Current Performance of MALDI–TOF Mass Spectrometry Databases for the Identification of Dermatophyte Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains
2.2. MALDI–TOF Mass Spectrometry
2.3. Addition of New Reference Spectra to HGM Database
2.4. DNA Analysis
2.5. Protein Peak Analysis
2.6. Statistical Analysis
3. Results
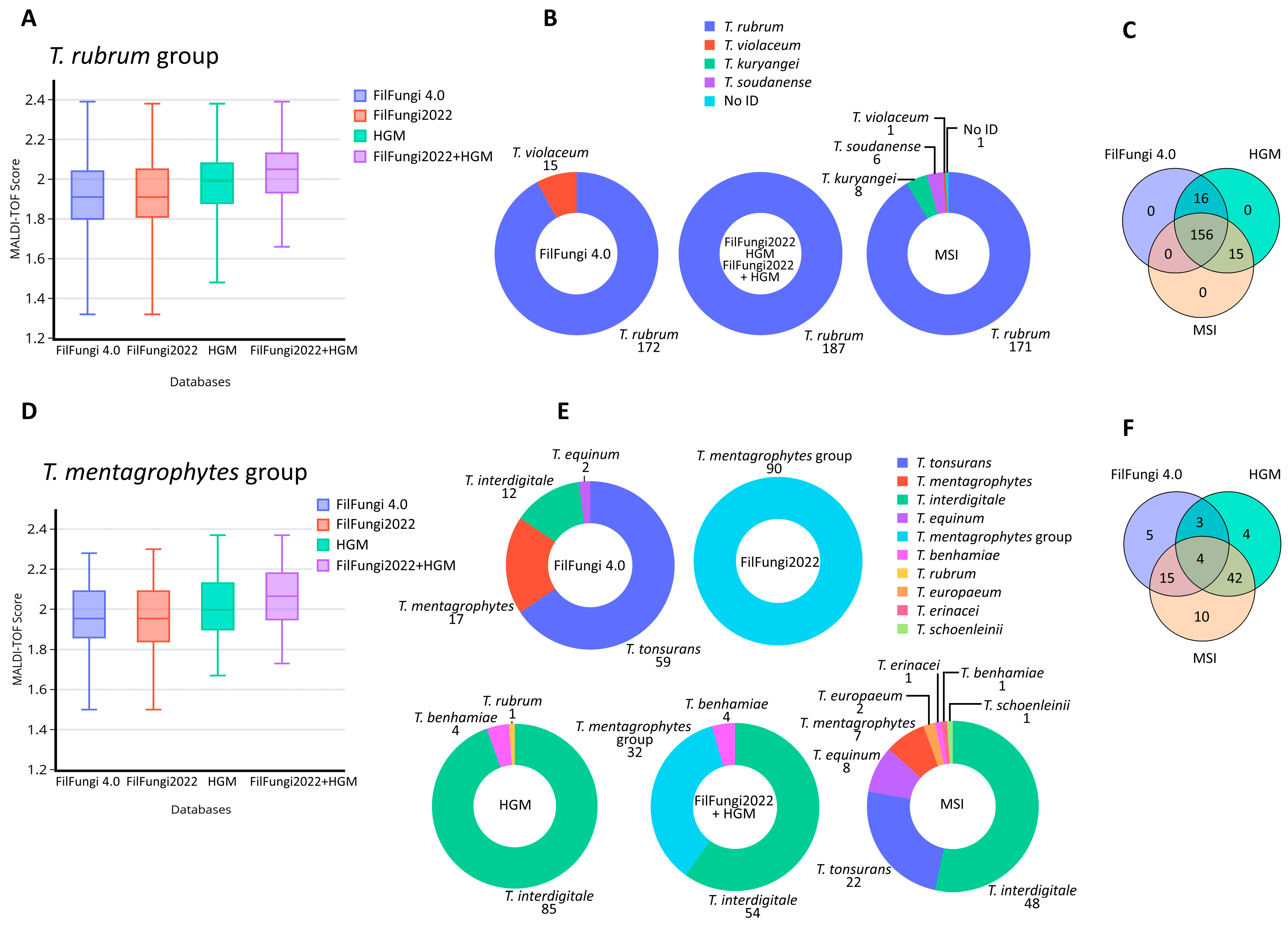
3.1. Comparison of Current Databases for T. rubrum Group
3.2. Comparison of Current Databases for T. mentagrophytes Group
3.3. Comparison of Current Databases for Other Genera
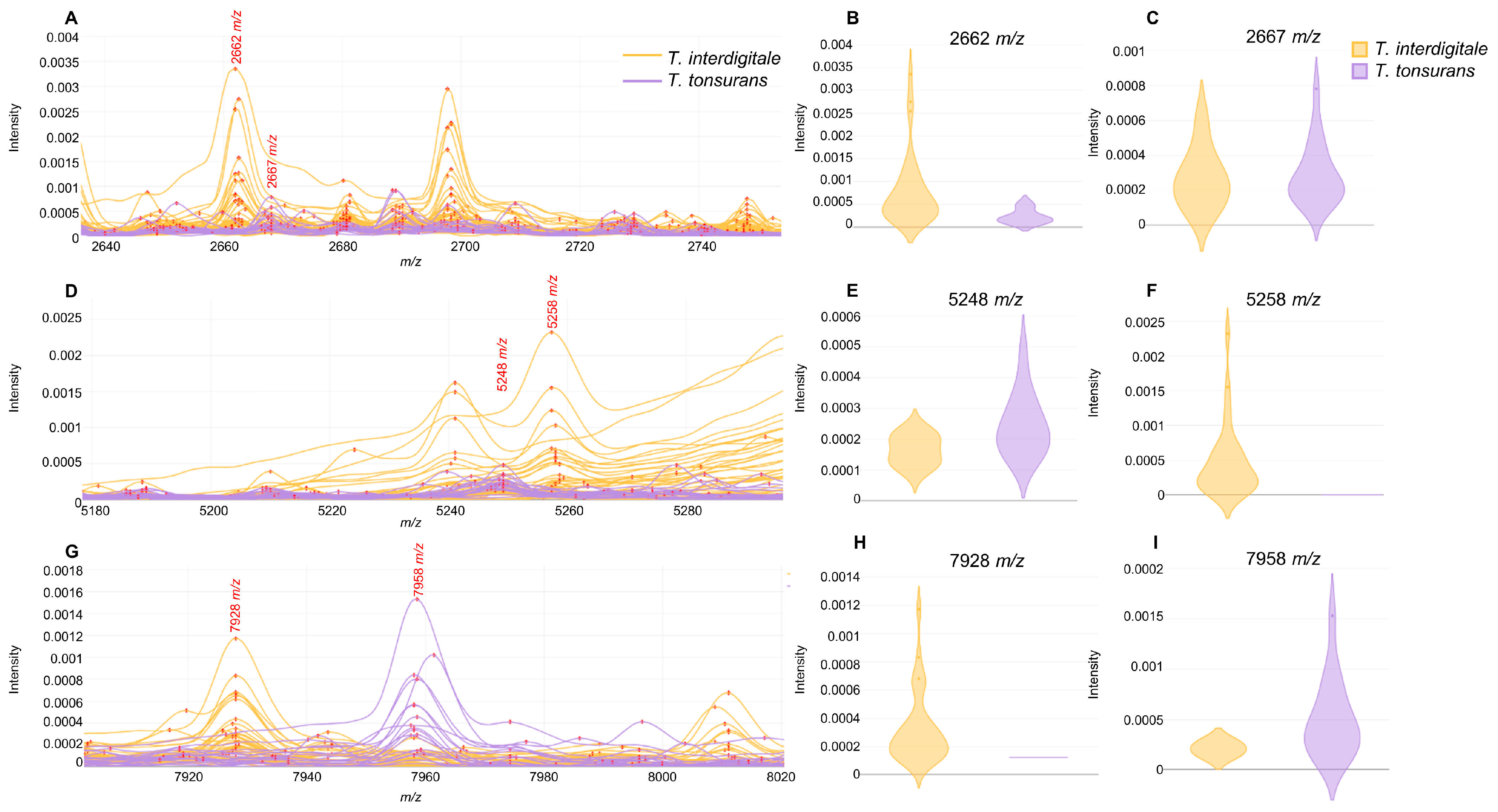
3.4. Peak Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weitzman, I.; Summerbell, R.C. The dermatophytes. Clin. Microbiol. Rev. 1995, 8, 240–259. [Google Scholar] [CrossRef] [PubMed]
- Bouchara, J.P.; Mignon, B.; Chaturvedi, V. Dermatophytes and Dermatophytoses: A Thematic Overview of State of the Art, and the Directions for Future Research and Developments. Mycopathologia 2017, 182, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Baumgardner, D.J. Fungal Infections From Human and Animal Contact. J. Patient Cent. Res. Rev. 2017, 4, 78–89. [Google Scholar] [CrossRef]
- de Hoog, G.S.; Dukik, K.; Monod, M.; Packeu, A.; Stubbe, D.; Hendrickx, M.; Kupsch, C.; Stielow, J.B.; Freeke, J.; Goker, M.; et al. Toward a Novel Multilocus Phylogenetic Taxonomy for the Dermatophytes. Mycopathologia 2017, 182, 5–31. [Google Scholar] [CrossRef]
- Seebacher, C.; Bouchara, J.-P.; Mignon, B. Updates on the Epidemiology of Dermatophyte Infections. Mycopathologia 2008, 166, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Ilkit, M.; Durdu, M. Tinea pedis: The etiology and global epidemiology of a common fungal infection. Crit. Rev. Microbiol. 2014, 41, 374–388. [Google Scholar] [CrossRef]
- Moskaluk, A.E.; VandeWoude, S. Current Topics in Dermatophyte Classification and Clinical Diagnosis. Pathogens 2022, 11, 957. [Google Scholar] [CrossRef]
- Pihet, M.; Le Govic, Y. Reappraisal of Conventional Diagnosis for Dermatophytes. Mycopathologia 2017, 182, 169–180. [Google Scholar] [CrossRef]
- Verrier, J.; Monod, M. Diagnosis of Dermatophytosis Using Molecular Biology. Mycopathologia 2017, 182, 193–202. [Google Scholar] [CrossRef]
- Lau, A.F.; Drake, S.K.; Calhoun, L.B.; Henderson, C.M.; Zelazny, A.M. Development of a clinically comprehensive database and a simple procedure for identification of molds from solid media by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2013, 51, 828–834. [Google Scholar] [CrossRef]
- Zvezdanova, M.E.; de Aledo, M.G.; Lopez-Mirones, J.I.; Ortega, J.; Canut, A.; Castro, C.; Gomez, C.; Hernaez, S.; Oviano, M.; Ercibengoa, M.; et al. Validation of an expanded, in-house library and an optimized preparation method for the identification of fungal isolates using MALDI-TOF mass spectrometry. Med. Mycol. 2023, 61, myad038. [Google Scholar] [CrossRef] [PubMed]
- Packeu, A.; Stubbe, D.; Roesems, S.; Goens, K.; Van Rooij, P.; de Hoog, S.; Hendrickx, M. Lineages Within the Trichophyton rubrum Complex. Mycopathologia 2020, 185, 123–136. [Google Scholar] [CrossRef] [PubMed]
- L’Ollivier, C.; Ranque, S. MALDI-TOF-Based Dermatophyte Identification. Mycopathologia 2017, 182, 183–192. [Google Scholar] [CrossRef]
- Čmoková, A.; Kolařík, M.; Dobiáš, R.; Hoyer, L.L.; Janouškovcová, H.; Kano, R.; Kuklová, I.; Lysková, P.; Machová, L.; Maier, T.; et al. Resolving the taxonomy of emerging zoonotic pathogens in the Trichophyton benhamiae complex. Fungal Divers. 2020, 104, 333–387. [Google Scholar] [CrossRef]
- Nenoff, P.; Erhard, M.; Simon, J.C.; Muylowa, G.K.; Herrmann, J.; Rataj, W.; Graser, Y. MALDI-TOF mass spectrometry—A rapid method for the identification of dermatophyte species. Med. Mycol. 2013, 51, 17–24. [Google Scholar] [CrossRef]
- Karabicak, N.; Karatuna, O.; Ilkit, M.; Akyar, I. Evaluation of the Bruker Matrix-Assisted Laser Desorption-Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF MS) System for the Identification of Clinically Important Dermatophyte Species. Mycopathologia 2015, 180, 165–171. [Google Scholar] [CrossRef]
- Maldonado, I.; Relloso, S.; Guelfand, L.; Fox, B.; Azula, N.; Romano, V.; Cantore, A.; Barrios, R.; Carnovale, S.; Nuske, E.; et al. Evaluation of the MALDI-TOF mass spectrometry technique for the identification of dermatophytes: Use of an extended database. Rev. Iberoam. Micol. 2023, 40, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.F.; Fan, Y.C.; Lu, J.J.; Chien, C.C.; Wang, H.Y.; Sun, P.L. Identification of Challenging Dermatophyte Species Using Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry. J. Fungi 2025, 11, 107. [Google Scholar] [CrossRef]
- Normand, A.C.; Blaize, M.; Imbert, S.; Packeu, A.; Becker, P.; Fekkar, A.; Stubbe, D.; Piarroux, R. Identification of Molds with Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry: Performance of the Newly Developed MSI-2 Application in Comparison with the Bruker Filamentous Fungi Database and MSI-1. J. Clin. Microbiol. 2021, 59, e0129921. [Google Scholar] [CrossRef]
- Normand, A.C.; Packeu, A.; Cassagne, C.; Hendrickx, M.; Ranque, S.; Piarroux, R. Nucleotide Sequence Database Comparison for Routine Dermatophyte Identification by Internal Transcribed Spacer 2 Genetic Region DNA Barcoding. J. Clin. Microbiol. 2018, 56, e00046-18. [Google Scholar] [CrossRef]
- Escribano, P.; Pelaez, T.; Recio, S.; Bouza, E.; Guinea, J. Characterization of clinical strains of Aspergillus terreus complex: Molecular identification and antifungal susceptibility to azoles and amphotericin B. Clin. Microbiol. Infect. 2012, 18, E24–E26. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.r-project.org/ (accessed on 2 May 2025).
- Calderaro, A.; Motta, F.; Montecchini, S.; Gorrini, C.; Piccolo, G.; Piergianni, M.; Buttrini, M.; Medici, M.C.; Arcangeletti, M.C.; Chezzi, C.; et al. Identification of Dermatophyte species after implementation of the in-house MALDI-TOF MS database. Int. J. Mol. Sci. 2014, 15, 16012–16024. [Google Scholar] [CrossRef] [PubMed]
- De Respinis, S.; Monnin, V.; Girard, V.; Welker, M.; Arsac, M.; Celliere, B.; Durand, G.; Bosshard, P.P.; Farina, C.; Passera, M.; et al. Matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry using the Vitek MS system for rapid and accurate identification of dermatophytes on solid cultures. J. Clin. Microbiol. 2014, 52, 4286–4292. [Google Scholar] [CrossRef] [PubMed]
- Patel, R. A Moldy Application of MALDI: MALDI-ToF Mass Spectrometry for Fungal Identification. J. Fungi 2019, 5, 4. [Google Scholar] [CrossRef]
- L’Ollivier, C.; Cassagne, C.; Normand, A.C.; Bouchara, J.P.; Contet-Audonneau, N.; Hendrickx, M.; Fourquet, P.; Coulibaly, O.; Piarroux, R.; Ranque, S. A MALDI-TOF MS procedure for clinical dermatophyte species identification in the routine laboratory. Med. Mycol. 2013, 51, 713–720. [Google Scholar] [CrossRef]
- Baumbach, C.M.; Muller, S.; Reuschel, M.; Uhrlass, S.; Nenoff, P.; Baums, C.G.; Schrodl, W. Identification of Zoophilic Dermatophytes Using MALDI-TOF Mass Spectrometry. Front. Cell Infect. Microbiol. 2021, 11, 631681. [Google Scholar] [CrossRef]
- Cornet, L.; D’Hooge, E.; Magain, N.; Stubbe, D.; Packeu, A.; Baurain, D.; Becker, P. The taxonomy of the Trichophyton rubrum complex: A phylogenomic approach. Microb. Genom. 2021, 7, 000707. [Google Scholar] [CrossRef]
- Hedayati, M.T.; Ansari, S.; Ahmadi, B.; Taghizadeh Armaki, M.; Shokohi, T.; Abastabar, M.; Er, H.; Ozhak, B.; Ogunc, D.; Ilkit, M.; et al. Identification of clinical dermatophyte isolates obtained from Iran by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Curr. Med. Mycol. 2019, 5, 22–26. [Google Scholar] [CrossRef]
- Shao, J.; Wan, Z.; Li, R.; Yu, J. Species identification of dermatophytes isolated in China by matrix-assisted laser desorption ionisation-time-of-flight mass spectrometry. Mycoses 2020, 63, 1352–1361. [Google Scholar] [CrossRef]
- Lecerf, P.; De Paepe, R.; Jazaeri, Y.; Normand, A.C.; Martiny, D.; Packeu, A. Evaluation of a Liquid Media MALDI-TOF MS Protocol for the Identification of Dermatophytes Isolated from Tinea capitis Infections. J. Fungi 2022, 8, 1248. [Google Scholar] [CrossRef] [PubMed]
- Baert, F.; Lefevere, P.; D’hooge, E.; Stubbe, D.; Packeu, A. A Polyphasic Approach to Classification and Identification of Species within the Trichophyton benhamiae Complex. J. Fungi 2021, 7, 602. [Google Scholar] [CrossRef] [PubMed]
- Jabet, A.; Normand, A.-C.; Moreno-Sabater, A.; Guillot, J.; Risco-Castillo, V.; Brun, S.; Demar, M.; Blaizot, R.; Nabet, C.; Packeu, A.; et al. Investigations upon the Improvement of Dermatophyte Identification Using an Online Mass Spectrometry Application. J. Fungi 2022, 8, 73. [Google Scholar] [CrossRef]
- Candela, A.; Guerrero-López, A.; Mateos, M.; Gómez-Asenjo, A.; Arroyo, M.J.; Hernandez-García, M.; del Campo, R.; Cercenado, E.; Cuénod, A.; Méndez, G.; et al. Automatic Discrimination of Species within the Enterobacter cloacae Complex Using Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry and Supervised Algorithms. J. Clin. Microbiol. 2023, 61, e0104922. [Google Scholar] [CrossRef] [PubMed]
- Normand, A.-C.; Chaline, A.; Mohammad, N.; Godmer, A.; Acherar, A.; Huguenin, A.; Ranque, S.; Tannier, X.; Piarroux, R. Identification of a clonal population of Aspergillus flavus by MALDI-TOF mass spectrometry using deep learning. Sci. Rep. 2022, 12, 1575. [Google Scholar] [CrossRef]
- Kano, R.; Kimura, U.; Kakurai, M.; Hiruma, J.; Kamata, H.; Suga, Y.; Harada, K. Trichophyton indotineae sp. nov.: A New Highly Terbinafine-Resistant Anthropophilic Dermatophyte Species. Mycopathologia 2020, 185, 947–958. [Google Scholar] [CrossRef]
| Database | Species Result | T. interdigitale (n = 53) | T. tonsurans (n = 25) | T. mentagrophytes (n = 8) | T. japonicum (n = 4) |
|---|---|---|---|---|---|
| FilFungi4.0 | T. tonsurans | 36 | 18 | 5 | - |
| T. mentagrophytes | 10 | 4 | 2 | 1 | |
| T. interdigitale | 7 | 1 | 1 | 3 | |
| T. equinum | - | 2 | - | - | |
| HGM | T. interdigitale | 53 | 25 | 7 | - |
| T. benhamiae | - | - | - | 4 | |
| T. rubrum | - | - | 1 | - | |
| MSI | T. interdigitale | 46 | - | 2 | - |
| T. tonsurans | 3 | 19 | - | - | |
| T. equinum | 3 | 5 | - | - | |
| T. mentagrophytes | 1 | - | 6 | - | |
| T. europaeum | - | - | - | 2 | |
| T. erinacei | - | - | - | 1 | |
| T. benhamiae | - | - | - | 1 | |
| T. schoenleinii | - | 1 | - | - |
| Peaks (m/z) | AUC | Species | t-Test p Value |
|---|---|---|---|
| 2512 | 0.9021 | T. tonsurans | <0.05 |
| 2662 | 0.8256 | T. interdigitale | <0.05 |
| 2667 | 0.9045 | T. tonsurans | <0.05 |
| 2747 | 0.8629 | T. interdigitale | <0.05 |
| 2887 | 0.8213 | T. interdigitale | <0.05 |
| 3630 | 0.8568 | T. tonsurans | <0.05 |
| 3740 | 0.6860 | T. interdigitale | >0.05 |
| 4664 | 0.8397 | T. tonsurans | <0.05 |
| 4749 | 0.8152 | T. tonsurans | >0.05 |
| 5248 | 0.7332 | T. tonsurans | <0.05 |
| 5258 | 0.8488 | T. interdigitale | <0.05 |
| 5329 | 0.8960 | T. interdigitale | <0.05 |
| 5400 | 0.8531 | T. interdigitale | <0.05 |
| 5629 | 0.8299 | T. tonsurans | <0.05 |
| 5852 | 0.8042 | T. interdigitale | <0.05 |
| 6357 | 0.8237 | T. tonsurans | <0.05 |
| 6458 | 0.7858 | T. interdigitale | <0.05 |
| 6533 | 0.8409 | T. tonsurans | <0.05 |
| 7304 | 0.8017 | T. tonsurans | <0.05 |
| 7327 | 0.8042 | T. tonsurans | <0.05 |
| 7865 | 0.8384 | T. tonsurans | >0.05 |
| 7928 | 0.8739 | T. interdigitale | <0.05 |
| 7958 | 0.9088 | T. tonsurans | <0.05 |
| 9367 | 0.8244 | T. tonsurans | <0.05 |
| 9499 | 0.8568 | T. tonsurans | <0.05 |
| 11,228 | 0.8397 | T. interdigitale | <0.05 |
| 12,251 | 0.8684 | T. tonsurans | >0.05 |
| 12,419 | 0.7607 | T. interdigitale | >0.05 |
| 12,435 | 0.8403 | T. tonsurans | >0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Temporal, D.; Adrados, D.; Alastruey-Izquierdo, A.; Alkorta, M.; Candela, A.; Canut, A.; Castro, C.; Cilla, C.G.; de Dios Caballero, J.; Ercibengoa, M.; et al. Current Performance of MALDI–TOF Mass Spectrometry Databases for the Identification of Dermatophyte Species. J. Fungi 2025, 11, 356. https://doi.org/10.3390/jof11050356
Rodriguez-Temporal D, Adrados D, Alastruey-Izquierdo A, Alkorta M, Candela A, Canut A, Castro C, Cilla CG, de Dios Caballero J, Ercibengoa M, et al. Current Performance of MALDI–TOF Mass Spectrometry Databases for the Identification of Dermatophyte Species. Journal of Fungi. 2025; 11(5):356. https://doi.org/10.3390/jof11050356
Chicago/Turabian StyleRodriguez-Temporal, David, Daniel Adrados, Ana Alastruey-Izquierdo, Miriam Alkorta, Ana Candela, Andrés Canut, Carmen Castro, Carlos Gustavo Cilla, Juan de Dios Caballero, María Ercibengoa, and et al. 2025. "Current Performance of MALDI–TOF Mass Spectrometry Databases for the Identification of Dermatophyte Species" Journal of Fungi 11, no. 5: 356. https://doi.org/10.3390/jof11050356
APA StyleRodriguez-Temporal, D., Adrados, D., Alastruey-Izquierdo, A., Alkorta, M., Candela, A., Canut, A., Castro, C., Cilla, C. G., de Dios Caballero, J., Ercibengoa, M., Fernández, M., Fradejas, I., Fraile, O., Goyanes, M. J., Gutiérrez, A., López, J. I., López, C., López-Calleja, A. I., López-Medrano, R., ... Rodríguez-Sánchez, B. (2025). Current Performance of MALDI–TOF Mass Spectrometry Databases for the Identification of Dermatophyte Species. Journal of Fungi, 11(5), 356. https://doi.org/10.3390/jof11050356









