High-Throughput Sequencing Uncovers Fungal Community Succession During Morchella sextelata Development
Abstract
1. Introduction
2. Materials and Methods
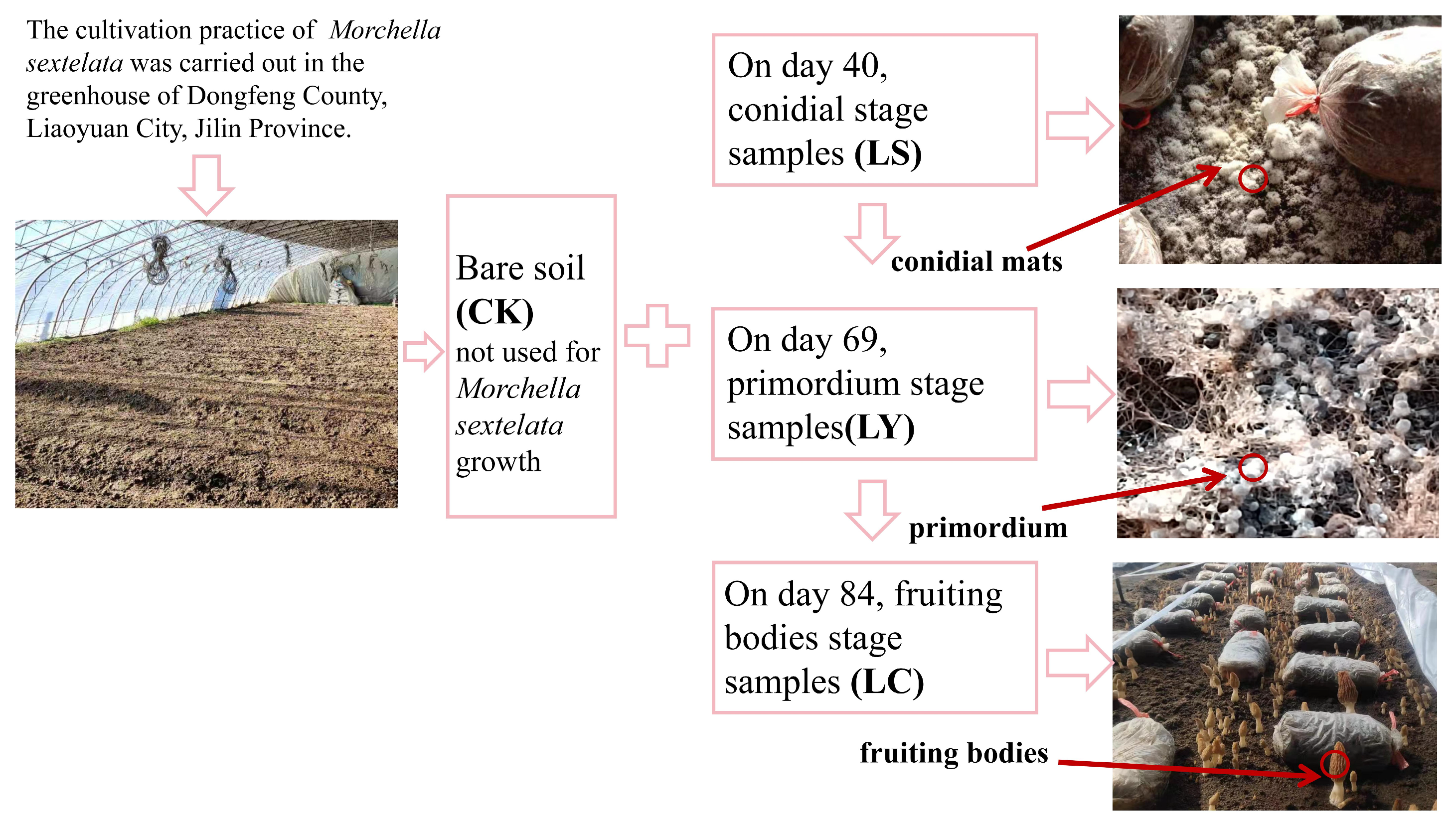
2.1. Experiment Description and Sampling
2.2. Soil DNA Extraction, PCR Amplification, and Sequencing
2.3. Statistical Analysis
3. Results
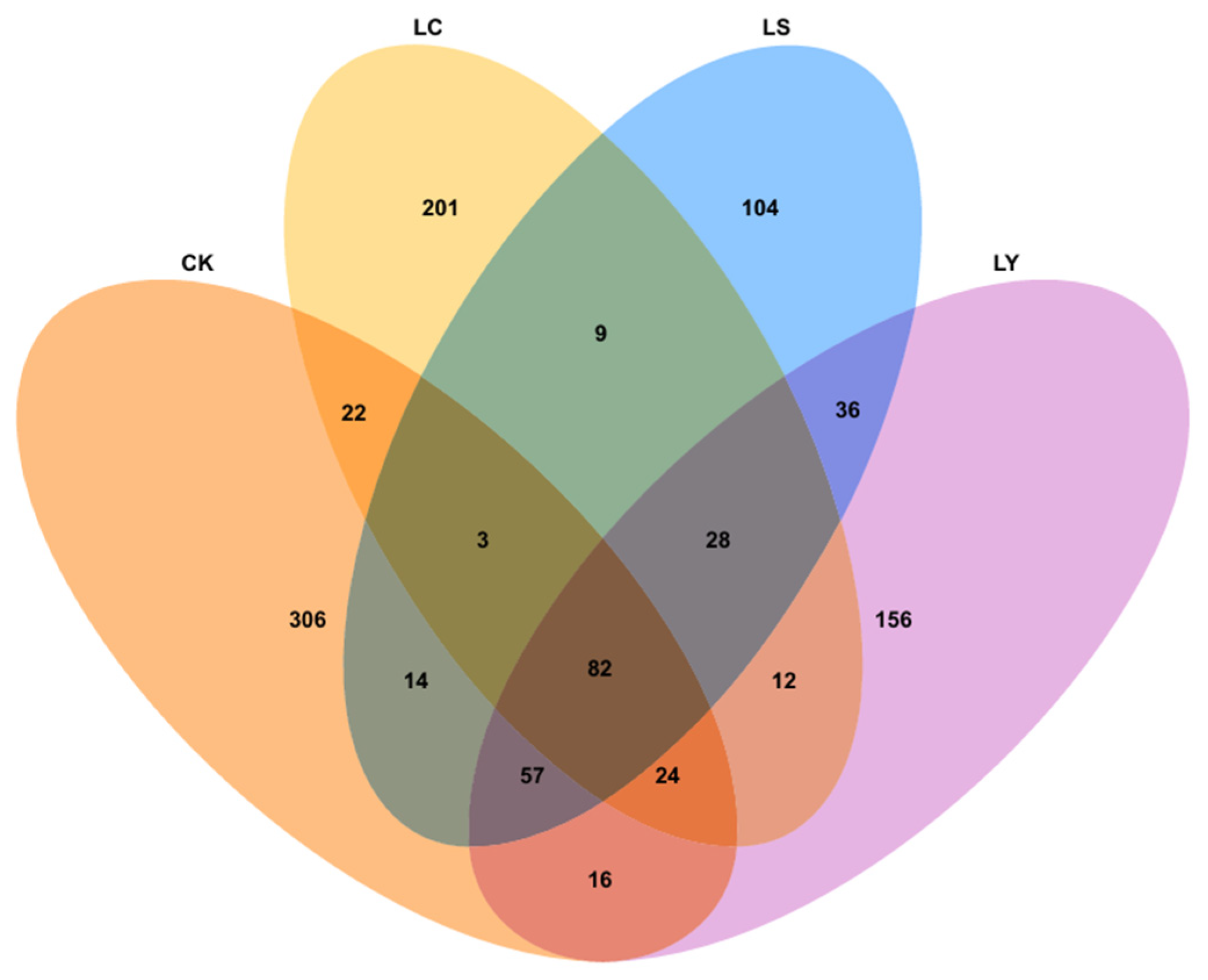
3.1. Analysis of Sequencing Data of Soil Samples
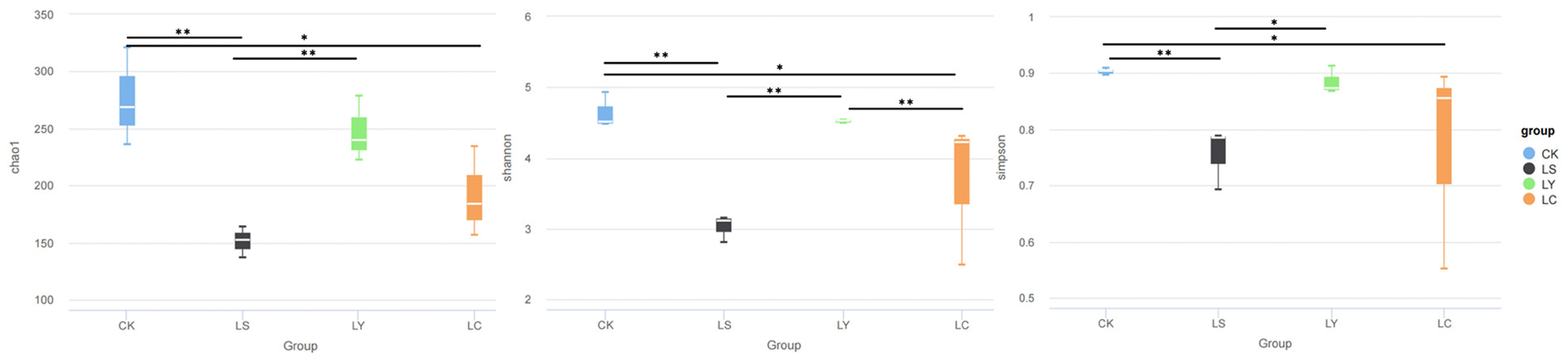
3.2. Changes in Fungal Community Diversity
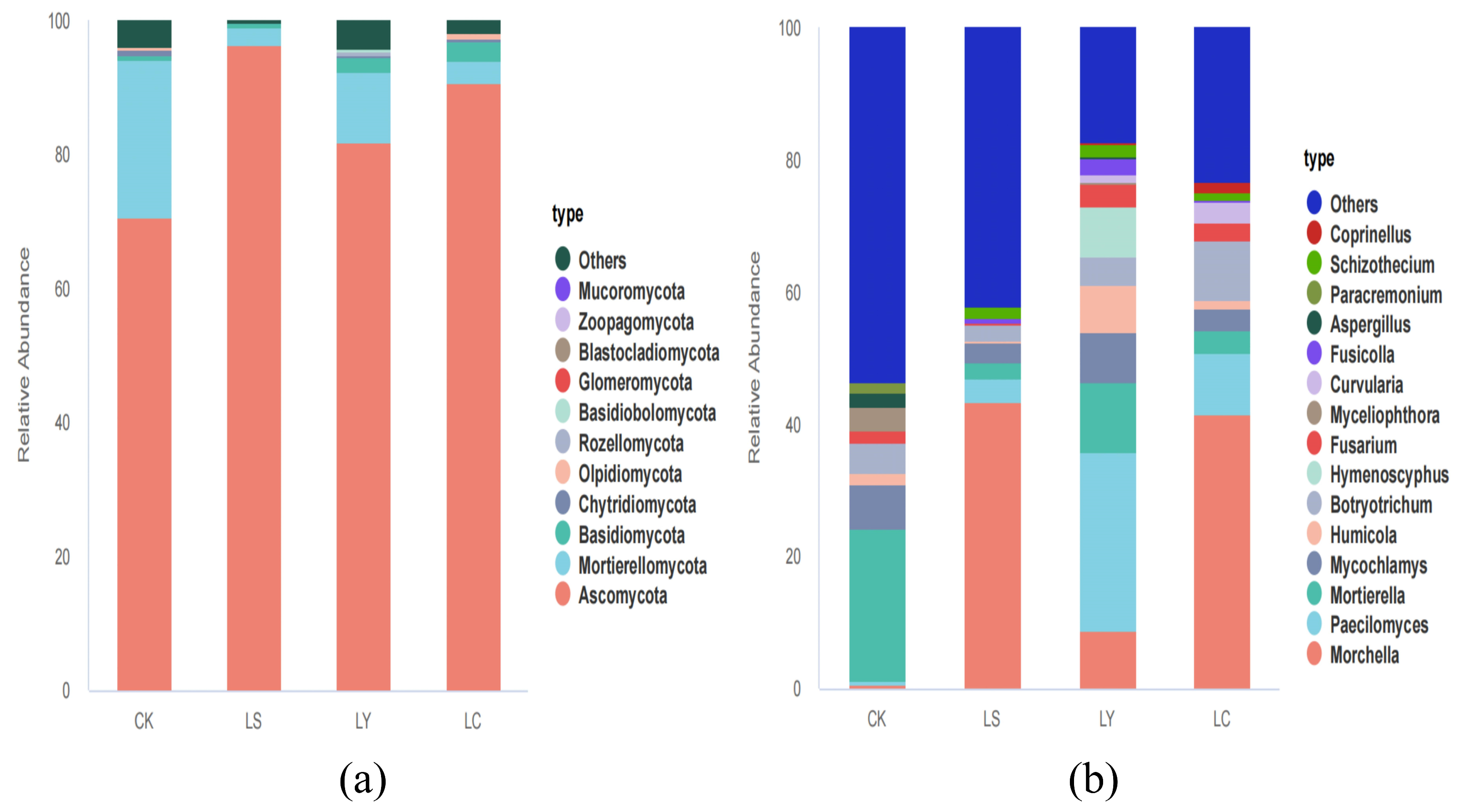
3.3. Changes in Fungal Community Composition
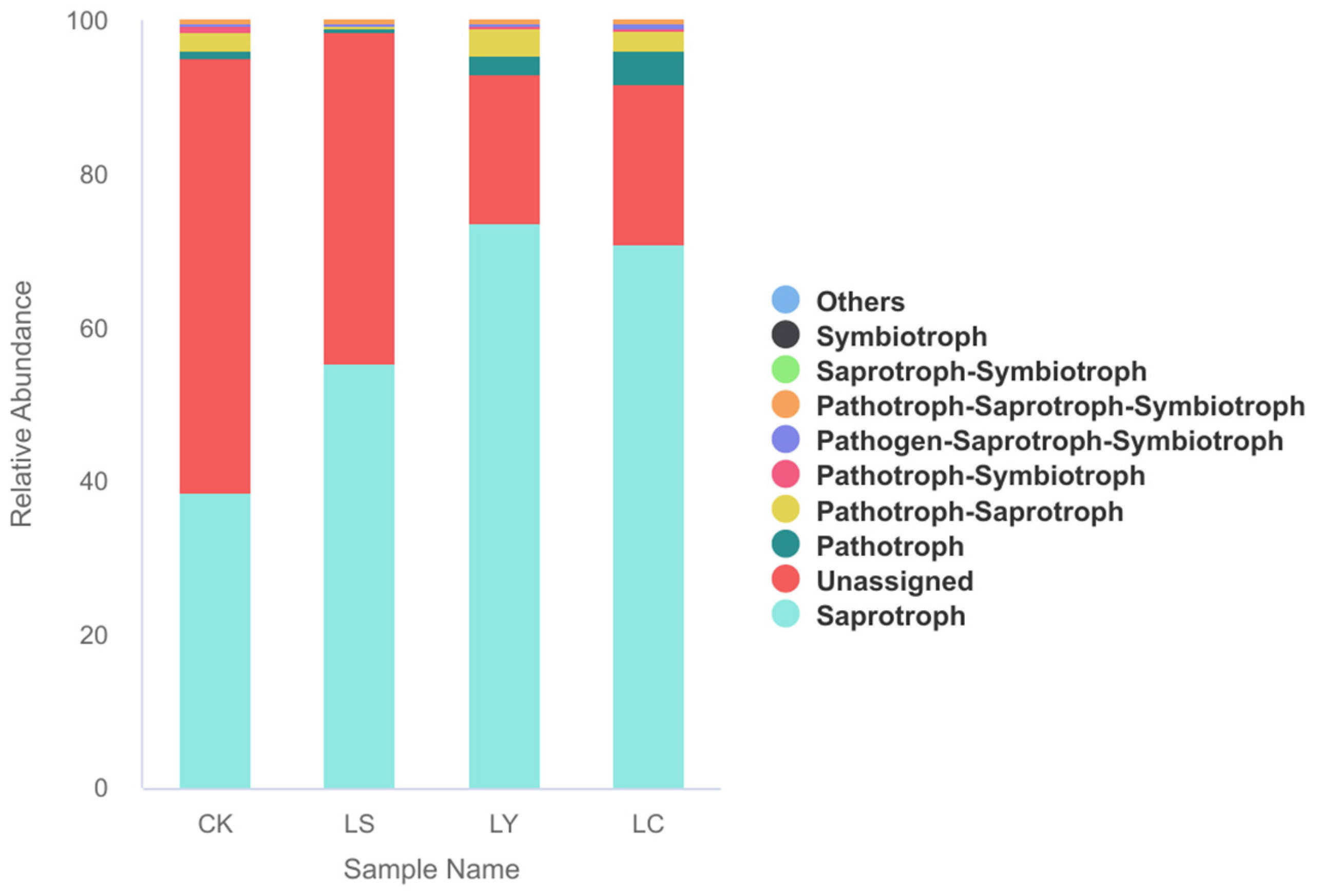
3.4. Functional Prediction of Microorganisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, X.H.; Zhao, Q.; Yang, Z.L. A Review on Research Advances, Issues, and Perspectives of Morchellas. Mycology 2015, 6, 78–85. [Google Scholar] [CrossRef]
- Tietel, Z.; Masaphy, S. True Morels (Morchella)—Nutritional and Phytochemical Composition, Health Benefits and Flavor: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1888–1901. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Sun, Y.; Deng, T.; Song, L.; Li, P.; Zeng, H.; Tang, X. Identification and Functional Characterization of a Novel Immunomodulatory Protein from Morchella conica SH. Front. Immunol. 2020, 11, 559770. [Google Scholar] [CrossRef]
- Sunil, C.; Xu, B. Mycochemical Profile and Health-Promoting Effects of Morel Mushroom Morchella esculenta (L.)—A Review. Food Res. Int. 2022, 159, 111571. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, H.; Zhang, Y.; Dong, C. Artificial Cultivation of True Morels: Current State, Issues and Perspectives. Crit. Rev. Biotechnol. 2018, 38, 259–271. [Google Scholar] [CrossRef] [PubMed]
- McGee, C.F. Microbial Ecology of the Agaricus bisporus Mushroom Cropping Process. Appl. Microbiol. Biotechnol. 2018, 102, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.-T.; Song, T.-T.; Shen, Y.-Y.; Jin, Q.-L.; Feng, W.-L.; Fan, L.J.; Cai, W.-M. Diversity of Bacterial and Fungal Communities in Wheat Straw Compost for Agaricus bisporus Cultivation. HortScience 2019, 54, 100–109. [Google Scholar] [CrossRef]
- Ren, F.; Zhang, Y.-G.; Yu, H.; Zhang, Y.-A. Ganoderma lucidum Cultivation Affects Microbial Community Structure of Soil, Wood Segments and Tree Roots. Sci. Rep. 2020, 10, 3435. [Google Scholar] [CrossRef]
- Zhang, B.; Yan, L.-J.; Li, Q.; Zou, J.; Tan, H.; Tan, W.; Peng, W.-H.; Li, X.-L.; Zhang, X.-P. Dynamic Succession of Substrate-Associated Bacterial Composition and Function during Ganoderma lucidum Growth. PeerJ 2018, 6, e4975. [Google Scholar] [CrossRef]
- Yang, R.-H.; Bao, D.-P.; Guo, T.; Li, Y.; Ji, G.-Y.; Ji, K.-P.; Tan, Q. Bacterial Profiling and Dynamic Succession Analysis of Phlebopus portentosus Casing Soil Using MiSeq Sequencing. Front. Microbiol. 2019, 10, 1927. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Y.-L.; Zhang, P.; Yu, F.-Q.; Perez-Moreno, J. Macrofungi Cultivation in Shady Forest Areas Significantly Increases Microbiome Diversity, Abundance and Functional Capacity in Soil Furrows. J. Fungi 2021, 7, 775. [Google Scholar] [CrossRef] [PubMed]
- Gohar, D.; Pent, M.; Poldmaa, K.; Bahram, M. Bacterial Community Dynamics across Developmental Stages of Fungal Fruiting Bodies. FEMS Microbiol. Ecol. 2020, 96, fiaa175. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, J.; Preston, G.M. Growing Edible Mushrooms: A Conversation between Bacteria and Fungi. Environ. Microbiol. 2020, 22, 858–872. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.-Z.; Zhang, P.-F.; Trivedi, P.; Riera, N.; Wang, Y.-Y.; Liu, X.; Fan, G.-Y.; Tang, J.-J.; Coletta-Filho, H.D.; et al. The Structure and Function of the Global Citrus Rhizosphere Microbiome. Nat. Commun. 2018, 9, 4894. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, S.; Luo, D.; Mao, P.; Rosazlina, R.; Martin, F.; Xu, L. Decline in Morel Production upon Continuous Cropping Is Related to Changes in Soil Mycobiome. J. Fungi 2023, 9, 492. [Google Scholar] [CrossRef]
- Tan, H.; Liu, T.; Yu, Y.; Tang, J.; Jiang, L.; Martin, F.M.; Peng, W. Morel Production Related to Soil Microbial Diversity and Evenness. Microbiol. Spectr. 2021, 9, e00229-21. [Google Scholar] [CrossRef]
- Wei-Ye, L.; Hong-Bo, G.; Ke-Xin, B.; Alekseevna, S.L.; Xiao-Jian, Q.; Xiao-Dan, Y. Determining Why Continuous Cropping Reduces the Production of the Morel Morchella sextelata. Front. Microbiol. 2022, 13, 903983. [Google Scholar] [CrossRef]
- Yu, F.-M.; Jayawardena, R.S.; Thongklang, N.; Lv, M.-L.; Zhu, X.-T.; Zhao, Q. Morel Production Associated with Soil Nitrogen-Fixing and Nitrifying Microorganisms. J. Fungi 2022, 8, 299. [Google Scholar] [CrossRef]
- Liu, W.; He, P.; Zhang, J.; Wu, L.; Er, L.; Shi, X.; Gu, Z.; Yu, F.; Pérez-Moreno, J. Ultrastructure and Physiological Characterization of Morchella Mitospores and Their Relevance in the Understanding of the Morel Life Cycle. Microorganisms 2023, 11, 345. [Google Scholar] [CrossRef]
- Luo, X.; Li, W.; Li, J.; Cao, Y.; Yang, L.; Shen, Z.; Lu, Q.; Li, R. Transportation Mechanism of Exogenous Nitrogen Nutrition in the Artificial Cultivation Process of Morchella. J. Edible Fungi 2022, 29, 28–31. (In Chinese) [Google Scholar]
- Lv, J.; Li, C.; Yang, Z.; Liu, P.; Lu, M.; Ren, Y.; Tian, K.; Zhao, X.; Chen, Z. Response of Soil Microbial Community to Land Use Change in Napahai Plateau Wetland. Soil Bull. 2023, 54, 682–694. [Google Scholar]
- Magoč, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E.; et al. Chimeric 16S rRNA Sequence Formation and Detection in Sanger and 454-Pyrosequenced PCR Amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef] [PubMed]
- QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [CrossRef]
- Chao, A. Nonparametric Estimation of the Number of Classes in a Population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Minchin, P.R. An Evaluation of the Relative Robustness of Techniques for Ecological Ordination. In Theory and Models in Vegetation Science, Proceedings of the Symposium, Uppsala, Sweden, 8–13 July 1985; Springer: Dordrecht, The Netherlands, 1987; Volume 69, pp. 89–107. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: http://www.R-project.org (accessed on 6 April 2023).
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, J.; Zied, D.C.; Pardo, J.E.; Preston, G.M.; Pardo-Giménez, A. Supplementation in Mushroom Crops and Its Impact on Yield and Quality. AMB Express 2018, 8, 146. [Google Scholar] [CrossRef]
- Clay, K.; Holah, J. Fungal Endophyte Symbiosis and Plant Diversity in Successional Fields. Science 1999, 285, 1742–1745. [Google Scholar] [CrossRef]
- Rai, N.; Kumari Keshri, P.; Verma, A.; Kamble, S.C.; Mishra, P.; Barik, S.; Kumar Singh, S.; Gautam, V. Plant Associated Fungal Endophytes as a Source of Natural Bioactive Compounds. Mycology 2021, 12, 139–159. [Google Scholar] [CrossRef]
- Shi, X.; Liu, D.; He, X.; Liu, W.; Yu, F. Epidemic Identification of Fungal Diseases in Morchella Cultivation across China. J. Fungi 2022, 8, 1107. [Google Scholar] [CrossRef]
- Wang, X.; Peng, J.; Sun, L.; Bonito, G.; Guo, Y.; Li, Y.; Fu, Y. Genome Sequencing of Paecilomyces penicillatus Provides Insights into Its Phylogenetic Placement and Mycoparasitism Mechanisms on Morel Mushrooms. Pathogens 2020, 9, 834. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Delgado-Baquerizo, M.; Guo, X.; Wang, D.; Zhu, Y.G.; Chu, H. Biodiversity of Keystone Phylotypes Determines Crop Production in a 4-Decade Fertilization Experiment. ISME J. 2020, 15, 550–561. [Google Scholar] [CrossRef]
- Egidi, E.; Delgado-Baquerizo, M.; Plett, J.M.; Wang, J.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K. A Few Ascomycota Taxa Dominate Soil Fungal Communities Worldwide. Nat. Commun. 2019, 10, 2369. [Google Scholar] [CrossRef]
- Ding, C.; Hu, W.G.; Zhang, X.; Qi, X.Y.; He, B.; Chen, X.M. Composition and Diversity of the Fungal Community in the Rhizosphere Soil of Halophytic Vegetation in Ebinur Lake Wetland. Environ. Sci. Pollut. Res. 2023, 30, 86097–86109. [Google Scholar] [CrossRef]
- Lang, X.; Xu, A.; Wang, Y.; Song, Z. Seasonal Variation of Aerosol Fungal Community Structure in Reed Constructed Wetlands. Environ. Sci. Pollut. Res. Int. 2022, 29, 19420–19431. [Google Scholar] [CrossRef]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial and Fungal Contributions to Carbon Sequestration in Agroecosystems. Soil Sci. Soc. Am. J. 2006, 70, 555–569. [Google Scholar] [CrossRef]
- Tedersoo, L.; Mikryukov, V.; Anslan, S.; Bahram, M.; Khalid, A.N.; Corrales, A.; Agan, A.; Vasco-Palacios, A.-M.; Saitta, A.; Antonelli, A.; et al. The Global Soil Mycobiome Consortium Dataset for Boosting Fungal Diversity Research. Fungal Divers. 2021, 111, 573–588. [Google Scholar] [CrossRef]
- Ekwomadu, T.I.; Mwanza, M. Fusarium Fungi Pathogens, Identification, Adverse Effects, Disease Management, and Global Food Security: A Review of the Latest Research. Agriculture 2023, 13, 1810. [Google Scholar] [CrossRef]
- Longley, R.; Benucci, G.M.N.; Mills, G.; Bonito, G. Fungal and Bacterial Community Dynamics in Substrates during the Cultivation of Morels (Morchella rufobrunnea) Indoors. FEMS Microbiol. Lett. 2019, 366, fnz215. [Google Scholar] [CrossRef]
- Yu, Y.; Tan, H.; Liu, T.; Liu, L.; Tang, J.; Peng, W. Dual RNA-Seq Analysis of the Interaction Between Edible Fungus Morchella sextelata and Its Pathogenic Fungus Paecilomyces penicillatus Uncovers the Candidate Defense and Pathogenic Factors. Front. Microbiol. 2021, 12, 760444. [Google Scholar] [CrossRef]
- He, X.L.; Peng, W.H.; Miao, R.Y.; Tang, J.; Chen, Y.; Liu, L.X.; Wang, D.; Gan, B.C. White Mold on Cultivated Morels Caused by Paecilomyces penicillatus. FEMS Microbiol. Lett. 2017, 364, fnx037. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, T.; Liu, L.; Chen, Y.; Tang, J.; Peng, W.; Tan, H. Application of the Mushroom Volatile 1-Octen-3-ol to Suppress a Morel Disease Caused by Paecilomyces penicillatus. Appl. Microbiol. Biotechnol. 2022, 106, 4787–4799. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Q.; Wang, P.; Liu, Z.; Yu, Y.; Tan, X.; Huang, X.; Wen, J.; Zhang, W. High-Throughput Sequencing Uncovers Fungal Community Succession During Morchella sextelata Development. J. Fungi 2025, 11, 364. https://doi.org/10.3390/jof11050364
Yan Q, Wang P, Liu Z, Yu Y, Tan X, Huang X, Wen J, Zhang W. High-Throughput Sequencing Uncovers Fungal Community Succession During Morchella sextelata Development. Journal of Fungi. 2025; 11(5):364. https://doi.org/10.3390/jof11050364
Chicago/Turabian StyleYan, Qi, Peng Wang, Zhushan Liu, Ya Yu, Xiao Tan, Xiao Huang, Jiawei Wen, and Weidong Zhang. 2025. "High-Throughput Sequencing Uncovers Fungal Community Succession During Morchella sextelata Development" Journal of Fungi 11, no. 5: 364. https://doi.org/10.3390/jof11050364
APA StyleYan, Q., Wang, P., Liu, Z., Yu, Y., Tan, X., Huang, X., Wen, J., & Zhang, W. (2025). High-Throughput Sequencing Uncovers Fungal Community Succession During Morchella sextelata Development. Journal of Fungi, 11(5), 364. https://doi.org/10.3390/jof11050364




