Pediatric Candida Manifestations in the Orofacial Region: A Retrospective Analysis of Different Forms, Risk Factors and Species Distribution
Abstract
1. Introduction
2. Materials and Methods
3. Results
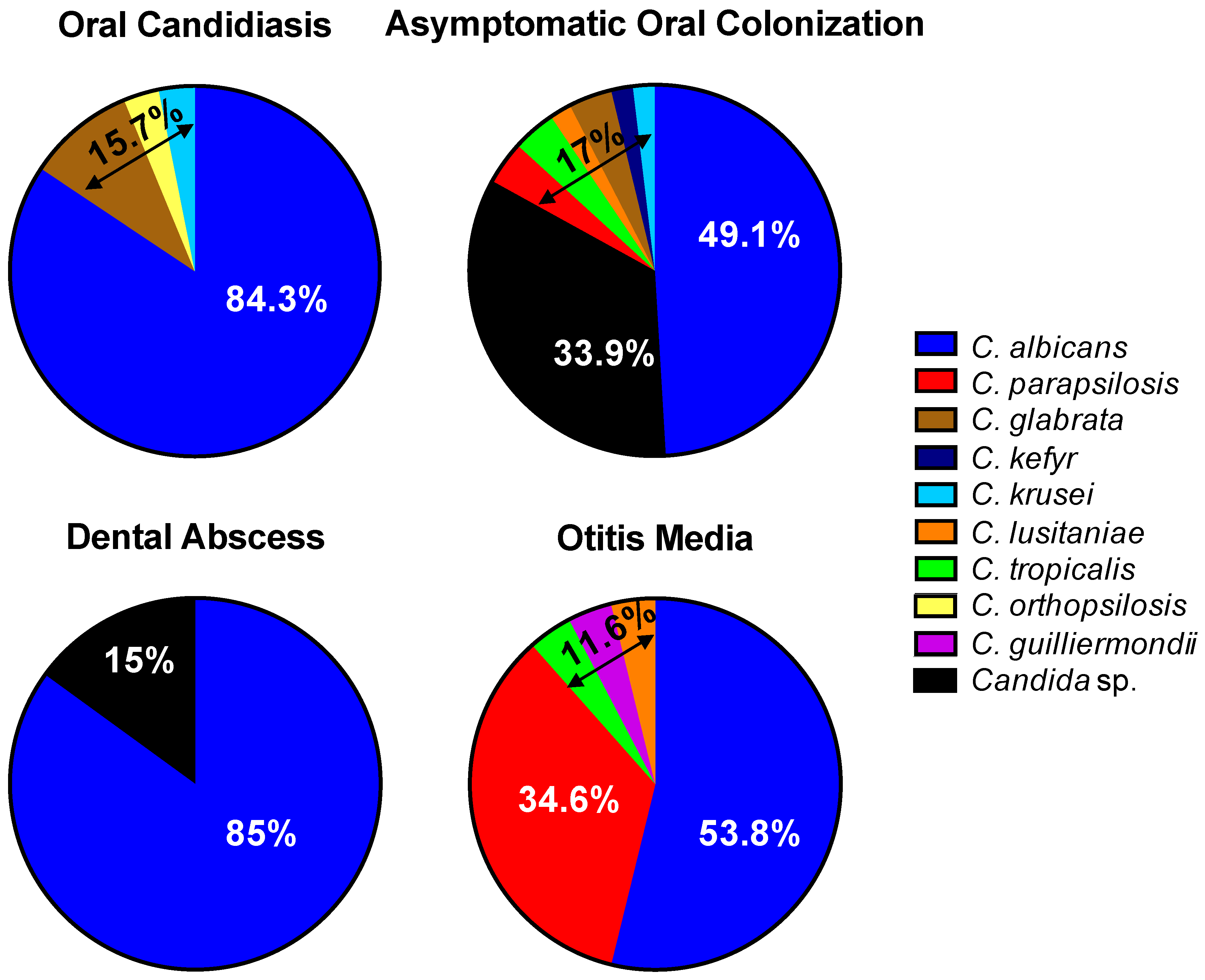
3.1. Oral Candidiasis
3.2. Asymptomatic Oral Candida Colonization
3.3. Dental Abscesses and Candida Detection
3.4. Otitis Media in Cleft Patients
3.5. Microbiological Candida Spectrum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Findley, K.; Oh, J.; Yang, J.; Conlan, S.; Deming, C.; Meyer, J.A.; Schoenfeld, D.; Nomicos, E.; Park, M.; NIH Intramural Sequencing Center Comparative Sequencing Program; et al. Topographic diversity of fungal and bacterial communities in human skin. Nature 2013, 498, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Kuhbacher, A.; Burger-Kentischer, A.; Rupp, S. Interaction of Candida Species with the Skin. Microorganisms 2017, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Roth, R.R.; James, W.D. Microbial ecology of the skin. Annu. Rev. Microbiol. 1988, 42, 441–464. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.D.; Chaffin, W.L. Oral colonization by Candida albicans. Crit. Rev. Oral. Biol. Med. 1999, 10, 359–383. [Google Scholar] [CrossRef]
- Wisplinghoff, H.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Inflammatory response and clinical course of adult patients with nosocomial bloodstream infections caused by Candida spp. Clin. Microbiol. Infect. 2006, 12, 170–177. [Google Scholar] [CrossRef]
- Delaloye, J.; Calandra, T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence 2014, 5, 161–169. [Google Scholar] [CrossRef]
- Walsh, T.J.; Katragkou, A.; Chen, T.; Salvatore, C.M.; Roilides, E. Invasive Candidiasis in Infants and Children: Recent Advances in Epidemiology, Diagnosis, and Treatment. J. Fungi. 2019, 5, 11. [Google Scholar] [CrossRef]
- Sipsas, N.V.; Lewis, R.E.; Tarrand, J.; Hachem, R.; Rolston, K.V.; Raad, I.I.; Kontoyiannis, D.P. Candidemia in patients with hematologic malignancies in the era of new antifungal agents (2001–2007): Stable incidence but changing epidemiology of a still frequently lethal infection. Cancer 2009, 115, 4745–4752. [Google Scholar] [CrossRef]
- McCort, M.E.; Tsai, H. Epidemiology of Invasive Candidiasis in Patients with Hematologic Malignancy on Antifungal Prophylaxis. Mycopathologia 2023, 188, 885–892. [Google Scholar] [CrossRef]
- Li, D.; Li, T.; Bai, C.; Zhang, Q.; Li, Z.; Li, X. A predictive nomogram for mortality of cancer patients with invasive candidiasis: A 10-year study in a cancer center of North China. BMC Infect. Dis. 2021, 21, 76. [Google Scholar] [CrossRef]
- Mantadakis, E.; Pana, Z.D.; Zaoutis, T. Candidemia in children: Epidemiology, prevention and management. Mycoses 2018, 61, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Steinbach, W.J.; Roilides, E.; Berman, D.; Hoffman, J.A.; Groll, A.H.; Bin-Hussain, I.; Palazzi, D.L.; Castagnola, E.; Halasa, N.; Velegraki, A.; et al. Results from a prospective, international, epidemiologic study of invasive candidiasis in children and neonates. Pediatr. Infect. Dis. J. 2012, 31, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Noni, M.; Stathi, A.; Vaki, I.; Velegraki, A.; Zachariadou, L.; Michos, A. Changing Epidemiology of Invasive Candidiasis in Children during a 10-Year Period. J. Fungi. 2019, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Sutcu, M.; Acar, M.; Genc, G.E.; Kokcu, I.; Akturk, H.; Atay, G.; Torun, S.H.; Salman, N.; Erturan, Z.; Somer, A. Evaluation of Candida species and antifungal susceptibilities among children with invasive candidiasis. Turk. Pediatri Ars. 2017, 52, 145–153. [Google Scholar] [CrossRef]
- Chow, B.D.; Linden, J.R.; Bliss, J.M. Candida parapsilosis and the neonate: Epidemiology, virulence and host defense in a unique patient setting. Expert. Rev. Anti Infect. Ther. 2012, 10, 935–946. [Google Scholar] [CrossRef]
- Dotis, J.; Prasad, P.A.; Zaoutis, T.; Roilides, E. Epidemiology, risk factors and outcome of Candida parapsilosis bloodstream infection in children. Pediatr. Infect. Dis. J. 2012, 31, 557–560. [Google Scholar] [CrossRef]
- Pammi, M.; Holland, L.; Butler, G.; Gacser, A.; Bliss, J.M. Candida parapsilosis is a significant neonatal pathogen: A systematic review and meta-analysis. Pediatr. Infect. Dis. J. 2013, 32, e206–e216. [Google Scholar] [CrossRef]
- Wang, Y.S.; Hsu, J.F.; Lee, W.J.; Wang, S.H.; Chu, S.M.; Huang, H.R.; Yang, P.H.; Fu, R.H.; Tsai, M.H. Invasive Candida parapsilosis Bloodstream Infections in Children: The Antifungal Susceptibility, Clinical Characteristics and Impacts on Outcomes. Microorganisms 2023, 11, 1149. [Google Scholar] [CrossRef]
- Warris, A. Candida auris, what do paediatricians need to know? Arch. Dis. Child. 2018, 103, 891–894. [Google Scholar] [CrossRef]
- Vasileiou, E.; Apsemidou, A.; Vyzantiadis, T.A.; Tragiannidis, A. Invasive candidiasis and candidemia in pediatric and neonatal patients: A review of current guidelines. Curr. Med. Mycol. 2018, 4, 28–33. [Google Scholar] [CrossRef]
- Millsop, J.W.; Fazel, N. Oral candidiasis. Clin. Dermatol. 2016, 34, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Hellstein, J.W.; Marek, C.L. Candidiasis: Red and White Manifestations in the Oral Cavity. Head. Neck Pathol. 2019, 13, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Brizuela, M.; Raja, A. Oral Candidiasis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Akpan, A.; Morgan, R. Oral candidiasis. Postgrad. Med. J. 2002, 78, 455–459. [Google Scholar] [CrossRef]
- Goregen, M.; Miloglu, O.; Buyukkurt, M.C.; Caglayan, F.; Aktas, A.E. Median rhomboid glossitis: A clinical and microbiological study. Eur. J. Dent. 2011, 5, 367–372. [Google Scholar] [CrossRef]
- Budtz-Jorgensen, E. Etiology, pathogenesis, therapy, and prophylaxis of oral yeast infections. Acta Odontol. Scand. 1990, 48, 61–69. [Google Scholar] [CrossRef]
- Patil, S.; Rao, R.S.; Majumdar, B.; Anil, S. Clinical Appearance of Oral Candida Infection and Therapeutic Strategies. Front. Microbiol. 2015, 6, 1391. [Google Scholar] [CrossRef]
- Humbert, L.; Cornu, M.; Proust-Lemoine, E.; Bayry, J.; Wemeau, J.L.; Vantyghem, M.C.; Sendid, B. Chronic Mucocutaneous Candidiasis in Autoimmune Polyendocrine Syndrome Type 1. Front. Immunol. 2018, 9, 2570. [Google Scholar] [CrossRef]
- Vila, T.; Sultan, A.S.; Montelongo-Jauregui, D.; Jabra-Rizk, M.A. Oral Candidiasis: A Disease of Opportunity. J. Fungi 2020, 6, 15. [Google Scholar] [CrossRef]
- Okada, S.; Puel, A.; Casanova, J.L.; Kobayashi, M. Chronic mucocutaneous candidiasis disease associated with inborn errors of IL-17 immunity. Clin. Transl. Immunol. 2016, 5, e114. [Google Scholar] [CrossRef]
- Dhalla, F.; Fox, H.; Davenport, E.E.; Sadler, R.; Anzilotti, C.; van Schouwenburg, P.A.; Ferry, B.; Chapel, H.; Knight, J.C.; Patel, S.Y. Chronic mucocutaneous candidiasis: Characterization of a family with STAT-1 gain-of-function and development of an ex-vivo assay for Th17 deficiency of diagnostic utility. Clin. Exp. Immunol. 2016, 184, 216–227. [Google Scholar] [CrossRef]
- Frede, N.; Rojas-Restrepo, J.; Caballero Garcia de Oteyza, A.; Buchta, M.; Hubscher, K.; Gamez-Diaz, L.; Proietti, M.; Saghafi, S.; Chavoshzadeh, Z.; Soler-Palacin, P.; et al. Genetic Analysis of a Cohort of 275 Patients with Hyper-IgE Syndromes and/or Chronic Mucocutaneous Candidiasis. J. Clin. Immunol. 2021, 41, 1804–1838. [Google Scholar] [CrossRef] [PubMed]
- Stinnett, E.A.; Childers, N.K.; Wright, J.T.; Rodu, B.K.; Bradley, E.L., Jr. The detection of oral Candida in pediatric leukemia patients. Pediatr. Dent. 1992, 14, 236–239. [Google Scholar] [PubMed]
- Shirazian, S.; Manifar, S.; Nodehi, R.S.; Shabani, M. Oropharyngeal Candida Colonization in Patients with Acute Myeloid Leukemia. Front. Dent. 2020, 17, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Alberth, M.; Majoros, L.; Kovalecz, G.; Borbas, E.; Szegedi, I.; Márton, I.J.; Kiss, C. Significance of oral Candida infections in children with cancer. Pathol. Oncol. Res. 2006, 12, 237–241. [Google Scholar] [CrossRef]
- Berdicevsky, I.; Ben-Aryeh, H.; Szargel, R.; Gutman, D. Oral Candida in children. Oral. Surg. Oral. Med. Oral. Pathol. 1984, 57, 37–40. [Google Scholar] [CrossRef]
- Rozkiewicz, D.; Daniluk, T.; Zaremba, M.L.; Cylwik-Rokicka, D.; Stokowska, W.; Pawinska, M.; Dabrowska, E.; Marczuk-Kolada, G.; Waszkiel, D. Oral Candida albicans carriage in healthy preschool and school children. Adv. Med. Sci. 2006, 51 (Suppl. S1), 187–190. [Google Scholar]
- Barnett, J.A. A history of research on yeasts 12: Medical yeasts part 1, Candida albicans. Yeast 2008, 25, 385–417. [Google Scholar] [CrossRef]
- Raja, M.; Hannan, A.; Ali, K. Association of oral candidal carriage with dental caries in children. Caries Res. 2010, 44, 272–276. [Google Scholar] [CrossRef]
- Koo, H.; Bowen, W.H. Candida albicans and Streptococcus mutans: A potential synergistic alliance to cause virulent tooth decay in children. Future Microbiol. 2014, 9, 1295–1297. [Google Scholar] [CrossRef]
- Thomas, A.; Mhambrey, S.; Chokshi, K.; Chokshi, A.; Jana, S.; Thakur, S.; Jose, D.; Bajpai, G. Association of Oral Candida albicans with Severe Early Childhood Caries—A Pilot Study. J. Clin. Diagn. Res. 2016, 10, ZC109–ZC112. [Google Scholar] [CrossRef]
- Alberti, A.; Corbella, S.; Taschieri, S.; Francetti, L.; Fakhruddin, K.S.; Samaranayake, L.P. Fungal species in endodontic infections: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0255003. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, J.C.; Watts, C.M.; Xia, T. Occurrence of Candida albicans in infections of endodontic origin. J. Endod. 2000, 26, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.J.; Kim, A.R.; Perinpanayagam, H.; Han, S.H.; Kum, K.Y. Candida albicans Virulence Factors and Pathogenicity for Endodontic Infections. Microorganisms 2020, 8, 1300. [Google Scholar] [CrossRef] [PubMed]
- Vieira Colombo, A.P.; Magalhaes, C.B.; Hartenbach, F.A.; Martins do Souto, R.; Maciel da Silva-Boghossian, C. Periodontal-disease-associated biofilm: A reservoir for pathogens of medical importance. Microb. Pathog. 2016, 94, 27–34. [Google Scholar] [CrossRef]
- Walia, I.S.; Borle, R.M.; Mehendiratta, D.; Yadav, A.O. Microbiology and antibiotic sensitivity of head and neck space infections of odontogenic origin. J. Maxillofac. Oral. Surg. 2014, 13, 16–21. [Google Scholar] [CrossRef]
- Rega, A.J.; Aziz, S.R.; Ziccardi, V.B. Microbiology and antibiotic sensitivities of head and neck space infections of odontogenic origin. J. Oral. Maxillofac. Surg. 2006, 64, 1377–1380. [Google Scholar] [CrossRef]
- Chunduri, N.S.; Madasu, K.; Goteki, V.R.; Karpe, T.; Reddy, H. Evaluation of bacterial spectrum of orofacial infections and their antibiotic susceptibility. Ann. Maxillofac. Surg. 2012, 2, 46–50. [Google Scholar] [CrossRef]
- Uppada, U.K.; Sinha, R. Outcome of Odontogenic Infections in Rural Setup: Our Experience in Management. J. Maxillofac. Oral. Surg. 2020, 19, 113–118. [Google Scholar] [CrossRef]
- Sanchez, R.; Mirada, E.; Arias, J.; Pano, J.R.; Burgueno, M. Severe odontogenic infections: Epidemiological, microbiological and therapeutic factors. Med. Oral. Patol. Oral. Cir. Bucal 2011, 16, e670–e676. [Google Scholar] [CrossRef]
- Eisler, L.; Wearda, K.; Romatoski, K.; Odland, R.M. Morbidity and cost of odontogenic infections. Otolaryngol. Head. Neck Surg. 2013, 149, 84–88. [Google Scholar] [CrossRef]
- Al-Nawas, B.; Maeurer, M. Severe versus local odontogenic bacterial infections: Comparison of microbial isolates. Eur. Surg. Res. 2008, 40, 220–224. [Google Scholar] [CrossRef]
- Bottger, S.; Zechel-Gran, S.; Schmermund, D.; Streckbein, P.; Wilbrand, J.F.; Knitschke, M.; Pons-Kuhnemann, J.; Hain, T.; Weigel, M.; Imirzalioglu, C.; et al. Clinical Relevance of the Microbiome in Odontogenic Abscesses. Biology 2021, 10, 916. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Shih, C.C.; Yen, K.L.; Wang, S.M.; Kuo, Y.S.; Kuo, M.Y.; Chiang, C.P. Facial Candida albicans cellulitis occurring in a patient with oral submucous fibrosis and unknown diabetes mellitus after local corticosteroid injection treatment. J. Oral. Pathol. Med. 2004, 33, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Peker, E.; Zor, F.; Toprak, M.E.; Baris, E. Facial Candidal Abscess in a Patient with Unknown Type 2 Diabetes Mellitus. J. Maxillofac. Oral. Surg. 2015, 14, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Kwak, O.S.; Kang, M.I.; Kim, J.B.; Kim, M.W.; Kim, Y.K. A rare case of facial Candida albicans cellulitis in an uncontrolled diabetic patient. Mycoses 2009, 52, 379–381. [Google Scholar] [CrossRef]
- Sung, J.Y.; Kim, J.M.; Lee, J.U.; Lee, Y.H.; Lee, S.B. Multiple facial candidal abscesses after self-administered acupuncture in a patient with undiagnosed diabetes mellitus: A case report. BMC Complement. Med. Ther. 2021, 21, 170. [Google Scholar] [CrossRef]
- Even-Tov, E.; Niv, A.; Kraus, M.; Nash, M. Candida parotitis with abscess formation. Acta Otolaryngol. 2006, 126, 334–336. [Google Scholar] [CrossRef]
- Enache-Angoulvant, A.; Torti, F.; Tassart, M.; Poirot, J.L.; Jafari, A.; Roux, P.; Hennequin, C. Candidal abscess of the parotid gland due to Candida glabrata: Report of a case and literature review. Med. Mycol. 2010, 48, 402–405. [Google Scholar] [CrossRef]
- Codere, F.; Anderson, R.L. Bilateral Candida albicans dacryocystitis with facial cellulitis. Can. J. Ophthalmol. 1982, 17, 176–177. [Google Scholar]
- Klotz, S.A.; Penn, C.C.; Negvesky, G.J.; Butrus, S.I. Fungal and parasitic infections of the eye. Clin. Microbiol. Rev. 2000, 13, 662–685. [Google Scholar] [CrossRef]
- Ranjith, K.; Sontam, B.; Sharma, S.; Joseph, J.; Chathoth, K.N.; Sama, K.C.; Murthy, S.I.; Shivaji, S. Candida Species From Eye Infections: Drug Susceptibility, Virulence Factors, and Molecular Characterization. Invest. Ophthalmol. Vis. Sci. 2017, 58, 4201–4209. [Google Scholar] [CrossRef] [PubMed]
- Obi, E.; Roy, A.; Bates, V.; Sandy, C. Bilateral chronic fungal dacryocystitis caused by Candida dubliniensis in a neutropenic patient. J. Clin. Pathol. 2006, 59, 1194–1195. [Google Scholar] [CrossRef]
- Purgason, P.A.; Hornblass, A.; Loeffler, M. Atypical presentation of fungal dacryocystitis. A report of two cases. Ophthalmology 1992, 99, 1430–1432. [Google Scholar] [CrossRef] [PubMed]
- Bojanovic, M.; Stalevic, M.; Arsic-Arsenijevic, V.; Ignjatovic, A.; Randelovic, M.; Golubovic, M.; Zivkovic-Marinkov, E.; Koracevic, G.; Stamenkovic, B.; Otasevic, S. Etiology, Predisposing Factors, Clinical Features and Diagnostic Procedure of Otomycosis: A Literature Review. J. Fungi 2023, 9, 662. [Google Scholar] [CrossRef] [PubMed]
- Westby, D.; O’Connell, N.; Powell, J.; Fenton, J.E. The changing nature of paediatric otomycosis in the mid-west of Ireland. J. Laryngol. Otol. 2020, 134, 592–596. [Google Scholar] [CrossRef]
- Flynn, T.; Moller, C.; Jonsson, R.; Lohmander, A. The high prevalence of otitis media with effusion in children with cleft lip and palate as compared to children without clefts. Int. J. Pediatr. Otorhinolaryngol. 2009, 73, 1441–1446. [Google Scholar] [CrossRef]
- Pereira, M.; Rao, K.; Sharin, F.; Tanweer, F.; Mair, M.; Rea, P. Topical Antibiotic-Induced Otomycosis—A Systematic Review of Aetiology and Risk Factors. Indian. J. Otolaryngol. Head. Neck Surg. 2024, 76, 3766–3776. [Google Scholar] [CrossRef]
- Alansari, N.; Abed, H.; Abid, M. Oral flora and functional dysbiosis of cleft lip and palate patients: A scoping review. Spec. Care Dentist 2023, 44, 255–268. [Google Scholar] [CrossRef]
- Khan, I.; Ahmad, T.; Manzoor, N.; Rizvi, M.A.; Raza, U.; Premchandani, S. Evaluating the role of local host factors in the candidal colonization of oral cavity: A review update. Natl. J. Maxillofac. Surg. 2020, 11, 169–175. [Google Scholar] [CrossRef]
- de Souza, P.; Goncalves-Wilhelmsen, N.C.V.; Rosa, R.T.; Correia, C.; Pereira, T.M.; Kitahara, A.B.P.; Ignacio, S.A.; Azevedo-Alanis, L.R.; Rosa, E.A.R. Oral Colonization and Virulence Factors of Candida spp. in Babies with Cleft Palate. Cleft Palate Craniofac J. 2022, 59, 1056–1063. [Google Scholar] [CrossRef]
- Rawashdeh, M.A.; Ayesh, J.A.; Darwazeh, A.M. Oral candidal colonization in cleft patients as a function of age, gender, surgery, type of cleft, and oral health. J. Oral. Maxillofac. Surg. 2011, 69, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, H.N.; Hatipoglu, S.; Erdem, B.; Can, B.; Kadir, T. Adherence frequency of Candida albicans on nasoalveolar molding (NAM) appliances. J. Stomatol. Oral. Maxillofac. Surg. 2020, 121, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, R.; Durmaz, B.; Ari, O.; Abdulmajed, O.; Celik, S.; Kalcioglu, M.T. Mycobiome in the Middle Ear Cavity with and Without Otitis Media with Effusion. Turk. Arch. Otorhinolaryngol. 2021, 59, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Obeidat, R.; Noureldin, A.; Bitouni, A.; Abdellatif, H.; Lewis-Miranda, S.; Liu, S.; Badner, V.; Timothe, P. Oral health needs of U.S. children with developmental disorders: A population-based study. BMC Public. Health 2022, 22, 861. [Google Scholar] [CrossRef]
- Carlstedt, K.; Krekmanova, L.; Dahllof, G.; Ericsson, B.; Braathen, G.; Modeer, T. Oral carriage of Candida species in children and adolescents with Down’s syndrome. Int. J. Paediatr. Dent. 1996, 6, 95–100. [Google Scholar] [CrossRef]
- Willis, J.R.; Iraola-Guzman, S.; Saus, E.; Ksiezopolska, E.; Cozzuto, L.; Bejarano, L.A.; Andreu-Somavilla, N.; Alloza-Trabado, M.; Puig-Sola, A.; Blanco, A.; et al. Oral microbiome in down syndrome and its implications on oral health. J. Oral. Microbiol. 2020, 13, 1865690. [Google Scholar] [CrossRef]
- Lepesqueur, L.S.S.; Tanaka, M.H.; Lima, G.M.G.; Chiba, S.M.; Mota, A.J.; Santos, S.F.; Koga-Ito, C.Y. Oral prevalence and antifungal susceptibility of Candida species in cystic fibrosis patients. Arch. Oral. Biol. 2020, 116, 104772. [Google Scholar] [CrossRef]
- Dogan, M.; Sahiner, U.M.; Atac, A.S.; Ballikaya, E.; Soyer, O.U.; Sekerel, B.E. Oral health status of asthmatic children using inhaled corticosteroids. Turk. J. Pediatr. 2021, 63, 77–85. [Google Scholar] [CrossRef]
- Schnabl, D.; Fleischer, F.; Riedmann, M.; Laimer, J.; Gassner, R. Prevalence and distribution of deep caries and abscess formation in children who required emergency dental general anaesthesia. A retrospective analysis. Eur. J. Paediatr. Dent. 2019, 20, 119–122. [Google Scholar] [CrossRef]
- Doll, C.; Carl, F.; Neumann, K.; Voss, J.O.; Hartwig, S.; Waluga, R.; Heiland, M.; Raguse, J.D. Odontogenic Abscess-Related Emergency Hospital Admissions: A Retrospective Data Analysis of 120 Children and Young People Requiring Surgical Drainage. Biomed Res. Int. 2018, 2018, 3504727. [Google Scholar] [CrossRef]


| Oral Candidiasis | |||||||||||||||||||||||||||||
| Pseudomembraneous | Erythematous | Mucocutaneous | Angular Cheilitis | ||||||||||||||||||||||||||
| Total | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | |
| Oral mucosa | 28 | x a | x a | x a | x a | x a | x c | x c | x c | x a | x a | x a | x a | x a | x c | x c | x a | x a | x c | x c | x c | x c | x c | x c | x c | x c | x c | x a | x a |
| 6 | x | x | x | x | x | x | ||||||||||||||||||||||
| 19 | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | |||||||||
| 13 | x | x | x | x | x | x | x | x | x | x | x | x | x | |||||||||||||||
| 3 | x | x | x | |||||||||||||||||||||||||
| Other sites * | 18 | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||||||||
| 4 | x | x | x | x | ||||||||||||||||||||||||
| 3 | x | x | x | |||||||||||||||||||||||||
| 7 | x | x | x | x | x | x | x | |||||||||||||||||||||
| 1 | x | |||||||||||||||||||||||||||
| 4 | x | x | x | x | ||||||||||||||||||||||||
| 1 | x | |||||||||||||||||||||||||||
| 1 | x | |||||||||||||||||||||||||||
| 4 | x | x | x | x | ||||||||||||||||||||||||
| 3 | x | x | x | |||||||||||||||||||||||||
| 4 | x | x | x | x | ||||||||||||||||||||||||
| 2 | x | x | ||||||||||||||||||||||||||
| 11 | x | x | x | x | x | x | x | x | x | x | x | |||||||||||||||||
| 1 | x | |||||||||||||||||||||||||||
| 7 | x | x | x | x | x | x | x | |||||||||||||||||||||
| Oral Candidiasis n = 28 | Asymptomatic Colonization n = 47 | Dental Abscess n = 40 | Otitis media n = 19 | |
| No risk factors | 2 (7.1%) | 9 (19.1%) | 27 (67.5%) | 0 |
| Risk Factors | 26 (92.9%) | 36 (80.9%) | 13 (32.5%) | 19 (100%) |
| Type of risk factors (multiple entries per child possible): | ||||
| Immunological disorders or malignancies | 11 | 3 | 2 | 0 |
| 3 | - | - | - |
| 2 | - | 1 | - |
| 2 | - | - | - |
| - | 1 | 1 | - |
| 3 | - | - | - |
| 1 | - | 2 | - |
| - | 2 | - | - |
| Facial deformities | 14 | 25 | 1 | 19 |
| 9 | 17 | 1 | 19 |
| 1 | 3 | - | - |
| 1 | 5 | - | - |
| 3 | 6 | - | 2 |
| Neurological or neuromuscular disorders | 15 | 7 | 2 | 0 |
| 2 | 3 | - | - |
| 5 | 2 | - | - |
| - | 1 | - | - |
| 3 | 1 | 1 | - |
| 2 | 1 | - | - |
| 3 | 2 | 2 | - |
| Lung disease | 0 | 8 | 3 | 0 |
| - | 4 | 3 | - |
| - | 4 | - | - |
| Hemophilia | 0 | 0 | 1 | 0 |
| Metabolic disorders | 2 | 1 | 1 | 0 |
| 1 | - | 1 | - |
| 2 | 1 | - | - |
| Cardiovascular disease | 1 | 2 | 3 | 0 |
| Chronic skin disease | 4 | 0 | 0 | 0 |
| Chromosomal aberrations | 5 | 3 | 1 | 0 |
| PEG (percutaneous endoscopic gastrostomy) | 7 | 8 | 0 | 0 |
| Asymptomatic Oral Colonization | ||||||||||||||||||||||||||||||||||||||||||||||||
| Total | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | 44 | 45 | 46 | 47 | |
| Oral mucosa | 47 | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x |
| Other sites * | 27 | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||||||||||||||||||
| 3 | x | x | x | ||||||||||||||||||||||||||||||||||||||||||||
| 8 | x | x | x | x | x | x | x | x | |||||||||||||||||||||||||||||||||||||||
| 17 | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | ||||||||||||||||||||||||||||||
| 0 | |||||||||||||||||||||||||||||||||||||||||||||||
| 6 | x | x | x | x | x | x | |||||||||||||||||||||||||||||||||||||||||
| 1 | x | ||||||||||||||||||||||||||||||||||||||||||||||
| 0 | |||||||||||||||||||||||||||||||||||||||||||||||
| 2 | x | x | |||||||||||||||||||||||||||||||||||||||||||||
| 0 | |||||||||||||||||||||||||||||||||||||||||||||||
| 0 | |||||||||||||||||||||||||||||||||||||||||||||||
| 1 | x | ||||||||||||||||||||||||||||||||||||||||||||||
| 1 | x | ||||||||||||||||||||||||||||||||||||||||||||||
| 2 | x | x | |||||||||||||||||||||||||||||||||||||||||||||
| 5 | x | x | x | x | x | ||||||||||||||||||||||||||||||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakoschke, S.C.; Fleschutz, S.; Ruff, E.; Dichtl, K.; Groeger, M.; Schoen, C.; Otto, S.; Kakoschke, T.K. Pediatric Candida Manifestations in the Orofacial Region: A Retrospective Analysis of Different Forms, Risk Factors and Species Distribution. J. Fungi 2025, 11, 363. https://doi.org/10.3390/jof11050363
Kakoschke SC, Fleschutz S, Ruff E, Dichtl K, Groeger M, Schoen C, Otto S, Kakoschke TK. Pediatric Candida Manifestations in the Orofacial Region: A Retrospective Analysis of Different Forms, Risk Factors and Species Distribution. Journal of Fungi. 2025; 11(5):363. https://doi.org/10.3390/jof11050363
Chicago/Turabian StyleKakoschke, Sara Carina, Sara Fleschutz, Elisabeth Ruff, Karl Dichtl, Moritz Groeger, Carola Schoen, Sven Otto, and Tamara Katharina Kakoschke. 2025. "Pediatric Candida Manifestations in the Orofacial Region: A Retrospective Analysis of Different Forms, Risk Factors and Species Distribution" Journal of Fungi 11, no. 5: 363. https://doi.org/10.3390/jof11050363
APA StyleKakoschke, S. C., Fleschutz, S., Ruff, E., Dichtl, K., Groeger, M., Schoen, C., Otto, S., & Kakoschke, T. K. (2025). Pediatric Candida Manifestations in the Orofacial Region: A Retrospective Analysis of Different Forms, Risk Factors and Species Distribution. Journal of Fungi, 11(5), 363. https://doi.org/10.3390/jof11050363






