Airborne Fungal Spore Diversity Assessment Using Culture-Dependent and Metabarcoding Approaches in Bat-Inhabited Natural and Anthropogenic Roosts in Portugal
Abstract
1. Introduction
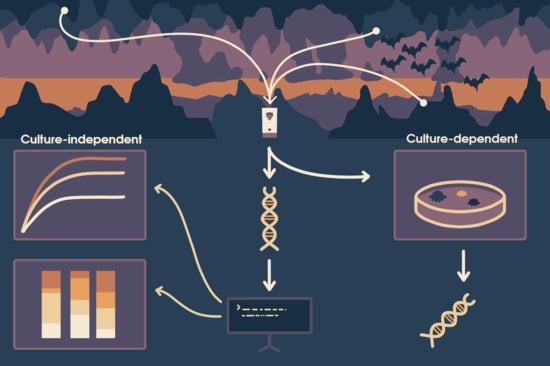
2. Material and Methods
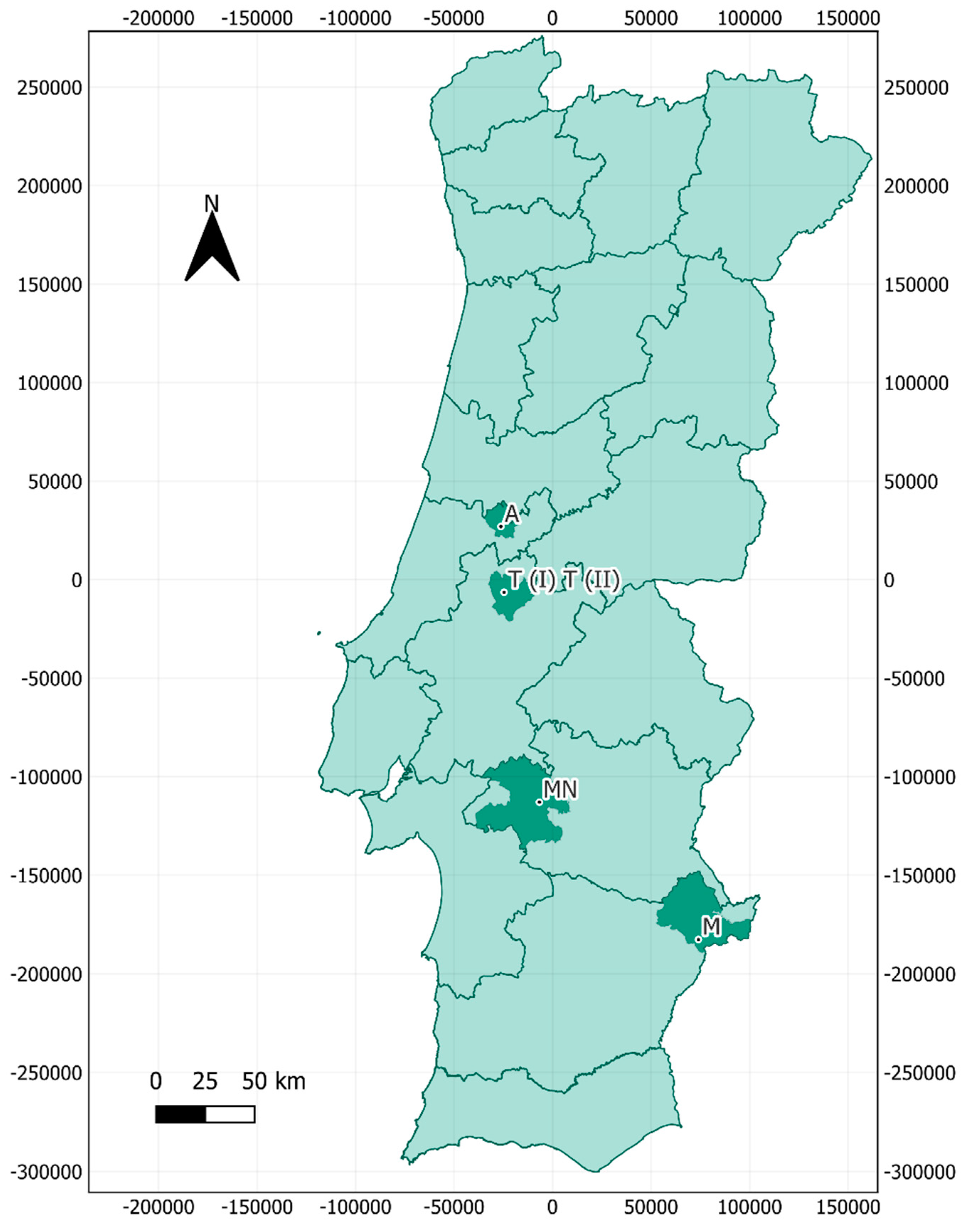
2.1. Sampling Location
2.2. Fungal Culture-Dependent Characterization
2.3. Sequencing of Fungal Isolates
2.4. DNA Extraction and Molecular Identification of the Air Samples
2.5. Oxford Nanopore Sequencing of the ITS
2.6. Taxonomic Classification and Refinement
2.7. Data Analysis
2.8. Custom Scripts for Data Manipulation and Visualization
3. Results
3.1. Culturable Results
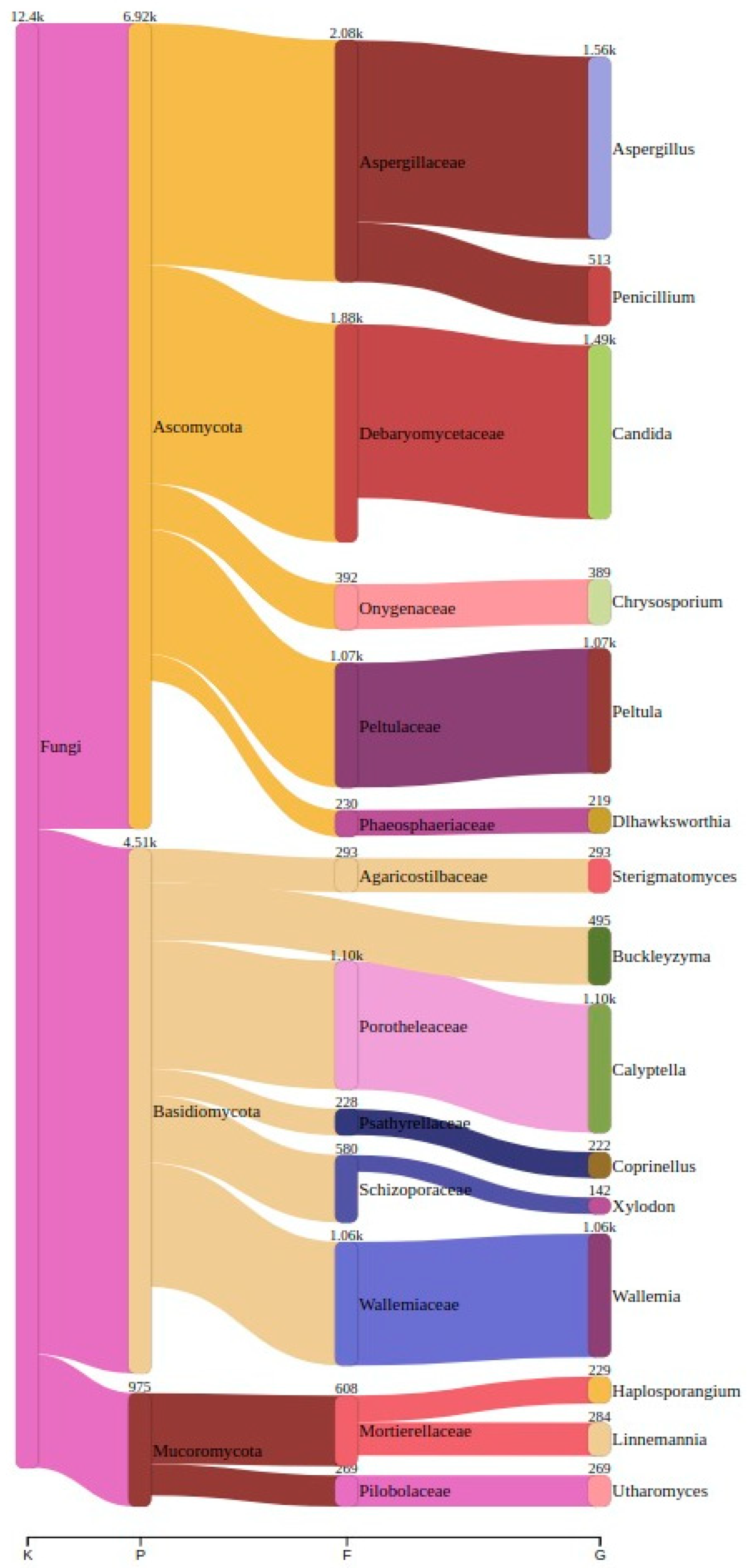
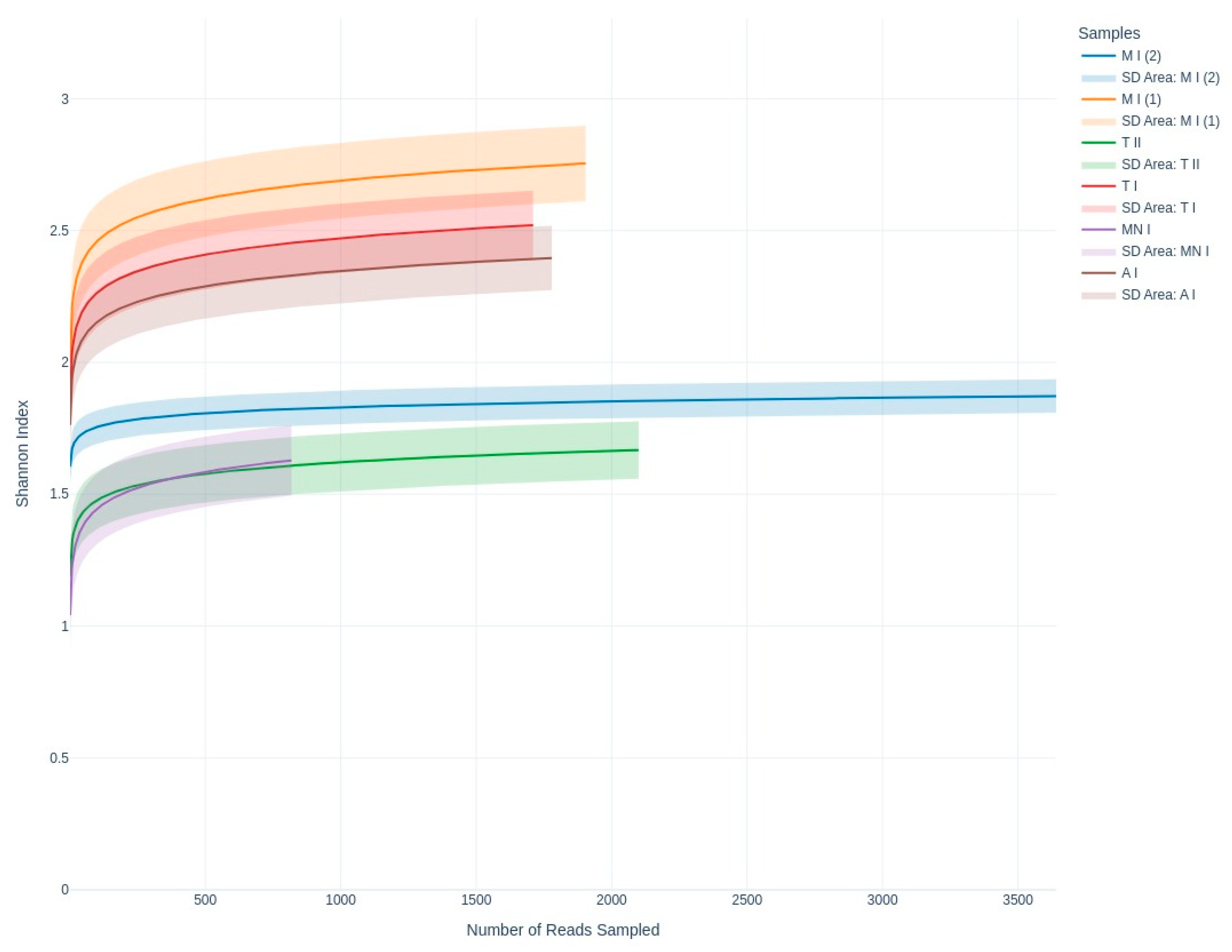
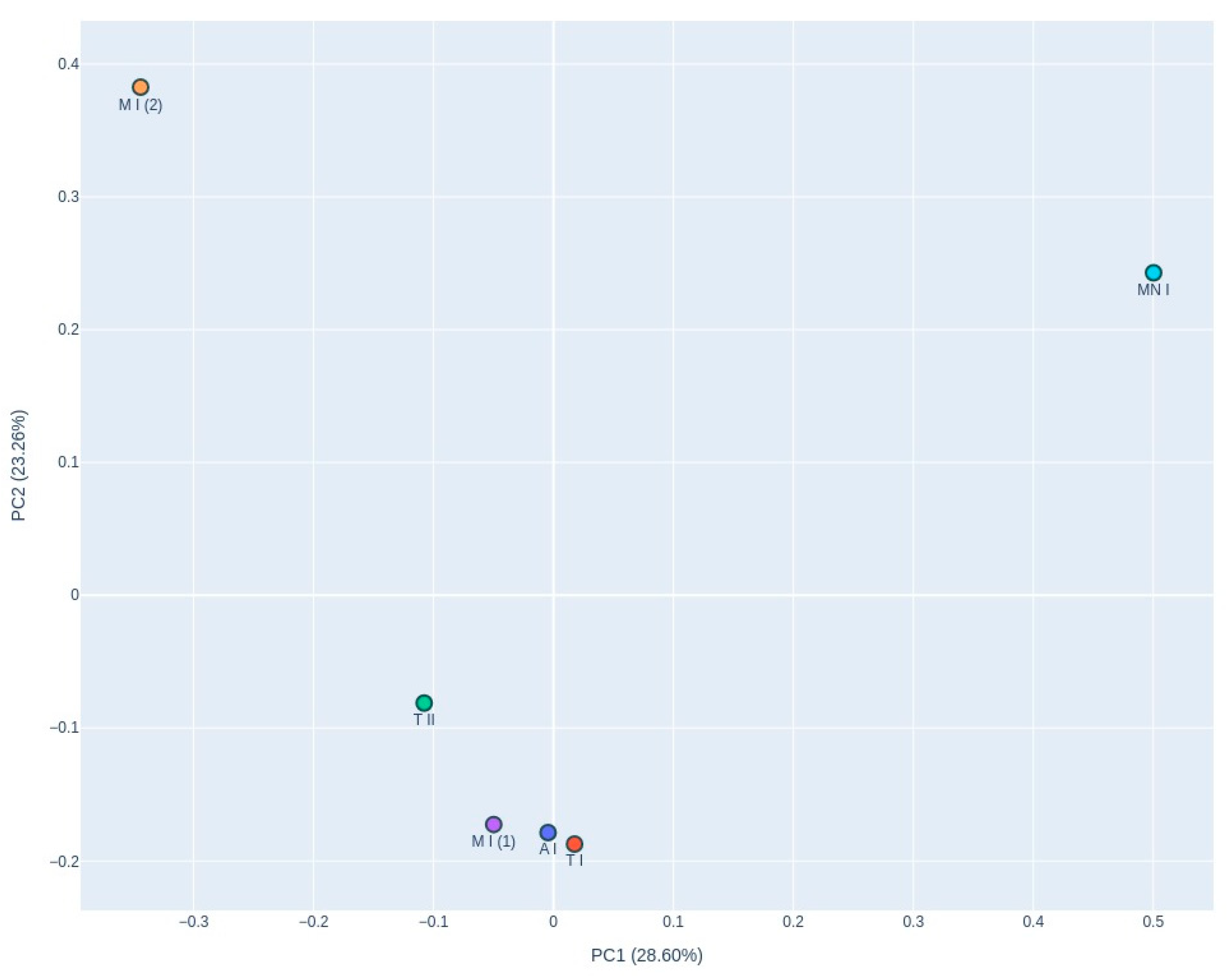
3.2. Fungal Taxonomy Determination Through Metabarcoding
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jurado, V.; Laiz, L.; Rodriguez-Nava, V.; Boiron, P.; Hermosin, B.; Sanchez-Moral, S.; Saiz-Jimenez, C. Pathogenic and Opportunistic Microorganisms in Caves. Int. J. Speleol. 2010, 39, 15–24. [Google Scholar] [CrossRef]
- De Mandal, S.; Zothansanga; Panda, A.K.; Bisht, S.S.; Senthil Kumar, N. First Report of Bacterial Community from a Bat Guano Using Illumina Next-Generation Sequencing. Genomics Data 2015, 4, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Mühldorfer, K. Bats and Bacterial Pathogens: A Review. Zoonoses Public Health 2013, 60, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Dimkić, I.; Fira, D.; Janakiev, T.; Kabić, J.; Stupar, M.; Nenadić, M.; Unković, N.; Grbić, M.L. The Microbiome of Bat Guano: For What Is This Knowledge Important? Appl. Microbiol. Biotechnol. 2021, 105, 1407–1419. [Google Scholar] [CrossRef] [PubMed]
- Kokurewicz, T.; Ogórek, R.; Pusz, W.; Matkowski, K. Bats Increase the Number of Cultivable Airborne Fungi in the “Nietoperek” Bat Reserve in Western Poland. Microb. Ecol. 2016, 72, 36–48. [Google Scholar] [CrossRef]
- Borzęcka, J.; Piecuch, A.; Kokurewicz, T.; Lavoie, K.H.; Ogórek, R. Greater Mouse-Eared Bats (Myotis Myotis) Hibernating in the Nietoperek Bat Reserve (Poland) as a Vector of Airborne Culturable Fungi. Biology 2021, 10, 593. [Google Scholar] [CrossRef]
- Voigt, C.C.; Phelps, K.L.; Aguirre, L.F.; Schoeman, M.C.; Vanitharani, J.; Zubaid, A. Bats and Buildings: The Conservation of Synanthropic Bats. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Springer Nature: Berlin/Heidelberg, Germany, 2016; pp. 427–453. ISBN 9783319252186. [Google Scholar]
- Wasti, I.G.; Khan, F.A.A.; Bernard, H.; Hassan, N.H.; Fayle, T.; Sathiya Seelan, J.S. Fungal Communities in Bat Guano, Speleothem Surfaces, and Cavern Water in Madai Cave, Northern Borneo (Malaysia). Mycology 2021, 12, 188–202. [Google Scholar] [CrossRef]
- Zhao, D.L.; Wang, D.; Tian, X.Y.; Cao, F.; Li, Y.Q.; Zhang, C.S. Anti-Phytopathogenic and Cytotoxic Activities of Crude Extracts and Secondary Metabolites of Marine-Derived Fungi. Mar. Drugs 2018, 16, 36. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Liu, F.; Zhou, X.; Liu, X.Z.; Liu, S.J.; Cai, L. Culturable Mycobiota from Karst Caves in China, with Descriptions of 20 New Species. Persoonia 2017, 39, 1–31. [Google Scholar] [CrossRef]
- Soares, D.; Niemiller, M.L. Extreme Adaptation in Caves. Anat. Rec. 2020, 303, 15–23. [Google Scholar] [CrossRef]
- Gabriel, C.R.; Northup, D.E. Microbial Ecology: Caves as an Extreme Habitat. In Cave Microbiomes: A Novel Resource for Drug Discovery; Cheeptham, N., Ed.; Springer: New York, NY, USA, 2013; pp. 85–108. ISBN 978-1-4614-5206-5. [Google Scholar]
- Zhang, Z.F.; Zhao, P.; Cai, L. Origin of Cave Fungi. Front. Microbiol. 2018, 9, 1407. [Google Scholar] [CrossRef] [PubMed]
- Ogórek, R.; Lejman, A.; Matkowski, K. Fungi Isolated from Niedźwiedzia Cave in Kletno (Lower Silesia, Poland). Int. J. Speleol. 2013, 42, 9. [Google Scholar] [CrossRef]
- Cigna, A.A. Tourism and Show Caves. Z. Geomorphol. 2016, 60, 217–233. [Google Scholar] [CrossRef]
- Mammola, S.; Meierhofer, M.B.; Borges, P.A.V.; Colado, R.; Culver, D.C.; Deharveng, L.; Delić, T.; Di Lorenzo, T.; Dražina, T.; Ferreira, R.L.; et al. Towards Evidence-Based Conservation of Subterranean Ecosystems. Biol. Rev. 2022, 97, 1476–1510. [Google Scholar] [CrossRef] [PubMed]
- Piano, E.; Biagioli, F.; Nicolosi, G.; Coleine, C.; Poli, A.; Prigione, V.; Zanellati, A.; Addesso, R.; Varese, G.C.; Selbmann, L.; et al. Tourism Affects Microbial Assemblages in Show Caves. Sci. Total Environ. 2023, 871, 162106. [Google Scholar] [CrossRef]
- Porca, E.; Jurado, V.; Martin-Sanchez, P.M.; Hermosin, B.; Bastian, F.; Alabouvette, C.; Saiz-Jimenez, C. Aerobiology: An Ecological Indicator for Early Detection and Control of Fungal Outbreaks in Caves. Ecol. Indic. 2011, 11, 1594–1598. [Google Scholar] [CrossRef]
- Ando, K.; Murakami, T. Detection of Human-Associated Bacteria in Water from Akiyoshi-Do Cave, Japan. Water Environ. Res. 2020, 92, 1866–1873. [Google Scholar] [CrossRef]
- Kukla, J.; Holec, M.; Trögl, J.; Holcová, D.; Hofmanová, D.; Kuráň, P.; Popelka, J.; Pacina, J.; Kříženecká, S.; Ust’ak, S.; et al. Tourist Traffic Significantly Affects Microbial Communities of Sandstone Cave Sediments in the Protected Landscape Area “Labské Pískovce” (Czech Republic): Implications for Regulatory Measures. Sustainability 2018, 10, 396. [Google Scholar] [CrossRef]
- Bercea, S.; Năstase-Bucur, R.; Moldovan, O.T.; Kenesz, M.; Constantin, S. Yearly Microbial Cycle of Human Exposed Surfaces in Show Caves. Subterr. Biol. 2019, 31, 1–14. [Google Scholar] [CrossRef]
- Hoyos, M.; Cañaveras, J.C.; Sánchez-Moral, S.; Sanz-Rubio, E.; Soler, V. Microclimatic Characterization of a Karstic Cave: Human Impact on Microenvironmental Parameters of a Prehistoric Rock Art Cave (Candamo Cave, Northern Spain). Environ. Geol. 1998, 33, 231–242. [Google Scholar] [CrossRef]
- Ogórek, R. Mycological Air Pollutions in Gold Mine (Gertruda’s Adit) in Złoty Stok. Proc. 36th Conf. Agric. Students Vet. Med. with Int. Particip. 2012, 1, 2–710. [Google Scholar]
- Griffin, D.W.; Gray, M.A.; Lyles, M.B.; Northup, D.E. The Transport of Nonindigenous Microorganisms Into Caves by Human Visitation: A Case Study at Carlsbad Caverns National Park. Geomicrobiol. J. 2014, 31, 175–185. [Google Scholar] [CrossRef]
- Stupar, M.; Savković, Ž.; Popović, S.; Simić, G.S.; Grbić, M.L. Speleomycology of Air in Stopića Cave (Serbia). Microb. Ecol. 2023, 86, 2021–2031. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; Beale, M.A.; Hagen, F.; Fisher, M.C.; Terra, P.P.D.; de Hoog, S.; Brilhante, R.S.N.; de Aguiar Cordeiro, R.; de Souza Collares Maia Castelo-Branco, D.; Rocha, M.F.G.; et al. The Global Epidemiology of Emerging Histoplasma Species in Recent Years. Stud. Mycol. 2020, 97, 100095. [Google Scholar] [CrossRef]
- Ogórek, R.; Kozak, B.; Višňovská, Z.; Tančinová, D. Phenotypic and Genotypic Diversity of Airborne Fungal Spores in Demänovská Ice Cave (Low Tatras, Slovakia). Aerobiologia 2018, 34, 13–28. [Google Scholar] [CrossRef]
- Hemnani, M.; da Silva, P.G.; Thompson, G.; Poeta, P.; Rebelo, H.; Mesquita, J.R. First Report of Alphacoronavirus Circulating in Cavernicolous Bats from Portugal. Viruses 2023, 15, 1521. [Google Scholar] [CrossRef]
- Raper, K.B. The Genus Aspergillus; Williams Wilkins Co.: Philadelphia, PA, USA, 1965. [Google Scholar]
- Domsch, K.H. Compendium of Soil Fungi; IHW-Verlag 1: Eching, Germany, 1993; pp. 630–643. [Google Scholar]
- Klich, M.A. Biogeography of Aspergillus Species in Soil and Litter. Mycologia 2002, 94, 21–27. [Google Scholar] [CrossRef]
- Kim, S.; Kim, N.; Park, W.B.; Kang, C.K.; Park, J.H.; Lee, S.-T.; Jung, K.-H.; Park, K.-I.; Lee, S.K.; Moon, J.; et al. Real-Time Application of ITS and D1-D3 Nanopore Amplicon Metagenomic Sequencing in Fungal Infections: Enhancing Fungal Infection Diagnostics. Int. J. Med. Microbiol. 2024, 316, 151630. [Google Scholar] [CrossRef]
- De Coster, W.; Rademakers, R. {NanoPack2}: Population-Scale Evaluation of Long-Read Sequencing Data. Bioinformatics 2023, 39, btad311. [Google Scholar] [CrossRef]
- Wick, R. Rrwick/{Porechop}. Available online: https://github.com/rrwick/Porechop (accessed on 25 November 2024).
- Wood, D.E.; Lu, J.; Langmead, B. Improved Metagenomic Analysis with {Kraken} 2. Genome Biol. 2019, 20, 257. [Google Scholar] [CrossRef]
- Abarenkov, K.; Zirk, A.; Piirmann, T.; Pöhönen, R.; Ivanov, F.; Nilsson, R.H.; Kõljalg, U. UNITE General FASTA Release for Fungi. Version 04.04.2024. 2024. Available online: https://unite.ut.ee/repository.php (accessed on 10 February 2025).
- Lu, J.; Breitwieser, F.P.; Thielen, P.; Salzberg, S.L. Bracken: Estimating Species Abundance in Metagenomics Data. PeerJ Comput. Sci. 2017, 3, e104. [Google Scholar] [CrossRef] [PubMed]
- Moreira, G. GmoreiraVet/Guitools: V1.1.1. 2025. Available online: https://github.com/GmoreiraVet/Guitools (accessed on 12 February 2025).
- Cantrell, S.A.; Dianese, J.C.; Fell, J.; Gunde-Cimerman, N.; Zalar, P. Unusual Fungal Niches. Mycologia 2011, 103, 1161–1174. [Google Scholar] [CrossRef]
- Siqueira, J.P.Z.; Sutton, D.A.; Gené, J.; García, D.; Wiederhold, N.; Guarro, J. Species of Aspergillus Section Aspergillus from Clinical Samples in the United States. Med. Mycol. 2018, 56, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.; Groenewald, M.; de Vries, M.; Gehrmann, T.; Stielow, B.; Eberhardt, U.; Al-Hatmi, A.; Groenewald, J.Z.; Cardinali, G.; Houbraken, J.; et al. Large-Scale Generation and Analysis of Filamentous Fungal DNA Barcodes Boosts Coverage for Kingdom Fungi and Reveals Thresholds for Fungal Species and Higher Taxon Delimitation. Stud. Mycol. 2019, 92, 135–154. [Google Scholar] [CrossRef]
- Chen, M.; Ding, Z.; Zhou, M.; Shang, Y.; Li, C.; Li, Q.; Bu, T.; Tang, Z.; Chen, H. The Diversity of Endophytic Fungi in Tartary Buckwheat (Fagopyrum Tataricum) and Its Correlation with Flavonoids and Phenotypic Traits. Front. Microbiol. 2024, 15, 1360988. [Google Scholar] [CrossRef]
- Gallego-Clemente, E.; Moreno-González, V.; Ibáñez, A.; Calvo-Peña, C.; Ghoreshizadeh, S.; Radišek, S.; Cobos, R.; Coque, J.J.R. Changes in the Microbial Composition of the Rhizosphere of Hop Plants Affected by Verticillium Wilt Caused by Verticillium Nonalfalfae. Microorganisms 2023, 11, 1819. [Google Scholar] [CrossRef]
- Moore, J.A.M.; Anthony, M.A.; Pec, G.J.; Trocha, L.K.; Trzebny, A.; Geyer, K.M.; van Diepen, L.T.A.; Frey, S.D. Fungal Community Structure and Function Shifts with Atmospheric Nitrogen Deposition. Glob. Change Biol. 2021, 27, 1349–1364. [Google Scholar] [CrossRef] [PubMed]
- Rabha, J.; Jha, D. Metabolic Diversity of Penicillium. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 217–234. [Google Scholar] [CrossRef]
- Krijgsheld, P.; Bleichrodt, R.; Veluw, G.; Wang, F.; Müller, W.; Dijksterhuis, J.; Wösten, H. Development in Aspergillus. Stud. Mycol. 2012, 74, 1–29. [Google Scholar] [CrossRef]
- Hagiwara, D.; Sakamoto, K.; Abe, K.; Gomi, K. Signaling Pathways for Stress Responses and Adaptation in Aspergillus Species: Stress Biology in the Post-Genomic Era. Biosci. Biotechnol. Biochem. 2016, 80, 1667–1680. [Google Scholar] [CrossRef]
- Morschhäuser, J. Adaptation of Candida Albicans to Specific Host Environments by Gain-of-Function Mutations in Transcription Factors. PLoS Pathog. 2024, 20, e1012643. [Google Scholar] [CrossRef]
- Keck, F.; Blackman, R.; Bossart, R.; Brantschen, J.; Couton, M.; Hürlemann, S.; Kirschner, D.; Locher, N.; Zhang, H.; Altermatt, F. Meta-analysis Shows Both Congruence and Complementarity of DNA and EDNA Metabarcoding to Traditional Methods for Biological Community Assessment. Mol. Ecol. 2022, 31, 1820–1835. [Google Scholar] [CrossRef] [PubMed]




| Bat Species |
|---|
| Myotis myotis |
| Miniopterus schreibersii |
| Rhinolophus ferrumequinum |
| Rhinolophus mehelyi |
| Fungal Taxa | Detected Sequences (Accession No.) | Bat Roosts | Query Cover (%) | Identities (%) | E Value | Highest Similarity Sequences (Accession No.) |
|---|---|---|---|---|---|---|
| Aspergillus pseudoglaucus | PV065740 | Tomar I | 100% | 100.00% | 0 | ON645262 |
| Botryotrichum murorum | PV065856 | Tomar I | 100% | 100.00% | 0 | MW563925 |
| Aspergillus sp. 1 | N.A. | Tomar I | N.A. | N.A. | N.A. | N.A. |
| Aspergillus pseudoglaucus | PV069224 | Tomar I | 100% | 100.00% | 0 | ON645262 |
| Aspergillus sp. 1 | N.A. | Tomar I | N.A. | N.A. | N.A. | N.A. |
| Chaetomium sp. 1 | N.A. | Tomar II | N.A. | N.A. | N.A. | N.A. |
| Chaetomium globosum | PV066994 | Moura I (2) | 100% | 100.00% | 0 | OKJ531968 |
| Aspergillus hiratsukae | PV067022 | Moura I (2) | 100% | 99.81% | 0 | OK94RE6FX016 |
| Aspergillus sp. 1 | N.A. | Montemor-o-Novo I | N.A. | N.A. | N.A. | N.A. |
| Penicillium shennongjianum | PV067057 | Montemor-o-Novo I | 100% | 100.00% | 0 | MH862167 |
| Penicillium shennongjianum | PV067130 | Montemor-o-Novo I | 100% | 100.00% | 0 | MH862167 |
| Sample | Unique Genera | First Top Genera | Reads | Second Top Genera | Reads | Third Top Genera | Reads |
|---|---|---|---|---|---|---|---|
| Ansião I | 99 | Wallemia | 405 (21.7%) | Aspergillus | 326 (18.2%) | Chrysosporium | 307 (16.9%) |
| Tomar I | 70 | Candida | 299 (14.6%) | Aspergillus | 240 (11.7%) | Coprinellus | 220 (10.7%) |
| Tomar II | 83 | Peltula | 1071 (50.9%) | Wallemia | 543 (25.7%) | Sistotrema | 91 (4.3%) |
| Moura I (1) | 119 | Utharomyces | 281 (13.9%) | Haplosporangium | 219 (11.3%) | Dlhawksworthia | 217 (11.2%) |
| Moura I (2) | 53 | Calyptella | 1079 (28.9%) | Candida | 1024 (27.5%) | Buckleyzyma | 405 (13.3%) |
| Montemor-o-Novo I | 91 | Aspergillus | 346 (42.0%) | Sterigmatomyces | 292 (35.6%) | Filobasidium | 69 (8.4%) |
| Top 3 Genera Across All The Samples | Reads |
|---|---|
| Aspergillus | 1563 (12.6%) |
| Candida | 1493 (12.3%) |
| Calyptella | 1101 (9.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bento, J.T.; Moreira, G.; Pinto, E.; Gomes da Silva, P.; Rebelo, H.; Mourão, J.; Sousa, S.I.V.; Mesquita, J.R. Airborne Fungal Spore Diversity Assessment Using Culture-Dependent and Metabarcoding Approaches in Bat-Inhabited Natural and Anthropogenic Roosts in Portugal. J. Fungi 2025, 11, 360. https://doi.org/10.3390/jof11050360
Bento JT, Moreira G, Pinto E, Gomes da Silva P, Rebelo H, Mourão J, Sousa SIV, Mesquita JR. Airborne Fungal Spore Diversity Assessment Using Culture-Dependent and Metabarcoding Approaches in Bat-Inhabited Natural and Anthropogenic Roosts in Portugal. Journal of Fungi. 2025; 11(5):360. https://doi.org/10.3390/jof11050360
Chicago/Turabian StyleBento, Jaqueline T., Guilherme Moreira, Eugénia Pinto, Priscilla Gomes da Silva, Hugo Rebelo, Joana Mourão, Sofia I. V. Sousa, and João R. Mesquita. 2025. "Airborne Fungal Spore Diversity Assessment Using Culture-Dependent and Metabarcoding Approaches in Bat-Inhabited Natural and Anthropogenic Roosts in Portugal" Journal of Fungi 11, no. 5: 360. https://doi.org/10.3390/jof11050360
APA StyleBento, J. T., Moreira, G., Pinto, E., Gomes da Silva, P., Rebelo, H., Mourão, J., Sousa, S. I. V., & Mesquita, J. R. (2025). Airborne Fungal Spore Diversity Assessment Using Culture-Dependent and Metabarcoding Approaches in Bat-Inhabited Natural and Anthropogenic Roosts in Portugal. Journal of Fungi, 11(5), 360. https://doi.org/10.3390/jof11050360