The Antifungal Effects of Equol Against Candida albicans Involve Mitochondrial Dysfunction
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Strains and Culture Conditions
2.3. Determination of Minimal Inhibitory Concentration (MIC)
2.4. Growth Inhibition Kinetics
2.5. Effects of Equol on Proliferation of Ana-1 Macrophages Cell
2.6. Measurement of Cell Membrane Permeability
2.7. Cell Wall Staining and Microscopic Examination
2.8. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
2.9. Measurement of ROS Concentrations
2.10. Measurement of Cytosolic Ca2+ Concentrations
2.11. Measurement of MMP
2.12. Transmission Electron Microscopy (TEM)
2.13. Statistical Analysis
3. Results
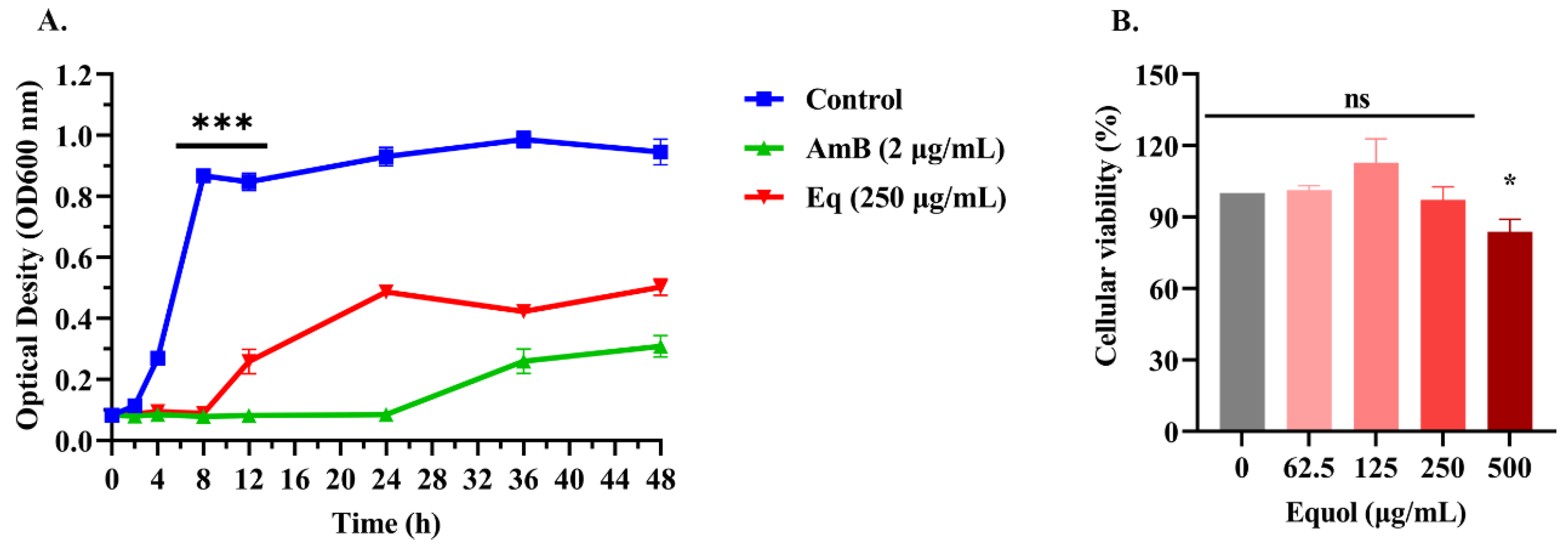
3.1. Equol Inhibited Growth of Fungal Cells
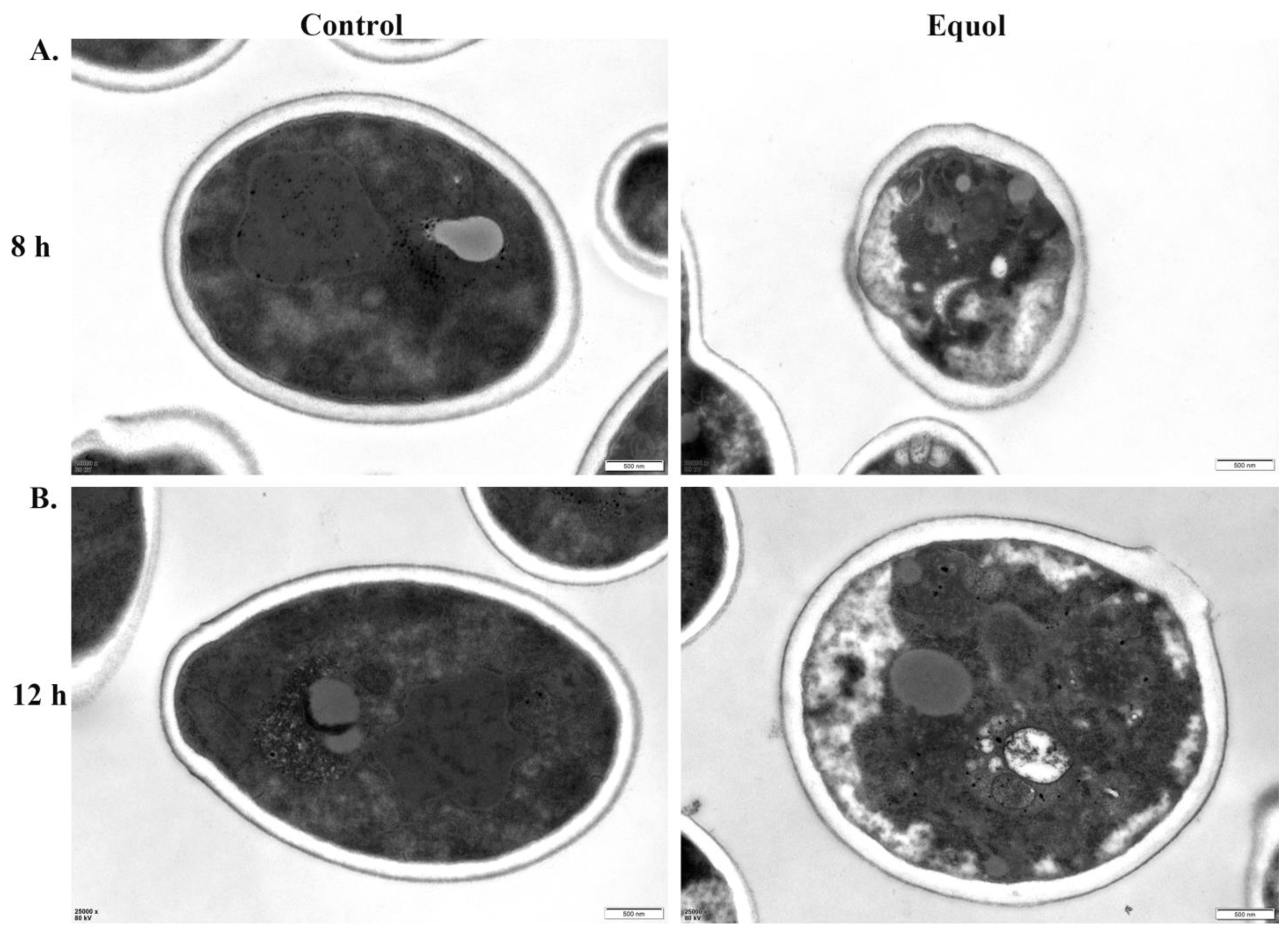
3.2. Equol Caused Ultrastructural Changes to Fungal Cells
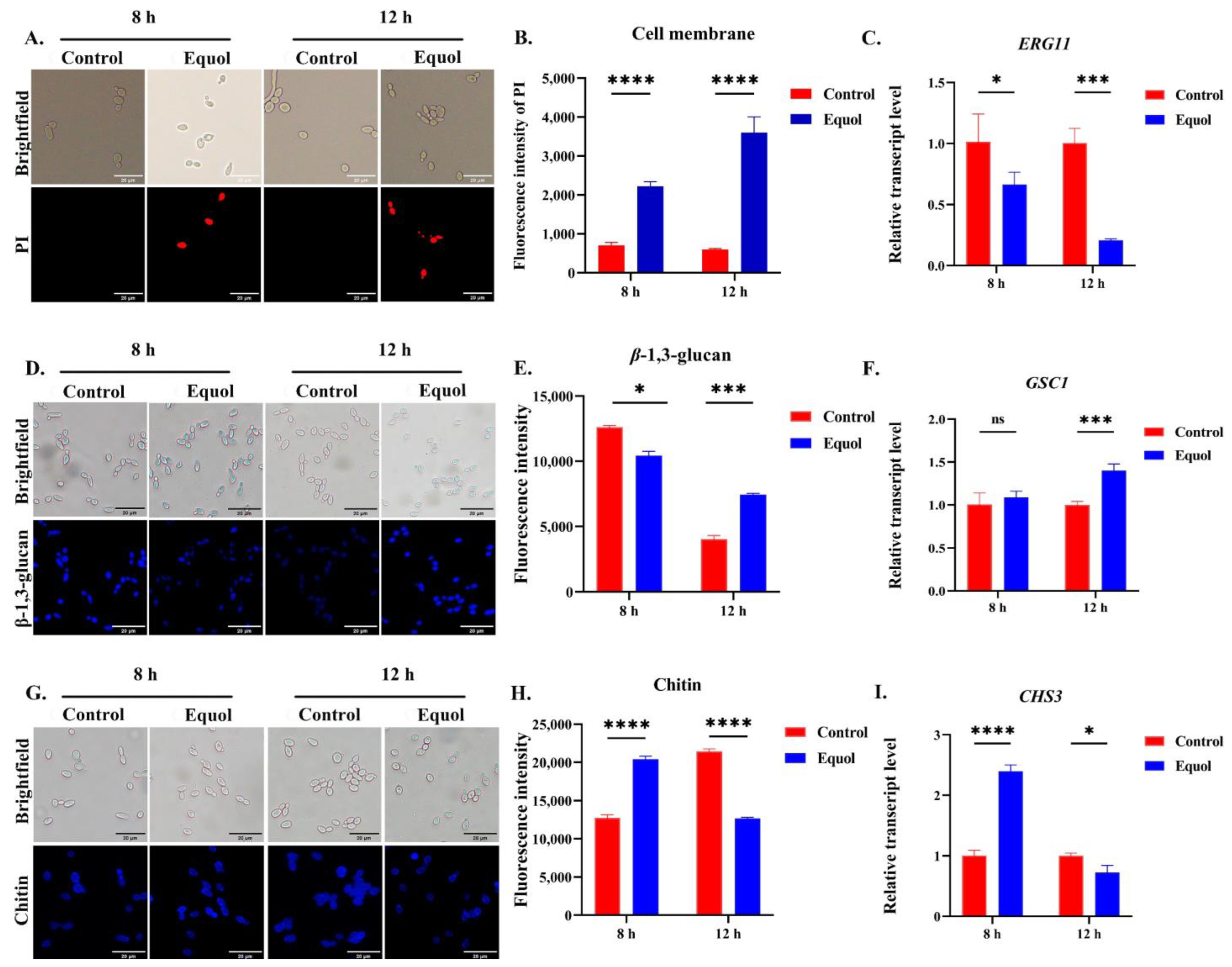
3.3. Equol Damaged the Fungal Cell Wall and Membrane
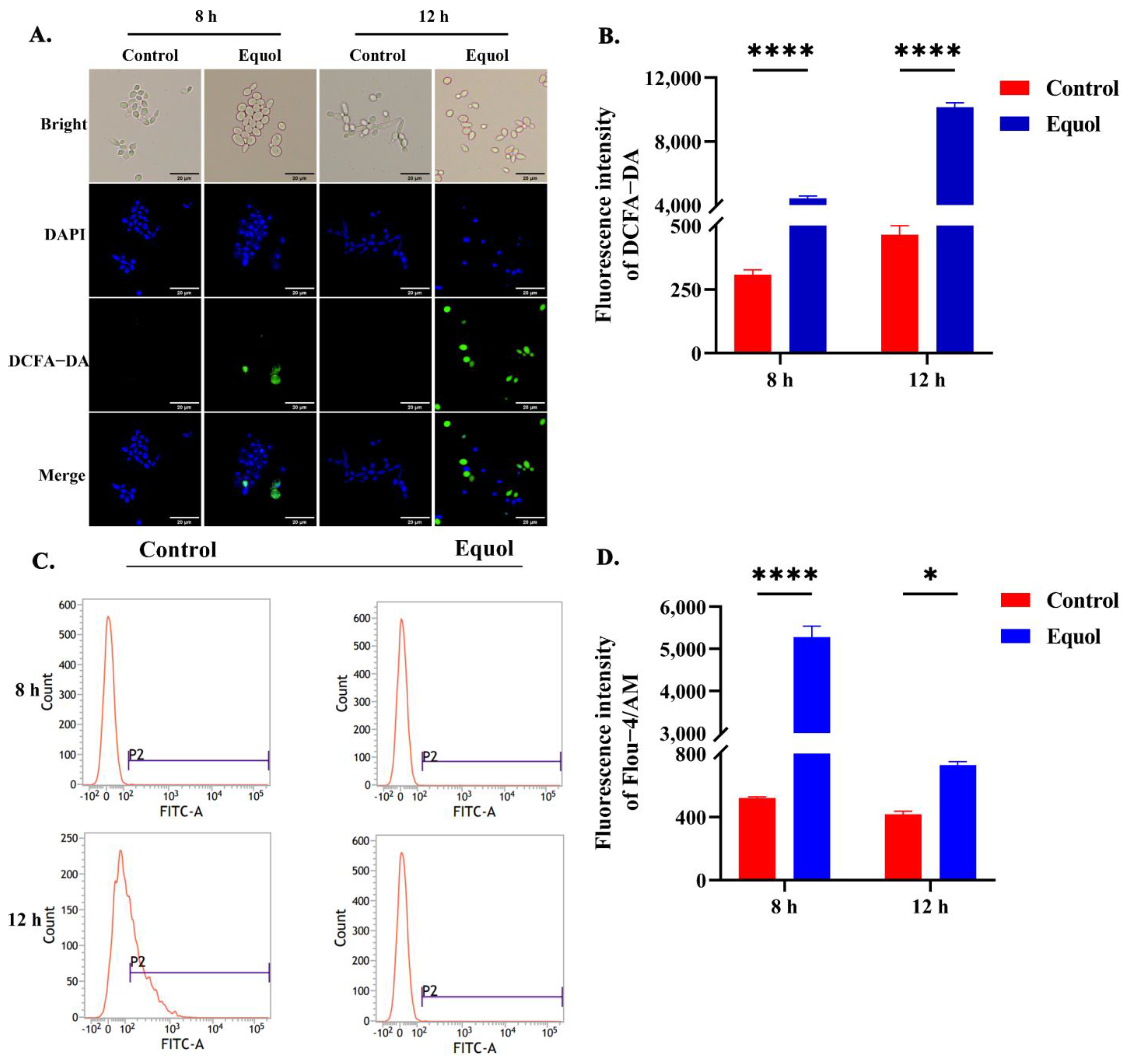
3.4. Equol Caused Intracellular ROS and Ca2+ Overload
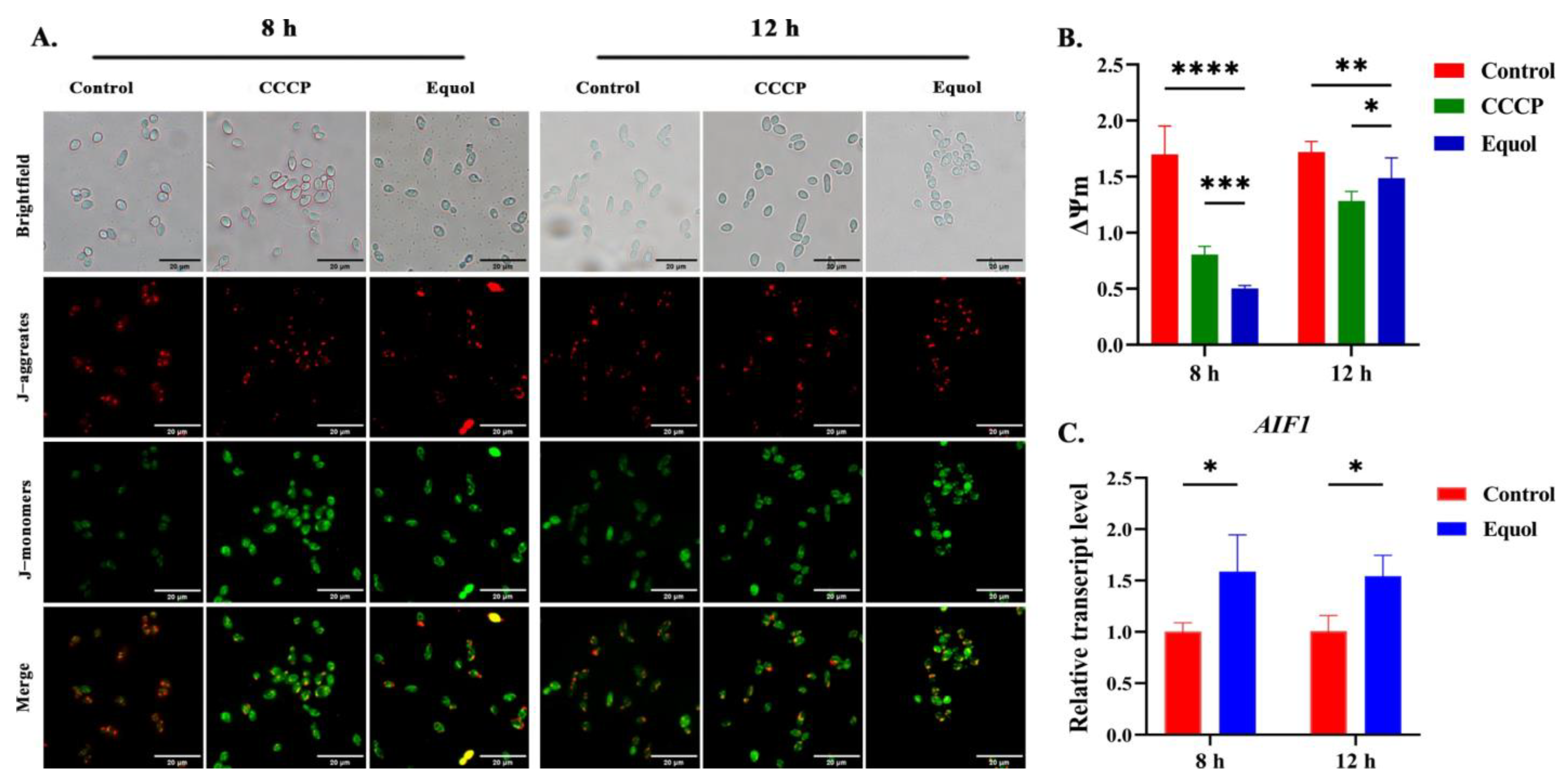
3.5. Equol Induced Mitochondrial Dysfunction
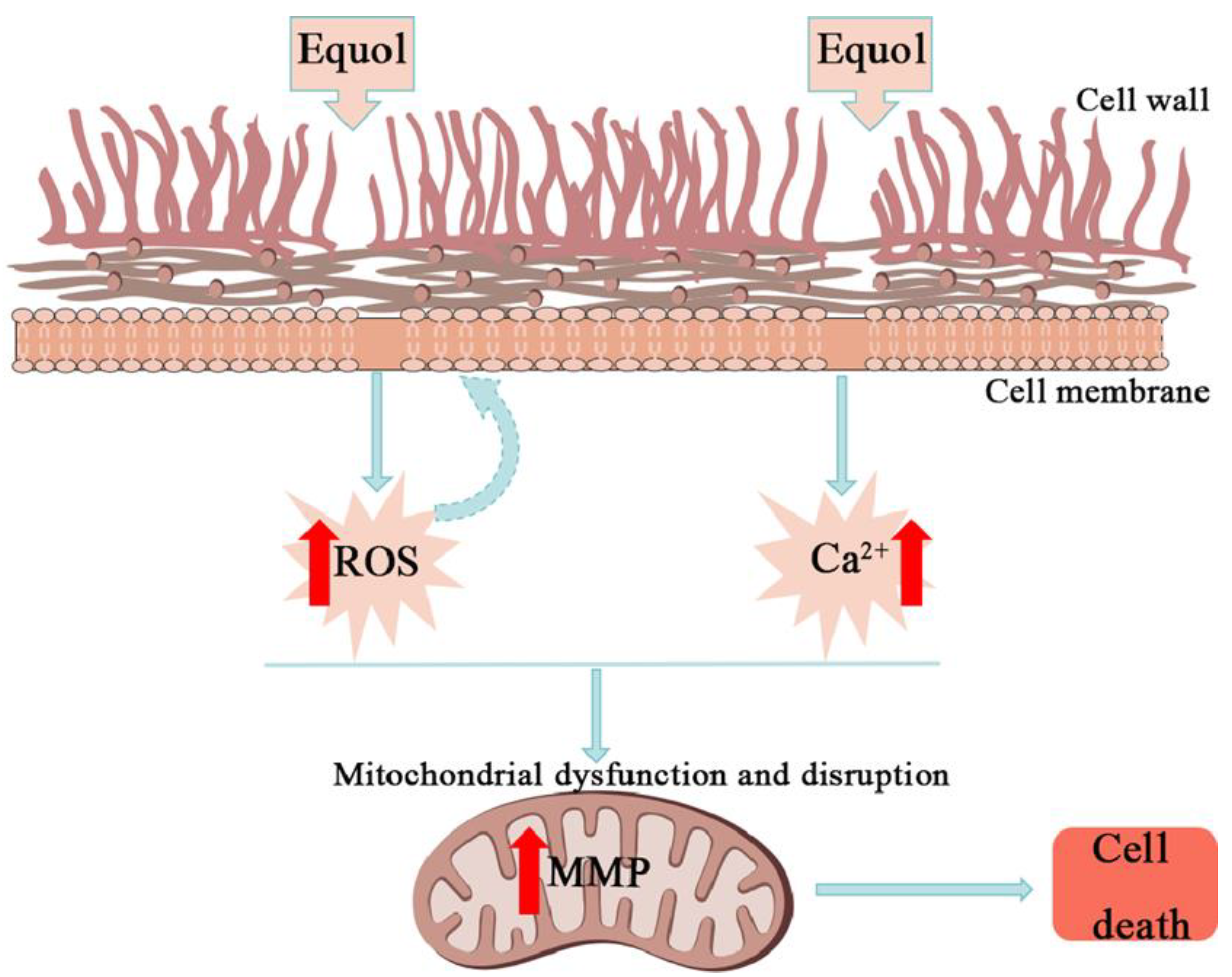
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katsipoulaki, M.; Stappers, M.H.T.; Malavia-Jones, D.; Brunke, S.; Hube, B.; Gow, N.A.R. Candida albicans and Candida glabrata: Global priority pathogens. Microbiol. Mol. Biol. Rev. 2024, 88, e0002123. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Fisher, M.C.; Denning, D.W. The WHO fungal priority pathogens list as a game-changer. Nat. Rev. Microbiol. 2023, 21, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.P.; Stylianou, M.; Nilsson, G.; Urban, C.F. Opportunistic pathogen Candida albicans elicits a temporal response in primary human mast cells. Sci. Rep. 2015, 5, 12287. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W.; Kneale, M.; Sobel, J.D.; Rautemaa-Richardson, R. Global burden of recurrent vulvovaginal candidiasis: A systematic review. Lancet Infect. Dis. 2018, 18, e339–e347. [Google Scholar] [CrossRef]
- Denning, D.W. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 2024, 24, e428–e438. [Google Scholar] [CrossRef]
- Bays, D.J.; Jenkins, E.N.; Lyman, M.; Chiller, T.; Strong, N.; Ostrosky-Zeichner, L.; Hoenigl, M.; Pappas, P.G.; Thompson, G.R., III. Epidemiology of Invasive Candidiasis. Clin. Epidemiol. 2024, 16, 549–566. [Google Scholar] [CrossRef]
- Ferngren, G.; Yu, D.; Unalan-Altintop, T.; Dinnétz, P.; Özenci, V. Epidemiological patterns of candidaemia: A comprehensive analysis over a decade. Mycoses 2024, 67, e13729. [Google Scholar] [CrossRef]
- Garvey, M.; Rowan, N.J. Pathogenic Drug Resistant Fungi: A Review of Mitigation Strategies. Int. J. Mol. Sci. 2023, 24, 1584. [Google Scholar] [CrossRef]
- Garcia-Rubio, R.; de Oliveira, H.C.; Rivera, J.; Trevijano-Contador, N. The Fungal Cell Wall: Candida, Cryptococcus, and Aspergillus Species. Front. Microbiol. 2019, 10, 2993. [Google Scholar] [CrossRef]
- Lima, S.L.; Colombo, A.L.; de Almeida Junior, J.N. Fungal Cell Wall: Emerging Antifungals and Drug Resistance. Front. Microbiol. 2019, 10, 2573. [Google Scholar] [CrossRef]
- Douglas, L.M.; Konopka, J.B. Plasma membrane organization promotes virulence of the human fungal pathogen Candida albicans. J. Microbiol. 2016, 54, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhang, B.; Li, J.; Zhang, B.; Wang, H.; Li, M. Endoplasmic reticulum-derived reactive oxygen species (ROS) is involved in toxicity of cell wall stress to Candida albicans. Free Radic. Biol. Med. 2016, 99, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Dias, L.P.; Santos, A.L.E.; Araújo, N.M.S.; Silva, R.R.S.; Santos, M.H.C.; Roma, R.R.; Rocha, B.A.M.; Oliveira, J.T.A.; Teixeira, C.S. Machaerium acutifolium lectin alters membrane structure and induces ROS production in Candida parapsilosis. Int. J. Biol. Macromol. 2020, 163, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, D.G. Naringin-generated ROS promotes mitochondria-mediated apoptosis in Candida albicans. IUBMB Life 2021, 73, 953–967. [Google Scholar] [CrossRef]
- Seyedjavadi, S.S.; Khani, S.; Eslamifar, A.; Ajdary, S.; Goudarzi, M.; Halabian, R.; Akbari, R.; Zare-Zardini, H.; Imani Fooladi, A.A.; Amani, J.; et al. The Antifungal Peptide MCh-AMP1 Derived From Matricaria chamomilla Inhibits Candida albicans Growth via Inducing ROS Generation and Altering Fungal Cell Membrane Permeability. Front. Microbiol. 2019, 10, 3150. [Google Scholar] [CrossRef]
- Sun, C.Q.; Peng, J.; Yang, L.B.; Jiao, Z.L.; Zhou, L.X.; Tao, R.Y.; Zhu, L.J.; Tian, Z.Q.; Huang, M.J.; Guo, G. A Cecropin-4 Derived Peptide C18 Inhibits Candida albicans by Disturbing Mitochondrial Function. Front. Microbiol. 2022, 13, 872322. [Google Scholar] [CrossRef]
- Carraro, M.; Bernardi, P. Calcium and reactive oxygen species in regulation of the mitochondrial permeability transition and of programmed cell death in yeast. Cell Calcium 2016, 60, 102–107. [Google Scholar] [CrossRef]
- Setchell, K.D.; Clerici, C. Equol: History, chemistry, and formation. J. Nutr. 2010, 140, 1355s–1362s. [Google Scholar] [CrossRef]
- Mayo, B.; Vázquez, L.; Flórez, A.B. Equol: A Bacterial Metabolite from The Daidzein Isoflavone and Its Presumed Beneficial Health Effects. Nutrients 2019, 11, 2231. [Google Scholar] [CrossRef]
- Fatima, A.; Khan, M.S.; Ahmad, M.W. Therapeutic Potential of Equol: A Comprehensive Review. Curr. Pharm. Des. 2020, 26, 5837–5843. [Google Scholar] [CrossRef]
- Kim, H.R.; Eom, Y.B. Synergistic Activity of Equol and Meropenem against Carbapenem-Resistant Escherichia coli. Antibiotics 2021, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kimura, S.; Ishii, Y.; Tateda, K. Equol inhibits growth and spore formation of Clostridioides difficile. J. Appl. Microbiol. 2019, 127, 932–940. [Google Scholar] [CrossRef]
- Kim, H.R.; Han, M.S.; Eom, Y.B. Anti-bacterial and Anti-biofilm Effects of Equol on Yersinia enterocolitica. Indian. J. Microbiol. 2022, 62, 401–410. [Google Scholar] [CrossRef]
- Wang, J.; Li, L.; Yin, Y.; Gu, Z.; Chai, R.; Wang, Y.; Sun, G. Equol, a Clinically Important Metabolite, Inhibits the Development and Pathogenicity of Magnaporthe oryzae, the Causal Agent of Rice Blast Disease. Molecules 2017, 22, 1799. [Google Scholar] [CrossRef]
- Lee, J.A.; Chee, H.Y. In Vitro Antifungal Activity of Equol against Candida albicans. Mycobiology 2010, 38, 328–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, J.; Zhang, Q.; Song, Z.; Xin, C. Antifungal activities of Equol against Candida albicans in vitro and in vivo. Virulence 2024, 15, 2404256. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Xiao, W.; Zhang, J.; Liu, F.; Xin, C.; Zhou, B.; Chen, W.; Song, Z. Antifungal activity of vitamin D(3) against Candida albicans in vitro and in vivo. Microbiol. Res. 2022, 265, 127200. [Google Scholar] [CrossRef]
- Huang, J.; Lei, J.; Ge, A.; Xiao, W.; Xin, C.; Song, Z.; Zhang, J. Antifungal Effect of Vitamin D(3) against Cryptococcus neoformans Coincides with Reduced Biofilm Formation, Compromised Cell Wall Integrity, and Increased Generation of Reactive Oxygen Species. J. Fungi 2023, 9, 772. [Google Scholar] [CrossRef]
- Lei, J.; Huang, J.; Xin, C.; Liu, F.; Zhang, J.; Xie, Y.; Mao, Y.; Chen, W.; Song, Z. Riboflavin Targets the Cellular Metabolic and Ribosomal Pathways of Candida albicans In Vitro and Exhibits Efficacy against Oropharyngeal Candidiasis. Microbiol. Spectr. 2023, 11, e0380122. [Google Scholar] [CrossRef]
- Stempinski, P.R.; Goughenour, K.D.; du Plooy, L.M.; Alspaugh, J.A.; Olszewski, M.A.; Kozubowski, L. The Cryptococcus neoformans Flc1 Homologue Controls Calcium Homeostasis and Confers Fungal Pathogenicity in the Infected Hosts. mBio 2022, 13, e0225322. [Google Scholar] [CrossRef]
- Zhou, R.; Chen, Y.; Li, S.; Wei, X.; Hu, W.; Tang, S.; Ding, J.; Fu, W.; Zhang, H.; Chen, F.; et al. TRPM7 channel inhibition attenuates rheumatoid arthritis articular chondrocyte ferroptosis by suppression of the PKCα-NOX4 axis. Redox Biol. 2022, 55, 102411. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Song, Y.; Chen, J.; Li, Q.; Gao, J.; Tan, H.; Zhu, Y.; Wang, Z.; Li, M.; Yang, H.; et al. Direct in vivo reprogramming with non-viral sequential targeting nanoparticles promotes cardiac regeneration. Biomaterials 2021, 276, 121028. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Liu, L.; Zhao, Y.; Yang, L.; Cheng, J.; Hua, R.; Zhang, Z.; Li, Q. Melatonin protects mouse testes from palmitic acid-induced lipotoxicity by attenuating oxidative stress and DNA damage in a SIRT1-dependent manner. J. Pineal Res. 2020, 69, e12690. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Lin, L.; Li, Q.; Liu, K.; Huang, Y.; Wang, X.; Cao, K.; Chen, X.; Cao, W.; Li, F.; et al. IGF-2 Preprograms Maturing Macrophages to Acquire Oxidative Phosphorylation-Dependent Anti-inflammatory Properties. Cell Metab. 2019, 29, 1363–1375.e1368. [Google Scholar] [CrossRef]
- Ma, H.; Yang, L.; Tian, Z.; Zhu, L.; Peng, J.; Fu, P.; Xiu, J.; Guo, G. Antimicrobial peptide AMP-17 exerts anti-Candida albicans effects through ROS-mediated apoptosis and necrosis. Int. Microbiol. 2023, 26, 81–90. [Google Scholar] [CrossRef]
- Derkacz, D.; Bernat, P.; Krasowska, A. K143R Amino Acid Substitution in 14-α-Demethylase (Erg11p) Changes Plasma Membrane and Cell Wall Structure of Candida albicans. Int. J. Mol. Sci. 2022, 23, 1631. [Google Scholar] [CrossRef]
- Tian, H.; Qu, S.; Wang, Y.; Lu, Z.; Zhang, M.; Gan, Y.; Zhang, P.; Tian, J. Calcium and oxidative stress mediate perillaldehyde-induced apoptosis in Candida albicans. Appl. Microbiol. Biotechnol. 2017, 101, 3335–3345. [Google Scholar] [CrossRef]
- Yun, D.G.; Lee, D.G. Silibinin triggers yeast apoptosis related to mitochondrial Ca2+ influx in Candida albicans. Int. J. Biochem. Cell Biol. 2016, 80, 1–9. [Google Scholar] [CrossRef]
- Lu, H.; Zhu, Z.; Dong, L.; Jia, X.; Sun, X.; Yan, L.; Chai, Y.; Jiang, Y.; Cao, Y. Lack of trehalose accelerates H2O2-induced Candida albicans apoptosis through regulating Ca2+ signaling pathway and caspase activity. PLoS ONE 2011, 6, e15808. [Google Scholar] [CrossRef]
- Ikuta, K.S.; Meštrović, T.; Naghavi, M. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 2024, 24, e268. [Google Scholar] [CrossRef]
- Thomas-Rüddel, D.O.; Schlattmann, P.; Pletz, M.; Kurzai, O.; Bloos, F. Risk Factors for Invasive Candida Infection in Critically Ill Patients: A Systematic Review and Meta-analysis. Chest 2022, 161, 345–355. [Google Scholar] [CrossRef]
- Gold, J.A.W.; Adjei, S.; Gundlapalli, A.V.; Huang, Y.A.; Chiller, T.; Benedict, K.; Toda, M. Increased Hospitalizations Involving Fungal Infections During COVID-19 Pandemic, United States, January 2020–December 2021. Emerg. Infect. Dis. 2023, 29, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Chowdhary, A.; Gold, J.A.W. The rapid emergence of antifungal-resistant human-pathogenic fungi. Nat. Rev. Microbiol. 2023, 21, 818–832. [Google Scholar] [CrossRef] [PubMed]
- Arita, G.S.; Faria, D.R.; Capoci, I.R.G.; Kioshima, E.S.; Bonfim-Mendonça, P.S.; Svidzinski, T.I.E. Cell wall associated proteins involved in filamentation with impact on the virulence of Candida albicans. Microbiol. Res. 2022, 258, 126996. [Google Scholar] [CrossRef] [PubMed]
- Razmi, M.; Kim, J.; Chinnici, J.; Busarajan, S.; Vuppalapaty, H.; Lankipalli, D.; Li, R.; Maddi, A. Candida albicans Mannosidases, Dfg5 and Dcw1, Are Required for Cell Wall Integrity and Pathogenesis. J. Fungi 2024, 10, 525. [Google Scholar] [CrossRef]
- Szymański, M.; Chmielewska, S.; Czyżewska, U.; Malinowska, M.; Tylicki, A. Echinocandins—Structure, mechanism of action and use in antifungal therapy. J. Enzyme Inhib. Med. Chem. 2022, 37, 876–894. [Google Scholar] [CrossRef]
- Ramzan, A.; Padder, S.A.; Masoodi, K.Z.; Shafi, S.; Tahir, I.; Rehman, R.U.; Prasad, R.; Shah, A.H. β-Nitrostyrene derivatives as broad range potential antifungal agents targeting fungal cell wall. Eur. J. Med. Chem. 2022, 240, 114609. [Google Scholar] [CrossRef]
- Parks, L.W.; Casey, W.M. Physiological implications of sterol biosynthesis in yeast. Annu. Rev. Microbiol. 1995, 49, 95–116. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Chandra, J. Candida biofilm resistance. Drug Resist. Updat. 2004, 7, 301–309. [Google Scholar] [CrossRef]
- Shahi, G.; Kumar, M.; Kumari, S.; Rudramurthy, S.M.; Chakrabarti, A.; Gaur, N.A.; Singh, A.; Prasad, R. A detailed lipidomic study of human pathogenic fungi Candida auris. FEMS Yeast Res. 2020, 20, foaa045. [Google Scholar] [CrossRef]
- Li, Y.; Shan, M.; Zhu, Y.; Yao, H.; Li, H.; Gu, B.; Zhu, Z. Kalopanaxsaponin A induces reactive oxygen species mediated mitochondrial dysfunction and cell membrane destruction in Candida albicans. PLoS ONE 2020, 15, e0243066. [Google Scholar] [CrossRef] [PubMed]
- Guirao-Abad, J.P.; Sánchez-Fresneda, R.; Alburquerque, B.; Hernández, J.A.; Argüelles, J.C. ROS formation is a differential contributory factor to the fungicidal action of Amphotericin B and Micafungin in Candida albicans. Int. J. Med. Microbiol. 2017, 307, 241–248. [Google Scholar] [CrossRef]
- Liao, M.; Xia, X.; Meng, Q.; Zhu, C.; Liao, B.; Wang, J.; Gou, L.; Zhou, X.; Yuan, W.; Cheng, L.; et al. Holotoxin A(1) from Apostichopus japonicus inhibited oropharyngeal and intra-abdominal candidiasis by inducing oxidative damage in Candida albicans. Br. J. Pharmacol. 2024, 181, 1857–1873. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Chang, C.K.; Lan, C.Y. Antimicrobial peptide LL-37 disrupts plasma membrane and calcium homeostasis in Candida albicans via the Rim101 pathway. Microbiol. Spectr. 2023, 11, e0255123. [Google Scholar] [CrossRef]
- Pinton, P.; Giorgi, C.; Siviero, R.; Zecchini, E.; Rizzuto, R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 2008, 27, 6407–6418. [Google Scholar] [CrossRef] [PubMed]
- King, M.M.; Kayastha, B.B.; Franklin, M.J.; Patrauchan, M.A. Calcium Regulation of Bacterial Virulence. Adv. Exp. Med. Biol. 2020, 1131, 827–855. [Google Scholar]
- Kong, F.; You, H.; Zheng, K.; Tang, R.; Zheng, C. The crosstalk between pattern-recognition receptor signaling and calcium signaling. Int. J. Biol. Macromol. 2021, 192, 745–756. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, X.; Ou, M.; Fu, X.; Lin, Q.; Tao, X.; Wang, Z.; Liu, A.; Li, G.; Xu, Y.; et al. Berbamine promotes macrophage autophagy to clear Mycobacterium tuberculosis by regulating the ROS/Ca2+ axis. mBio 2023, 14, e0027223. [Google Scholar] [CrossRef]
- Niu, C.; Wang, C.; Yang, Y.; Chen, R.; Zhang, J.; Chen, H.; Zhuge, Y.; Li, J.; Cheng, J.; Xu, K.; et al. Carvacrol Induces Candida albicans Apoptosis Associated with Ca2+/Calcineurin Pathway. Front. Cell Infect. Microbiol. 2020, 10, 192. [Google Scholar] [CrossRef]
- Han, G.; Lee, D.G. Indole propionic acid induced Ca2+-dependent apoptosis in Candida albicans. IUBMB Life 2022, 74, 235–244. [Google Scholar] [CrossRef]
- Zou, K.; Yin, K.; Ren, S.; Zhang, R.; Zhang, L.; Zhao, Y.; Li, R. Activity and mechanism of action of antimicrobial peptide ACPs against Candida albicans. Life Sci. 2024, 350, 122767. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Büttner, S.; Kroemer, G.; Madeo, F. The mitochondrial pathway in yeast apoptosis. Apoptosis 2007, 12, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Schofield, J.H.; Schafer, Z.T. Mitochondrial Reactive Oxygen Species and Mitophagy: A Complex and Nuanced Relationship. Antioxid. Redox Signal 2021, 34, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Zhang, Y.; Wang, Y.; Wan, Y.; Miao, Y.; Ma, T.; Yu, Q.; Li, M. Role of Aif1 in regulation of cell death under environmental stress in Candida albicans. Yeast 2016, 33, 493–506. [Google Scholar] [CrossRef]
- Reinhardt, C.; Arena, G.; Nedara, K.; Edwards, R.; Brenner, C.; Tokatlidis, K.; Modjtahedi, N. AIF meets the CHCHD4/Mia40-dependent mitochondrial import pathway. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165746. [Google Scholar] [CrossRef]
- Costa, I.; Lopes, I.; Morais, M.; Silva, R.; Remião, F.; Medeiros, R.; Alves, L.G.; Pinto, E.; Cerqueira, F. Disclosing the Antifungal Mechanisms of the Cyclam Salt H4[H2(4-CF3PhCH2)2Cyclam]Cl4 against Candida albicans and Candida krusei. Int. J. Mol. Sci. 2024, 25, 5209. [Google Scholar] [CrossRef]
| Strains | MIC90 (µg/mL) | |
|---|---|---|
| Equol | AmB | |
| C. albicans ATCC MYA-2876 | 250 | 1 |
| C. parapsilosis ATCC 22019 | 250 | 0.5 |
| C. neoformans ATCC 208821 | 250 | 0.5 |
| C. auris swcau | 125 | 0.5 |
| C. auris swcau1 | 125 | 0.5 |
| C. duobushaemulonii swcd | 500 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, A.; Zhou, H.; Yang, X.; Zhao, C.; Xin, C.; Song, Z. The Antifungal Effects of Equol Against Candida albicans Involve Mitochondrial Dysfunction. J. Fungi 2025, 11, 339. https://doi.org/10.3390/jof11050339
Ge A, Zhou H, Yang X, Zhao C, Xin C, Song Z. The Antifungal Effects of Equol Against Candida albicans Involve Mitochondrial Dysfunction. Journal of Fungi. 2025; 11(5):339. https://doi.org/10.3390/jof11050339
Chicago/Turabian StyleGe, Anni, Hao Zhou, Xi Yang, Chunling Zhao, Caiyan Xin, and Zhangyong Song. 2025. "The Antifungal Effects of Equol Against Candida albicans Involve Mitochondrial Dysfunction" Journal of Fungi 11, no. 5: 339. https://doi.org/10.3390/jof11050339
APA StyleGe, A., Zhou, H., Yang, X., Zhao, C., Xin, C., & Song, Z. (2025). The Antifungal Effects of Equol Against Candida albicans Involve Mitochondrial Dysfunction. Journal of Fungi, 11(5), 339. https://doi.org/10.3390/jof11050339







