Pneumocystis Infection in Pregnant Women: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
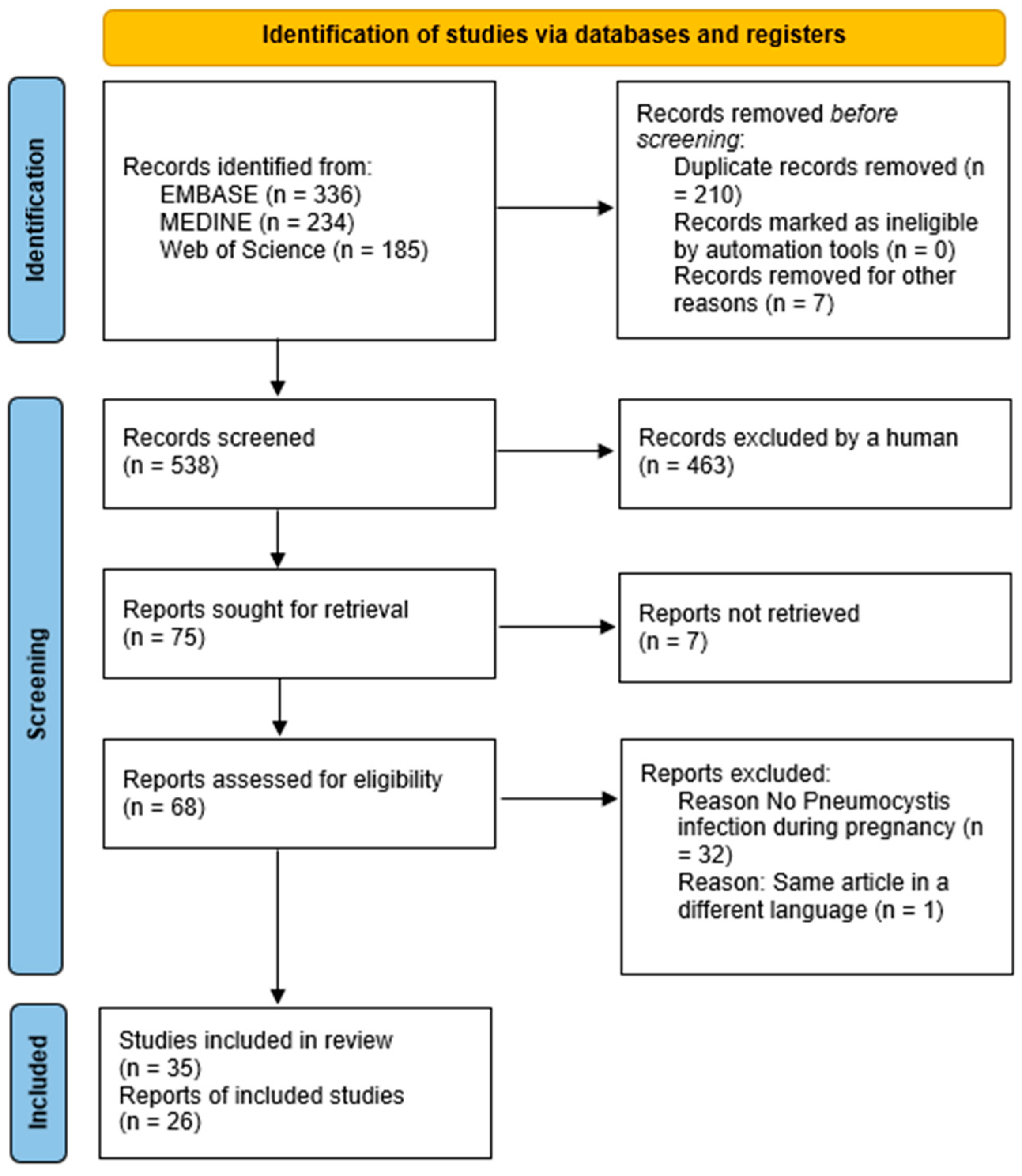
2.3. Study Screening and Selection
2.4. Data Extraction and Synthesis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calderón, E.J.; Friaza, V. Pneumocystis jirovecii in human disease: Just pneumonia? Rev. Clin. Esp. 2024, 224, 546–548. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.; Wei, K.; Afshar, K.; Huang, L. Epidemiology and clinical significance of pneumocystis colonization. J. Infect. Dis. 2008, 197, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Calderón, E.J. Epidemiology of Pneumocystis infection in Human. J. Med. Mycol. 2009, 19, 270–275. [Google Scholar] [CrossRef]
- Mori, S.; Cho, I.; Ichiyasu, H.; Sugimoto, M. Asymptomatic carriage of Pneumocystis jiroveci in elderly patients with rheumatoid arthritis in Japan: A possible association between colonization and development of Pneumocystis jiroveci pneumonia during low-dose MTX therapy. Mod. Rheumatol. 2008, 18, 240–246. [Google Scholar] [CrossRef]
- Ochoa, T.J.; Bustamante, B.; Garcia, C.; Neyra, E.; Mendoza, K.; Calderón, E.J.; Le Gal, S.; Miller, R.F.; Ponce, C.A.; Nevez, G.; et al. Pneumocystis primary infection in non- immunosuppressed infants in Lima, Peru. J. Mycol. Med. 2022, 32, 101202. [Google Scholar] [CrossRef]
- Mekinian, A.; Durand-Joly, I.; Hatron, P.Y.; Moranne, O.; Denis, G.; Dei-Cas, E.; Morell-Dubois, S.; Lambert, M.; Launay, D.; Delhaes, L.; et al. Pneumocystis jirovecii colonization in patients with systemic autoimmune diseases: Prevalence, risk factors of colonization and outcome. Rheumatology 2011, 50, 569–577. [Google Scholar] [CrossRef]
- Vera, C.; Rueda, Z.V. Transmission and Colonization of Pneumocystis jirovecii. J. Fungi 2021, 7, 979. [Google Scholar] [CrossRef]
- Alanazi, H.; Zhang, Y.; Fatunbi, J.; Luu, T.; Kwak-Kim, J. The impact of reproductive hormones on T cell immunity; normal and assisted reproductive cycles. J. Reprod. Immunol. 2024, 165, 104295. [Google Scholar] [CrossRef]
- Montes-Cano, M.A.; Chabe, M.; Fontillon-Alberdi, M.; de-Lahorra, C.; Respaldiza, N.; Medrano, F.J.; Varela, J.M.; Dei-Cas, E.; Calderon, E.J. Vertical transmission of Pneumocystis jirovecii in humans. Emerg. Infect. Dis. 2009, 15, 125–127. [Google Scholar] [CrossRef]
- Rojas, P.; Friaza, V.; García, E.; de la Horra, C.; Vargas, S.L.; Calderón, E.J.; Pavón, A. Early Acquisition of Pneumocystis jirovecii Colonization and Potential Association with Respiratory Distress Syndrome in Preterm Newborn Infants. Clin. Infect. Dis. 2017, 65, 976–981. [Google Scholar] [CrossRef]
- Szydłowicz, M.; Królak-Olejnik, B.; Vargas, S.L.; Zajączkowska, Ż.; Paluszyńska, D.; Szczygieł, A.; Matos, O.; Hendrich, A.B.; Kicia, M. Pneumocystis jirovecii Colonization in Preterm Newborns with Respiratory Distress Syndrome. J. Infect. Dis. 2022, 225, 1807–1810. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping Studies: Towards a methodological framework. Int. J. Social. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Pavlica, F. 1st observation on congenital Pneumocystic pneumonia in a mature, full-term stillborn child. Zentralblatt Allg. Pathol. Pathol. Anat. 1962, 103, 236–241. [Google Scholar]
- Pavlica, F. The first observation of congenital pneumocystic pneumonia in a fully developed stillborn child. Ann. Paediatr. 1962, 198, 177–184. [Google Scholar]
- Minkoff, H.; deRegt, R.H.; Landesman, S.; Schwarz, R. Pneumocystis carinii pneumonia associated with acquired immunodeficiency syndrome in pregnancy: A report of three maternal deaths. Obstet. Gynecol. 1986, 67, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Koonin, L.M.; Ellerbrock, T.V.; Atrash, H.K.; Rogers, M.F.; Smith, J.C.; Hogue, C.J.; Harris, M.A.; Chavkin, W.; Parker, A.L.; Halpin, G.J. Pregnancy-associated deaths due to AIDS in the United States. JAMA 1989, 261, 1306–1309. [Google Scholar] [CrossRef]
- Hicks, M.L.; Nolan, G.H.; Maxwell, S.L.; Mickle, C. Acquired immunodeficiency syndrome and Pneumocystis carinii infection in a pregnant woman. Obstet. Gynecol. 1990, 76 Pt 2, 480–481. [Google Scholar]
- Kell, P.D.; Barton, S.E.; Smith, D.E.; Nelson, M.; Marwood, R.P.; Gazzard, B. A maternal death caused by AIDS. Case report. Br. J. Obstet. Gynaecol. 1991, 98, 725–727. [Google Scholar] [CrossRef]
- Johnstone, F.D.; Willox, L.; Brettle, R.P. Survival time after AIDS in pregnancy. Br. J. Obstet. Gynaecol. 1992, 99, 633–636. [Google Scholar] [CrossRef]
- Gates, H.S.J.; Barker, C.D. Pneumocystis carinii pneumonia in pregnancy. A case report. J. Reprod. Med. 1993, 38, 483–486. [Google Scholar] [PubMed]
- Albino, J.; Shapiro, J. Respiratory-Failure in Pregnancy due to Pneumocystis-Carinii—Report of a Successful Outcome. Obstet. Gynecol. 1994, 83, 823–824. [Google Scholar] [PubMed]
- Schwebke, K.; Henry, K.; Balfour, H.H.J.; Olson, D.; Crane, R.T.; Jordan, M.C. Congenital cytomegalovirus infection as a result of nonprimary cytomegalovirus disease in a mother with acquired immunodeficiency syndrome. J. Pediatr. 1995, 126, 293–295. [Google Scholar] [CrossRef]
- Mortier, E.; Pouchot, J.; Bossi, P.; Molinié, V. Maternal-fetal transmission of Pneumocystis carinii in human immunodeficiency virus infection. N. Engl. J. Med. 1995, 332, 825. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, H.; Mehta, N.J.; Manikal, V.M.; Lamoste, T.J.; Chapnick, E.K.; Lutwick, L.I.; Sepkowitz, D.V. Pneumocystis carinii pneumonia in pregnancy. Chest 2001, 120, 666–671. [Google Scholar] [CrossRef]
- Hu, J.; Yao, Y.; Wang, J.; Fu, X.; Fu, B. Non-small cell lung cancer with bone metastasis and pneumocystis pneumonia in a pregnant woman: A case report and literature review. BMC Infect. Dis. 2023, 23, 792. [Google Scholar] [CrossRef]
- Onishi, C.; Nishikori, M.; Yakushijin, K.; Kurahashi, S.; Nakazawa, H.; Takamatsu, Y.; Hashimoto, Y.; Tatetsu, H.; Yuichiro, N.; Yoshida, M.; et al. Lymphoma during pregnancy in Japan: A multicenter retrospective cohort study. Int. J. Hematol. 2022, 115, 382–390. [Google Scholar] [CrossRef]
- Fritzsche, C.; Löbermann, M.; Bolz, M. Interstitial Pneumonia During Pregnancy. Dtsch. Arztebl. Int. 2021, 118, 224. [Google Scholar] [CrossRef]
- Trier-Mørch, S.; Sørensen, N.B.H.; Rostgaard-Knudsen, M.; Larsen, H.; Sørensen, A.N. [Life-threatening Pneumocystis pneumonia in a previously healthy pregnant woman]. Ugeskr. Laeger 2017, 179, V10150806. [Google Scholar]
- Fukutani, Y.; Chigusa, Y.; Kondoh, E.; Kawasaki, K.; Io, S.; Matsumura, N. Pneumocystis Pneumonia in Non-HIV Pregnant Women Receiving Chemotherapy for Malignant Lymphoma: Two Case Reports. Case Rep. Obstet. Gynecol. 2017, 2017, 1–4. [Google Scholar] [CrossRef]
- Bazhenov, A.; Galstian, G.M.; Troitskaya, V.V.; Parovichnikova, E.N.; Makhinya, S.; Drokov, M.Y.; Latishkevich, O.; Zvereva, A.; Savchenko, V.G. Outcomes of pregnant women with acute leukemia treated for life-threatening complications. Blood 2016, 128, 5976. [Google Scholar] [CrossRef]
- Galstyan, G.M.; Novikov, V.A.; Troitskaya, V.V.; Baryakh, E.A.; Makhinya, S.A.; Parovichnikova, E.N.; Savchenko, V.G. Lung ultrasound for the diagnosis of pneumonia in pregnant women with blood system tumors. Ter. Arkhiv 2015, 87, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, Y.; Higa, F.; Tasato, D.; Nakamura, H.; Uechi, K.; Tamayose, M.; Haranaga, S.; Yara, S.; Tateyama, M.; Fujita, J. Pneumocystis jirovecii pneumonia and alveolar hemorrhage in a pregnant woman with human T cell lymphotropic virus type-1 infection. Intern. Med. 2011, 50, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Parisaei, M.; Hemelaar, J.; Govind, A. HIV in pregnancy: A case of Pneumocystis (carinii) jiroveci pneumonia. Arch. Gynecol. Obstet. 2010, 281, 1–3. [Google Scholar] [CrossRef]
- Bera, E. Maternal outcomes following introduction of antiretroviral therapy in the public sector: A prospective study at a tertiary hospital in the Eastern Cape. S. Afr. J. Obs. Gyn 2009, 15, 26–33. [Google Scholar]
- McNally, L.M.; Jeena, P.M.; Lalloo, U.; Nyamande, K.; Gajee, K.; Sturm, A.W.; Goldblatt, D.; Tomkins, A.M.; Coovadia, H.M. Probable mother to infant transmission of Pneumocystis jiroveci from an HIV-infected woman to her HIV-uninfected infant. AIDS 2005, 19, 1548–1549. [Google Scholar] [CrossRef]
- Gervasoni, C.; Vaccarezza, M.; Ridolfo, A.L.; Moroni, M.; Galli, M. Pneumocystis carinii pneumonia after the discontinuation of long-term antiretroviral therapy in an HIV-1-infected pregnant woman. AIDS 2003, 17, 940–941. [Google Scholar] [CrossRef]
- Kumar, R.M.; Uduman, S.A.; Khurrana, A.K. Impact of pregnancy on maternal AIDS. J. Reprod. Med. 1997, 42, 429–434. [Google Scholar]
- Deresiewicz, R.L.; Katz, J.; Zaroff, J.; Pieciak, W. Pneumocystis carinii pneumonia in a pregnant woman without a predisposing condition. Infect. Dis. Clin. Pract. 1996, 5, 449–453. [Google Scholar] [CrossRef]
- Stratton, P.; Mofenson, L.M.; Willoughby, A.D. Human immunodeficiency virus infection in pregnant women under care at AIDS clinical trials centers in the United States. Obstet. Gynecol. 1992, 79, 364–368. [Google Scholar] [CrossRef]
- Bongain, A.; Fuzibet, J.G.; Gillet, J.Y. Pneumocystis carinii infection. Two cases responsible for maternal death during pregnancy. Presse Med. 1992, 21, 950. [Google Scholar] [PubMed]
- Constantopoulos, P.; Gabaude, B.; Duforestel, T.; Fuzibet, J.G.; Mourey, C.; Lefebvre, J.C.; Cassuto, J.P.; Gillet, J.Y. Positive HIV (human immunodeficiency virus) serology in the pregnant woman: Current data on its management. Apropos of a continuous series of 56 cases. Rev. Fr. Gynecol. Obstet. 1987, 82, 453–462. [Google Scholar]
- Kurennaia, S.S.; Shlopov, V.G.; Mirovich, D.I.; Demina, T.N.; Berko, A.T. Pneumocystosis in a pregnant patient complicated by transplacental infection and intrauterine fetal death. Akush Ginekol 1985, 4, 72–73. [Google Scholar]
- Vargas, S.L. Fungal colonization by Pneumocystis is highly frequent in the human placenta at birth. J. Pediatric Infect. Dis. Soc. 2022, 11, S12. [Google Scholar] [CrossRef]
- Garcia, C.; Ochoa, T.; Neyra, E.; Bustamante, B.; Ponce, C.; Calderon, E.J.; Vargas, S.L. Pneumocystis jirovecii colonisation in pregnant women and newborns in Lima, Peru. Rev. Iberoam. Micol. 2020, 37, 24–27. [Google Scholar] [CrossRef]
- Vera, C.; Aguilar, Y.A.; Vélez, L.A.; Rueda, Z.V. High transient colonization by Pneumocystis jirovecii between mothers and newborn. Eur. J. Pediatr. 2017, 176, 1619–1627. [Google Scholar] [CrossRef]
- Samitova, E.R.; Ermak, T.N.; Koltunov, I.E.; Kislyakov, A.N.; Karazhas, N.V.; Rybalkina, T.N.; Kornienko, M.Y. Intrauterine Pneumocystis infection. Ter. Arkh 2016, 88, 99–102. [Google Scholar] [CrossRef]
- Vargas, S.L.; Ponce, C.A.; Sanchez, C.A.; Ulloa, A.V.; Bustamante, R.; Juarez, G. Pregnancy and asymptomatic carriage of Pneumocystis jiroveci. Emerg. Infect. Dis. 2003, 9, 605–606. [Google Scholar] [CrossRef]
- Morris, A.; Norris, K.A. Colonization by Pneumocystis jirovecii and its role in disease. Clin. Microbiol. Rev. 2012, 25, 297–317. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Michalski, C.; Sadarangani, M.; Lavoie, P.M. Maternal Immunological Adaptation During Normal Pregnancy. Front. Immunol. 2020, 11, 575197. [Google Scholar] [CrossRef]
- Pereira-Díaz, E.; Moreno-Verdejo, F.; de la Horra, C.; Guerrero, J.A.; Calderón, E.J.; Medrano, F.J. Changing Trends in the Epidemiology and Risk Factors of Pneumocystis Pneumonia in Spain. Front. Public. Health 2019, 7, 275. [Google Scholar] [CrossRef] [PubMed]
- Kolbrink, B.; Scheikholeslami-Sabzewari, J.; Borzikowsky, C.; von Samson-Himmelstjerna, F.A.; Ullmann, A.J.; Kunzendorf, U.; Schulte, K. Evolving epidemiology of pneumocystis pneumonia: Findings from a longitudinal population-based study and a retrospective multi-center study in Germany. Lancet Reg. Health Eur. 2022, 18, 100400. [Google Scholar] [CrossRef]
- Ahmadpour, E.; Valilou, S.; Ghanizadegan, M.A.; Seyfi, R.; Hosseini, S.A.; Hatam-Nahavandi, K.; Hosseini, H.; Behravan, M.; Barac, A.; Morovati, H. Global prevalence, mortality, and main characteristics of HIV-associated pneumocystosis: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0297619. [Google Scholar] [CrossRef]
- Calderón, E.J.; Gutiérrez-Rivero, S.; Durand-Joly, I.; Dei-Cas, E. Pneumocystis infection in humans: Diagnosis and treatment. Expert. Rev. Anti Infect. Ther. 2010, 8, 683–701. [Google Scholar] [CrossRef] [PubMed]
- Murji, A.; Crosier, R.; Rasuli, P. Non-obstetric diagnostic imaging in pregnancy. CMAJ 2015, 187, 1309. [Google Scholar] [CrossRef][Green Version]
- Bonnet, P.; Le Gal, S.; Calderon, E.; Delhaes, L.; Quinio, D.; Robert-Gangneux, F.; Ramel, S.; Nevez, G. Pneumocystis jirovecii in Patients with Cystic Fibrosis: A Review. Front. Cell Infect. Microbiol. 2020, 10, 571253. [Google Scholar] [CrossRef] [PubMed]
- Sheikholeslami, M.F.; Sadraei, J.; Farnia, P.; Forozandeh, M.; Emadi Kochak, H.; Tabarsi, P.; Nadji, S.A.; Pirestani, M.; Masjedi, M.R.; Velayati, A. Colonization of Pneumocystis jirovecii in Chronic Obstructive Pulmonary Disease (COPD) patients and the rate of Pneumocystis pneumonia in Iranian non-HIV (+) immunocompromised patients. Iran. J. Microbiol. 2013, 5, 411–417. [Google Scholar]
- Calderón, E.J.; Rivero, L.; Respaldiza, N.; Morilla, R.; Montes-Cano, M.A.; Friaza, V.; Muñoz-Lobato, F.; Varela, J.M.; Medrano, F.J.; Horra Cde, L. Systemic inflammation in patients with chronic obstructive pulmonary disease who are colonized with Pneumocystis jiroveci. Clin. Infect. Dis. 2007, 45, e17–e19. [Google Scholar] [CrossRef]
- Cere, N.; Drouet-Viard, F.; Dei-Cas, E.; Chanteloup, N.; Coudert, P. In utero transmission of Pneumocystis carinii sp. f. oryctolagi. Parasite 1997, 4, 325–330. [Google Scholar] [CrossRef]
- Sanchez, C.A.; Chabé, M.; Moukhtar Aliouat, E.; Durand-Joly, I.; Gantois, N.; Conseil, V.; López, C.; Duriez, T.; Dei-Cas, E.; Vargas, S.L. Exploring transplacental transmission of Pneumocystis oryctolagi in first-time pregnant and multiparous rabbit does. Med. Mycol. 2007, 45, 701–707. [Google Scholar] [CrossRef]
- Ito, M.; Tsugane, T.; Kobayashi, K.; Kuramochi, T.; Hioki, K.; Furuta, T.; Nomura, T. Study on placental transmission of Pneumocystis carinii in mice using immunodeficient SCID mice as a new animal model. J. Protozool. 1991, 38, 218S–219S. [Google Scholar] [PubMed]
- Hong, S.T.; Park, Y.K.; Kim, J.; Kim, D.H.; Yun, C.K. Is Pneumocystis carinii vertically transmitted to neonatal rats? Korean, J. Parasitol. 1999, 37, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Icenhour, C.R.; Rebholz, S.L.; Collins, M.S.; Cushion, M.T. Early acquisition of Pneumocystis carinii in neonatal rats using targeted PCR and oral swabs. J. Eukaryot. Microbiol. 2001, 135S–136S. [Google Scholar] [CrossRef] [PubMed]
- Oladele, R.O.; Otu, A.A.; Richardson, M.D.; Denning, D.W. Diagnosis and Management of Pneumocystis Pneumonia in Resource-poor Settings. J. Health Care Poor Underserved 2018, 29, 107–158. [Google Scholar] [CrossRef]
- Miller, R.F.; Huang, L.; Walzer, P.D. The Relationship between Pneumocystis Infection in Animal and Human Hosts, and Climatological and Environmental Air Pollution Factors: A Systematic Review. OBM Genet. 2018, 2. [Google Scholar] [CrossRef]
| Database | Search Strategy |
|---|---|
| PubMed | (“pneumonia, pneumocystis” [MeSH Terms] OR (“pneumonia” [All Fields] AND “pneumocystis” [All Fields]) OR “pneumocystis pneumonia” [All Fields] OR “pneumocystis” [All Fields] OR “pneumocystis” [MeSH Terms]) AND (“pregnancy” [MeSH Terms] OR “pregnancy” [All Fields] OR “vertical transmission” [All Fields]) |
| EMBASE | (‘pneumocystosis’/exp OR ‘pneumocystosis’) AND (‘pregnancy’/exp OR ‘pregnancy’) |
| Web of Science | Pneumocystis and Pregnancy (Articles + case reports) excluded Review articles, Awarded Grant and Other |
| Authors [Ref] | Year | Country | Cases | Immunosuppression | Offspring |
|---|---|---|---|---|---|
| Hu et al. [26] | 2023 | China | 1 | Non-Small-Cell Lung Cancer | NAD |
| Onishi et al. [27] | 2022 | Japan | 3 | Lymphoma | NAD |
| Fritzsche et al. [28] | 2021 | Germany | 1 | HIV | Premature |
| Trier-Morch et al. [29] | 2017 | Denmark | 1 | No apparent | NAD |
| Fukutani et al. [30] | 2017 | Japan | 2 | Hodgkin’s lymphoma B-cell lymphoma | Emergent cesarean section Newborn; uneventful |
| Bazhenov et al. [31] | 2016 | Netherlands | 4 | Acute leukemia | 1 miscarriage |
| Galstyan et al. [32] | 2015 | Russia | 1 | Leukemia | NAD |
| Tamaki et al. [33] | 2011 | Japan | 1 | HTLV-I | NAD |
| Parisaei et al. [34] | 2010 | UK | 1 | HIV | NAD |
| Bera E. [35] | 2009 | South Africa | 2 | HIV | NAD |
| McNally et al. [36] | 2005 | UK | 1 | HIV | Pneumocystis infection |
| Gervasoni et al. [37] | 2003 | Italy | 1 | HIV | NAD |
| Ahmad et al. [25] | 2001 | USA | 22 | HIV | NAD |
| Kumar et al. [38] | 1997 | India | 5 | HIV | Fetal Death |
| Deresiewicz et al. [39] | 1996 | USA | 1 | No | NAD |
| Stratton et al. [40] | 1992 | USA | 35 | HIV | NAD |
| Bongain et al. [41] | 1992 | France | 2 | NAD | NAD |
| Constantopoulos et al. [42] | 1987 | France | 9 | HIV | NAD |
| Kurennaia et al. [43] | 1985 | Russia | 1 | NAD | Fetal death |
| Authors [Ref] | Year | Country | Cases/Population | Immunosuppression | Mother Sample | Offspring |
|---|---|---|---|---|---|---|
| Szydłowicz et al. [11] | 2022 | Poland | 7/31 (22.5%) | Non-immunosuppressed | Oral washes | 8/56 |
| Vargas S. [44] | 2022 | Chile | 35/91 (38.4%) | NAD | Placenta | NAD |
| García et al. [45] | 2020 | Perú | 5/92 (5.4%) | Non-immunosuppressed | Oropharyngeal washes, nasal swabs | 0/5 |
| Vera et al. [46] | 2017 | Colombia | 20/43 (46.5%) | Non-immunosuppressed | Nasopharyngeal swabs | 12/20 |
| Samitova et al. [47] | 2016 | Russia | 1/1 | NAD | Placenta | 1 PcP |
| Montes-Cano et al. [9] | 2009 | Spain | 8/20 (40%) | Non-immunosuppressed | Placenta | 5/8 |
| Vargas et al. [48] | 2003 | Chile | 5/33 (15.1%) | Non-immunosuppressed | Nasal swab | NAD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón-Baturone, I.; Salsoso, R.; Charpentier, E.; de Armas, Y.; Guadix, P.; Morilla, R.; Calderón, E.J.; Friaza, V. Pneumocystis Infection in Pregnant Women: A Scoping Review. J. Fungi 2025, 11, 327. https://doi.org/10.3390/jof11040327
Calderón-Baturone I, Salsoso R, Charpentier E, de Armas Y, Guadix P, Morilla R, Calderón EJ, Friaza V. Pneumocystis Infection in Pregnant Women: A Scoping Review. Journal of Fungi. 2025; 11(4):327. https://doi.org/10.3390/jof11040327
Chicago/Turabian StyleCalderón-Baturone, Irene, Rocío Salsoso, Elena Charpentier, Yaxsier de Armas, Pilar Guadix, Rubén Morilla, Enrique J. Calderón, and Vicente Friaza. 2025. "Pneumocystis Infection in Pregnant Women: A Scoping Review" Journal of Fungi 11, no. 4: 327. https://doi.org/10.3390/jof11040327
APA StyleCalderón-Baturone, I., Salsoso, R., Charpentier, E., de Armas, Y., Guadix, P., Morilla, R., Calderón, E. J., & Friaza, V. (2025). Pneumocystis Infection in Pregnant Women: A Scoping Review. Journal of Fungi, 11(4), 327. https://doi.org/10.3390/jof11040327







