agronomic traits and mineral elements of Auricularia heimuer cultivated on corncob substrates. Sci. Hortic. 2023, 314, 111942. [Google Scholar] [CrossRef]
- Fang, M.; Sun, X.; Yao, F.; Lu, L.; Ma, X.; Shao, K.; Kaimoyo, E. A combination of transcriptome and enzyme activity analysis unveils key genes and patterns of corncob lignocellulose degradation by Auricularia heimuer under cultivation conditions. J. Fungi 2024, 10, 545. [Google Scholar] [CrossRef]
- Cheng, Z.; Mu, C.; Li, X.; Cheng, W.; Cai, M.; Wu, C.; Jiang, J.; Fang, H.; Bai, Y.; Zheng, H.; et al. Single-cell transcriptome atlas reveals spatiotemporal developmental trajectories in the basal roots of moso bamboo (Phyllostachys edulis). Hortic. Res. 2023, 10, uhad122. [Google Scholar] [CrossRef]
- Sawarkar, A.D.; Shrimankar, D.D.; Kumar, M.; Kumar, P.; Singh, L. Bamboos as a cultivated medicinal grass for industries: A systematic review. Ind. Crops Prod. 2023, 203, 117210. [Google Scholar] [CrossRef]
- Dlamini, L.C.; Fakudze, S.; Makombe, G.G.; Muse, S.; Zhu, J. Bamboo as a valuable resource and its utilization in historical and modern-day China. Bioresources 2022, 17, 1926–1938. [Google Scholar] [CrossRef]
- Zhou, G.; Meng, C.; Jiang, P.; Xu, Q. Review of carbon fixation in bamboo forests in China. Bot. Rev. 2011, 77, 262–270. [Google Scholar] [CrossRef]
- Jin, L.; Wu, D.; Li, C.; Zhang, A.; Xiong, Y.; Wei, R.; Zhang, G.; Yang, S.; Deng, W.; Li, T.; et al. Bamboo nutrients and microbiome affect gut microbiome of giant panda. Symbiosis 2020, 80, 293–304. [Google Scholar] [CrossRef]
- Mo, W.; Li, B.; Liu, J.; Kong, F.; Chen, K. Short-term thermal drying-induced pore expansion effects of cellulosic fibers and its applications. Cellulose 2023, 30, 183–199. [Google Scholar] [CrossRef]
- Xiao, K.; Liang, X.; Lu, H.; Li, X.; Zhang, Z.; Lu, X.; Wang, H.; Meng, Y.; Roy, A.; Luo, W.; et al. Adaptation of gut microbiome and host metabolic systems to lignocellulosic degradation in bamboo rats. ISME J. 2022, 16, 1980–1992. [Google Scholar] [CrossRef]
- Liufu, Y.; Xi, F.; Wu, L.; Zhang, Z.; Wang, H.; Wang, H.; Zhang, J.; Wang, B.; Kou, W.; Gao, J.; et al. Inhibition of DNA and RNA methylation disturbs root development of moso bamboo. Tree Physiol. 2023, 43, 1653–1674. [Google Scholar] [CrossRef]
- Shinohara, Y.; Otsuki, K. Comparisons of soil-water content between a Moso bamboo (Phyllostachys pubescens) forest and an evergreen broadleaved forest in western Japan. Plant Species Biol. 2015, 30, 96–103. [Google Scholar] [CrossRef]
- Chutimanukul, P.; Phatthanamas, W.; Thepsilvisut, O.; Chantarachot, T.; Thongtip, A.; Chutimanukul, P. Commercial scale production of Yamabushitake mushroom (Hericium erinaceus (Bull.) Pers. 1797) using rubber and bamboo sawdust substrates in tropical regions. Sci. Rep. 2023, 13, 13316. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, B.; Yang, X.; Li, X.; Zhang, X.; Tan, W. Feasibility of Auricularia cornea cultivation with bamboo sawdust instead of wood sawdust and comprehensive evaluation of quality. Acta Agric. Zhejiangensis 2023, 35, 1416–1426. [Google Scholar]
- Zeng, F.; Ying, G.; Jiang, J.; Lv, M. Experiment of cultivating Lentinula edodes with Phyllostachys edulis sawdust. Edible Fungi China 2022, 41, 30–33, 42. [Google Scholar]
- Fu, L.; Cheng, J.; Hu, C.; Wei, H.; Li, H.; He, L. Study on Auricularia auricula-judae cultivation using waste of Phyllostachys edulis processing as substrate. Edible Fungi China 2015, 34, 36–38. [Google Scholar]
- Li, G.; Wang, Y.; Chen, Y.; Zhu, P.; Zhao, G.; Liu, C.; Zhao, H. Auricularia heimuer new cultivar ‘Wanheimuer 1(A3)’. Acta Hortic. Sin. 2023, 50, 89–90. [Google Scholar]
- Jacinto-Azevedo, B.; Valderrama, N.; Henriquez, K.; Aranda, M.; Aqueveque, P. Nutritional value and biological properties of Chilean wild and commercial edible mushrooms. Food Chem. 2021, 356, 129651. [Google Scholar] [CrossRef]
- Kruzselyi, D.; Kovacs, D.; Vetter, J. Chemical analysis of king oyster mushroom pleurotus eryngii fruitbodies. Acta Aliment. 2016, 45, 20–27. [Google Scholar] [CrossRef]
- Sudheep, N.M.; Sridhar, K.R. Nutritional composition of two wild mushrooms consumed by the tribals of the Western Ghats of India. Mycology 2014, 5, 64–72. [Google Scholar] [CrossRef]
- Guo, L.-Q.; Lin, J.-Y.; Lin, J.-F. Non-volatile components of several novel species of edible fungi in China. Food Chem. 2007, 100, 643–649. [Google Scholar] [CrossRef]
- Qi-Jing, L.; Pei-Yan, L. Comparison of phenol-sulfuric acid method and anthrone sulfate method for determining the content of Ganoderma lucidum polysaccharides in Ganoderma lucidum spore. China Food Saf. 2024, 14, 89–91,95. [Google Scholar]
- Yamauchi, M.; Sakamoto, M.; Yamada, M.; Hara, H.; Taib, S.M.; Rezania, S.; Din, M.F.M.; Hanafi, F.H.M. Cultivation of oyster mushroom (Pleurotus ostrreatus) on fermented moso bamboo sawdust. J. King Saud Univ. Sci. 2019, 31, 490–494. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, L.; Li, S.-p.; Zhang, Z.; Wu, Y.-l. The analysis of selenoprotein and Se-polysaccharide in selenium-enriched Lentinan edodes. In Proceedings of the 1st International Conference on Energy and Environmental Protection (ICEEP 2012), Hohhot, China, 23–24 June 2012; pp. 2325–2329. [Google Scholar]
- Khan, R.; Zeb, A.; Roy, N.; Magar, R.T.; Kim, H.J.; Lee, K.W.; Lee, S.-W. Biochemical and structural basis of triclosan resistance in a novel Enoyl-Acyl carrier protein reductase. Antimicrob. Agents Chemother. 2018, 62, e00648-18. [Google Scholar] [CrossRef]
- Khare, D.; Hale, W.A.; Tripathi, A.; Gu, L.; Sherman, D.H.; Gerwick, W.H.; Hakansson, K.; Smith, J.L. Structural basis for cyclopropanation by a unique Enoyl-Acyl carrier protein reductase. Structure 2015, 23, 2213–2223. [Google Scholar] [CrossRef]
- de Ramon-Carbonell, M.; Sanchez-Torres, P. Penicillium digitatum MFS transporters can display different roles during pathogen-fruit interaction. Int. J. Food Microbiol. 2021, 337, 108918. [Google Scholar] [CrossRef] [PubMed]
- Yan, N. Structural advances for the major facilitator superfamily (MFS) transporters. Trends Biochem. Sci. 2013, 38, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Quistgaard, E.M.; Low, C.; Guettou, F.; Nordlund, P. Understanding transport by the major facilitator superfamily (MFS): Structures pave the way. Nat. Rev. Mol. Cell Biol. 2016, 17, 123–132. [Google Scholar] [CrossRef]
- Dos Santos, S.C.; Teixeira, M.C.; Dias, P.J.; Isabel, S.-C. MFS transporters required for multidrug/multixenobiotic (MD/MX) resistance in the model yeast: Understanding their physiological function through post-genomic approaches. Front. Physiol. 2014, 5, 00180. [Google Scholar] [CrossRef] [PubMed]
- Jain, B.P.; Pandey, S. WD40 repeat proteins: Signalling scaffold with diverse functions. Protein J. 2018, 37, 391–406. [Google Scholar] [CrossRef]
- Neis, A.; Pinto, L.d.S. Glycosyl hydrolases family 5, subfamily 5: Relevance and structural insights for designing improved biomass degrading cocktails. Int. J. Biol. Macromol. 2021, 193, 980–995. [Google Scholar] [CrossRef]
- Fang, M.; Wang, X.; Chen, Y.; Wang, P.; Lu, L.; Lu, J.; Yao, F.; Zhang, Y. Genome Sequence Analysis of Auricularia heimuer Combined with Genetic Linkage Map. J. Fungi 2020, 6, 37. [Google Scholar] [CrossRef]
- Tyibilika, V.; Setati, M.E.; Bloem, A.; Divol, B.; Camarasa, C. Differences in the management of intracellular redox state between wine yeast species dictate their fermentation performances and metabolite production. Int. J. Food Microbiol. 2024, 411, 110537. [Google Scholar] [CrossRef]
- Karapetyan, S.; Dong, X. Redox and the circadian clock in plant immunity: A balancing act. Free Radic. Biol. Med. 2018, 119, 56–61. [Google Scholar] [CrossRef]
- Hartmann, S.K.; Stockdreher, Y.; Wandrey, G.; Tehrani, H.H.; Zambanini, T.; Meyer, A.J.; Buechs, J.; Blank, L.M.; Schwarzlaender, M.; Wierckx, N. Online in vivo monitoring of cytosolic NAD redox dynamics in Ustilago maydis. Biochim. Biophys. Acta-Bioenerg. 2018, 1859, 1015–1024. [Google Scholar] [CrossRef]
- Vallieres, C.; Golinelli-Cohen, M.-P.; Guittet, O.; Lepoivre, M.; Huang, M.-E.; Vernis, L. Redox-based strategies against infections by eukaryotic pathogens. Genes 2023, 14, 778. [Google Scholar] [CrossRef] [PubMed]
- Correa-Aragunde, N.; Foresi, N.; Lamattina, L. Nitric oxide is a ubiquitous signal for maintaining redox balance in plant cells: Regulation of ascorbate peroxidase as a case study. J. Exp. Bot. 2015, 66, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Liu, Y.; Hou, C.; Xie, Q.; Tang, D.; Liu, F.; Lou, B.; Zhu, J. The Keap1-Nrf2/ARE signaling pathway regulates redox balance and apoptosis in the small yellow croaker (Larimichthys polyactis) under hypoxic stress. Sci. Total Environ. 2024, 957, 177396. [Google Scholar] [CrossRef] [PubMed]
- Moulis, J.-M. Cellular dynamics of transition metal exchange on proteins: A challenge but a bonanza for coordination chemistry. Biomolecules 2020, 10, 1584. [Google Scholar] [CrossRef]
- Fones, H.; Davis, C.A.R.; Rico, A.; Fang, F.; Smith, J.A.C.; Preston, G.M. Metal hyperaccumulation armors plants against disease. PLoS Pathogens 2010, 6, e1001093. [Google Scholar] [CrossRef]
- Kim, S.K.; Yoon, D.; Lee, S.-C.; Kim, J. Mo2C/Graphene Nanocomposite As a Hydrodeoxygenation Catalyst for the Production of Diesel Range Hydrocarbons. ACS Catal. 2015, 5, 3292–3303. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, F.J.; Sun, W.J.; Fang, M.; Wu, C.S. Screening of reference genes for qRT-PCR amplification in Auricularia heimuer. Mycosystema 2020, 39, 1510–1519. [Google Scholar]


| Formula | Wood Sawdust (%) | Bamboo Sawdust (%) | Wheat Bran (%) | Gypsum (%) | Travertine (%) | C (%) | N (%) | C/N |
|---|---|---|---|---|---|---|---|---|
| CK | 86 | 0 | 12 | 1 | 1 | 26.36 | 0.756 | 34.86 |
| 1 | 66 | 20 | 12 | 1 | 1 | 26.24 | 0.773 | 33.96 |
| 2 | 56 | 30 | 12 | 1 | 1 | 26.18 | 0.781 | 33.52 |
| 3 | 46 | 40 | 12 | 1 | 1 | 26.12 | 0.789 | 33.09 |
| 4 | 36 | 50 | 12 | 1 | 1 | 26.07 | 0.798 | 32.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-H.; Yan, C.-S.; Deng, Y.-J.; Zhu, Z.-F.; Sun, H.-A.; Li, H.-P.; Zhao, H.-Y.; Li, G.-Q. Feasibility of Bamboo Sawdust as Sustainable Alternative Substrate for Auricularia heimuer Cultivation. J. Fungi 2025, 11, 387. https://doi.org/10.3390/jof11050387
Wang Y-H, Yan C-S, Deng Y-J, Zhu Z-F, Sun H-A, Li H-P, Zhao H-Y, Li G-Q. Feasibility of Bamboo Sawdust as Sustainable Alternative Substrate for Auricularia heimuer Cultivation. Journal of Fungi. 2025; 11(5):387. https://doi.org/10.3390/jof11050387
Chicago/Turabian StyleWang, Ya-Hui, Cong-Sheng Yan, Yong-Jin Deng, Zheng-Fu Zhu, Hua-An Sun, Hui-Ping Li, Hong-Yuan Zhao, and Guo-Qing Li. 2025. "Feasibility of Bamboo Sawdust as Sustainable Alternative Substrate for Auricularia heimuer Cultivation" Journal of Fungi 11, no. 5: 387. https://doi.org/10.3390/jof11050387
APA StyleWang, Y.-H., Yan, C.-S., Deng, Y.-J., Zhu, Z.-F., Sun, H.-A., Li, H.-P., Zhao, H.-Y., & Li, G.-Q. (2025). Feasibility of Bamboo Sawdust as Sustainable Alternative Substrate for Auricularia heimuer Cultivation. Journal of Fungi, 11(5), 387. https://doi.org/10.3390/jof11050387






