Molecular Characterisation of Fusarium Species Causing Common Bean Root Rot in Uganda
Abstract
1. Introduction
2. Materials and Methods
2.1. Origin of Fusarium Species Strains Used
2.2. DNA Extraction from Fusarium Species Strains
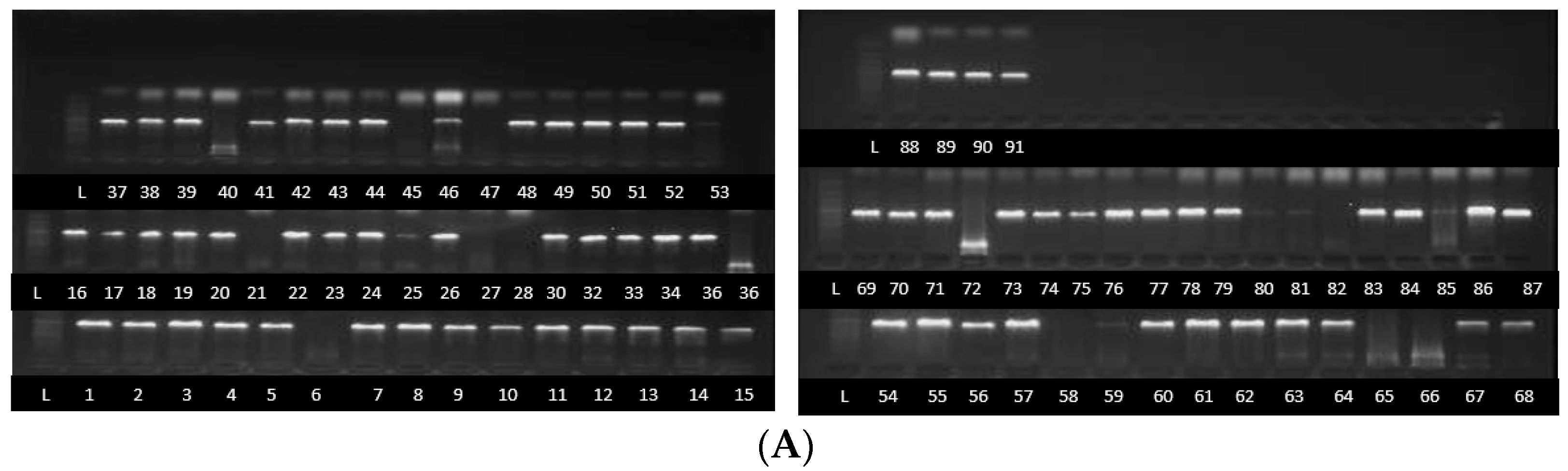
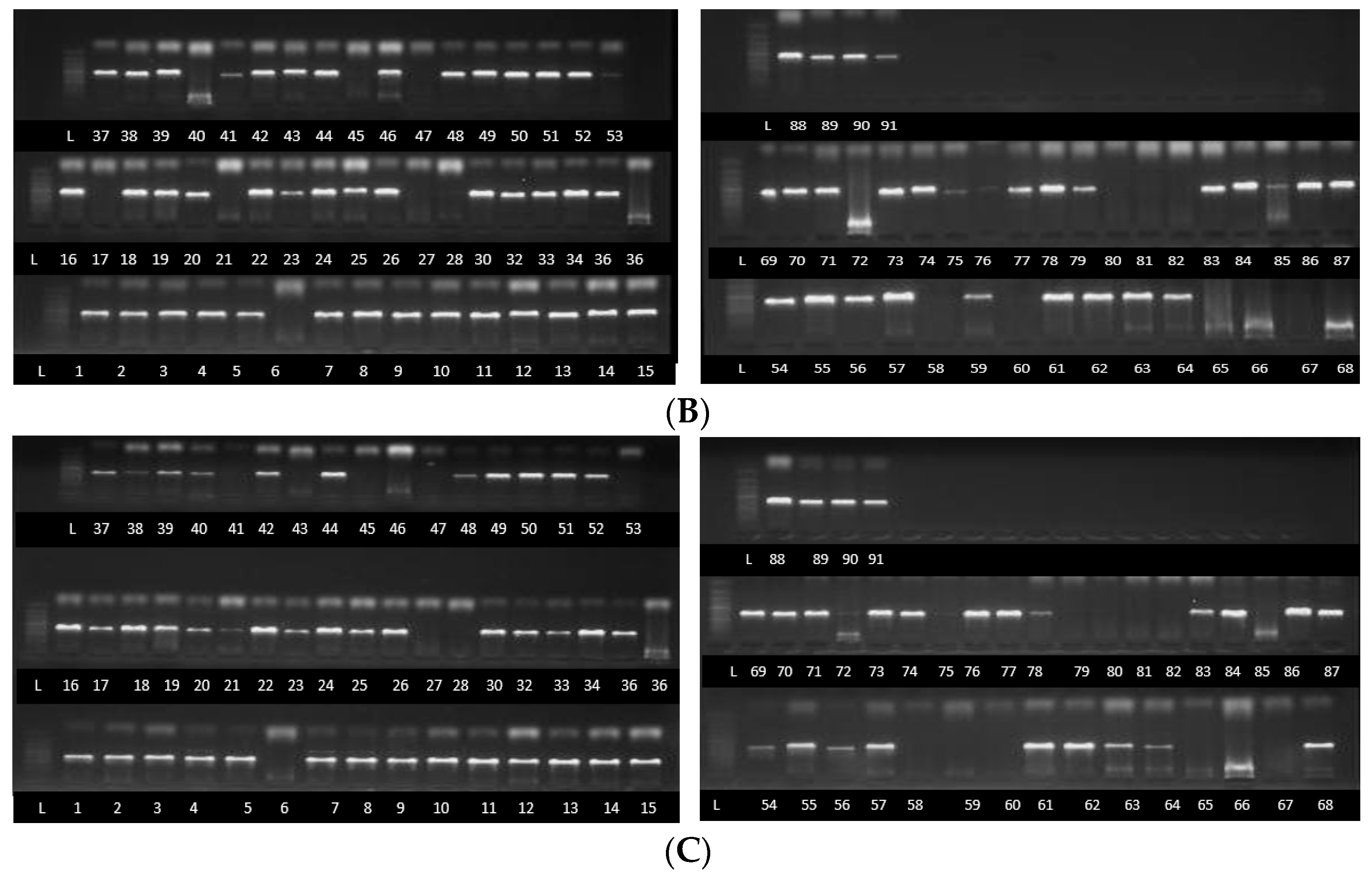
2.3. Fusarium Species Identification Using TEF1-α, β Tubulin and ITS Partial Sequences
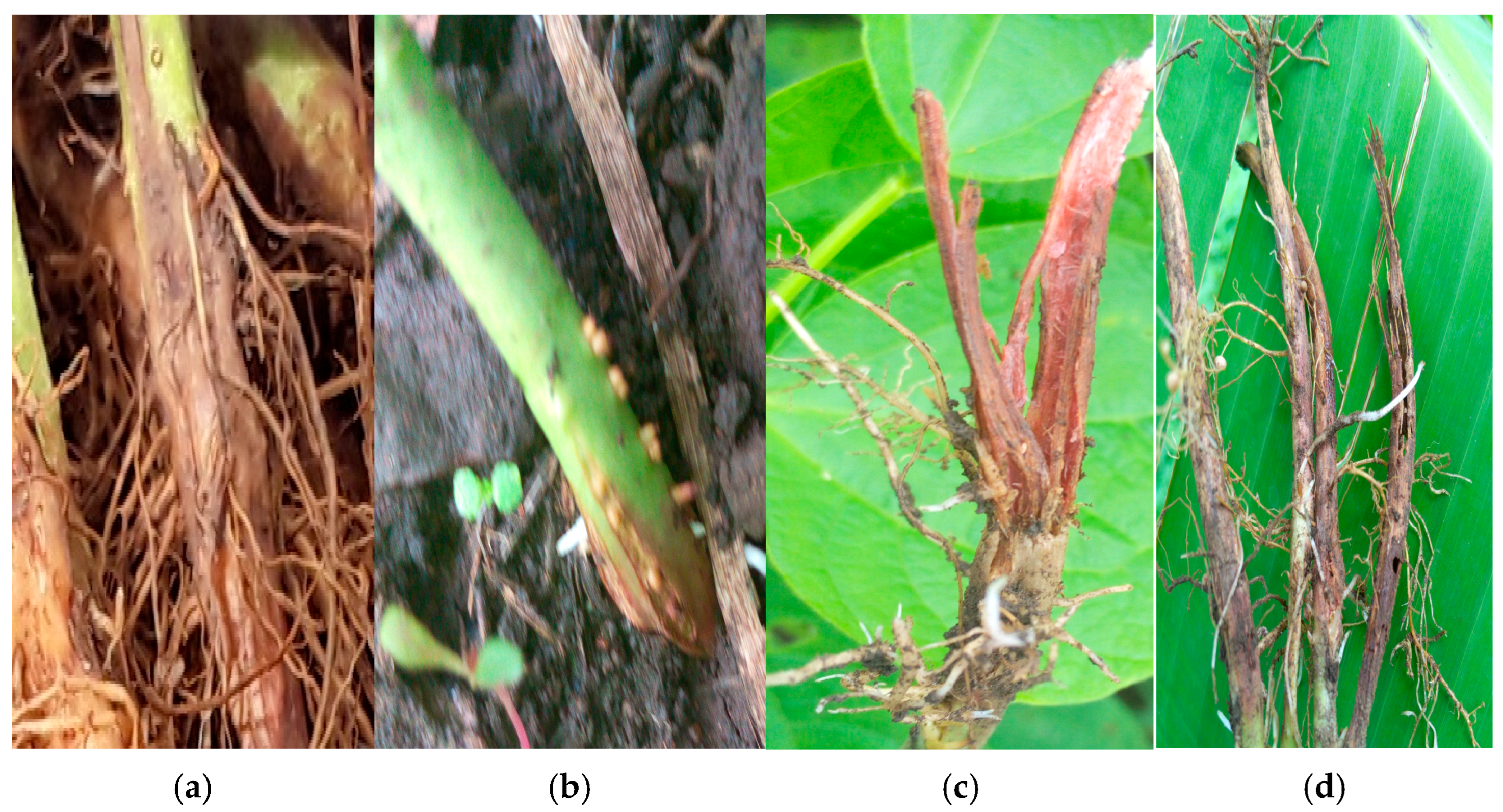
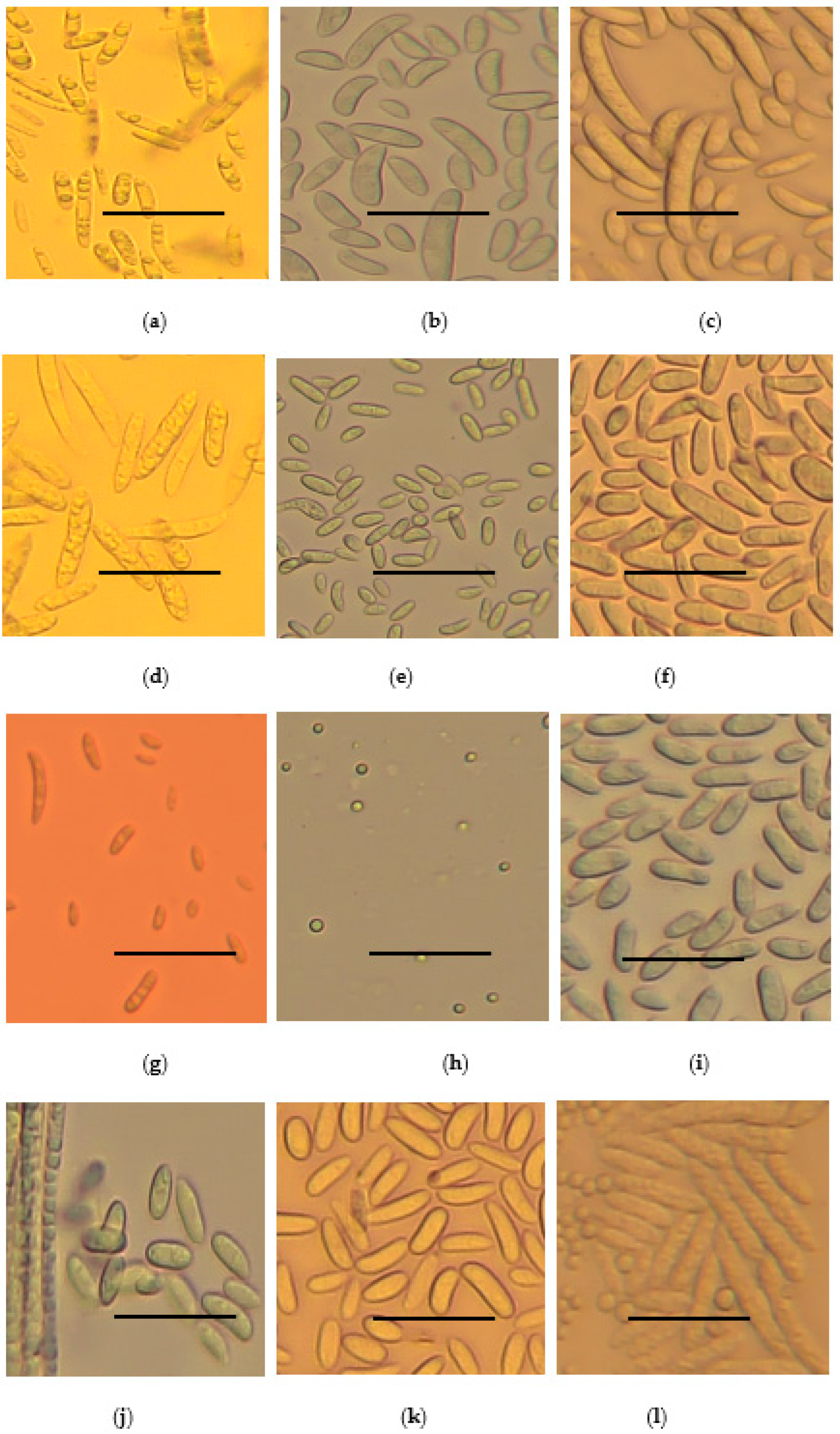
2.4. Growth Rate, Disease Severity Index (DSI) and Morphological Characteristics of Fusarium Species Strains
2.5. Data Analysis
3. Results
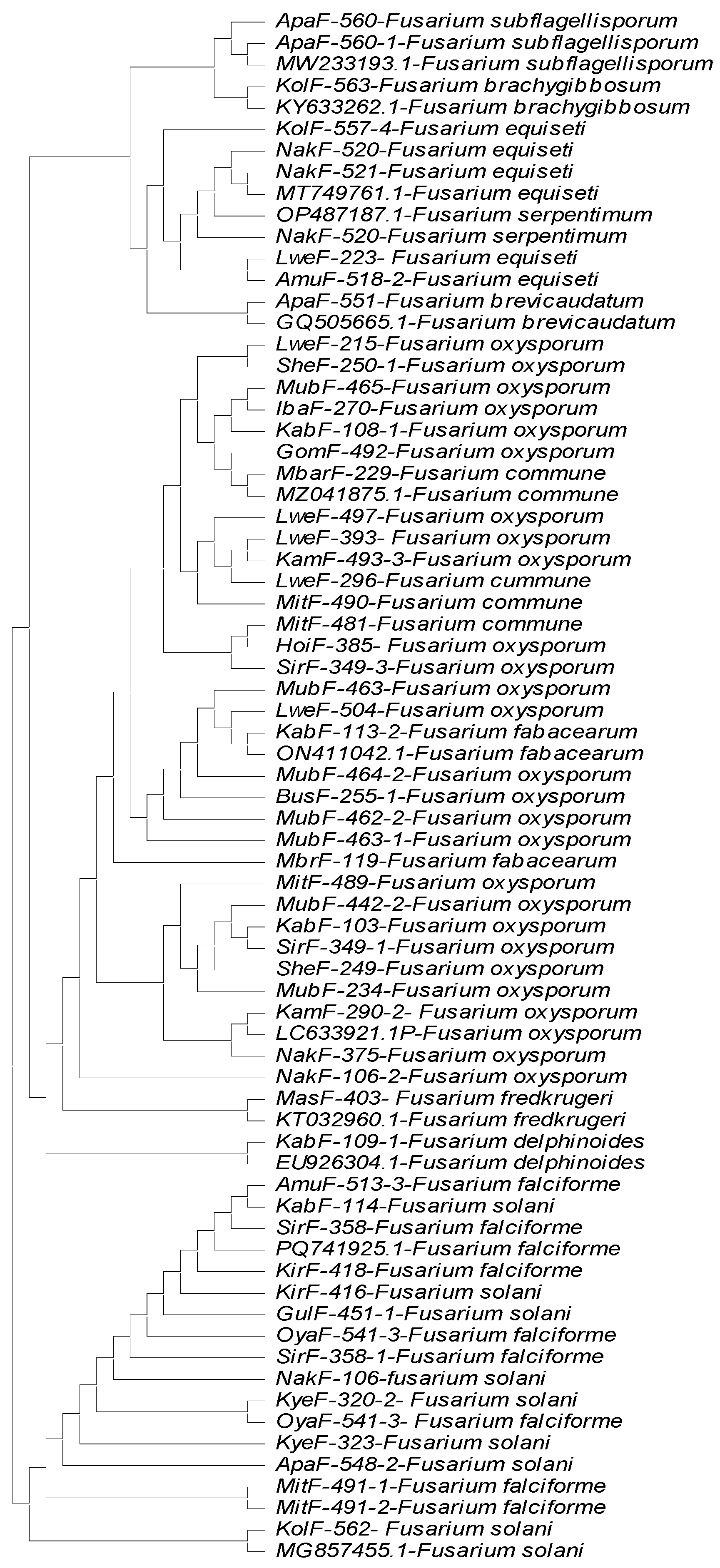
Identification of Fusarium Strains Using TEF1-α Gene, β Tubulin Gene and ITS Partial Sequences
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melotto, M.; Monteiro-Vitorello, C.B.; Bruschi, A.G.; Camargo, L.E. Comparative bioinformatic analysis of genes expressed in common bean (Phaseolus vulgaris L.) seedlings. Genome 2005, 48, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Food and Agricultural Organisation of United Nations (FAO). World Food and Agricultural Statistical Pocket Book; FAO: Rome, Italy, 2018; p. 28. [Google Scholar]
- CASA. Bean Sector Strategy-Uganda; CASA Uganda country team: Kampala, Uganda, 2020; p. 4, Unpublished. [Google Scholar]
- Miklas, P.N.; Kelly, J.D.; Beebe, S.E.; Blair, M.W. Common bean breeding for resistance against biotic and abiotic stress: From classical to MAS breeding. Euphytica 2006, 147, 105–131. [Google Scholar] [CrossRef]
- Li, Y.P.; You, M.P.; Barbetti, M.J. Species of Pythium associated with seedling root and hypocotyl disease on common bean (Phaseolus vulgaris) in Western Australia. Plant Dis. 2014, 98, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lu, X.H.; Li, S.D.; Wu, B.M. First report of common bean (Phaseolus vulgaris) root rot caused by Plectosphaerella cucumerina in China. Plant Dis. 2018, 102, 1849. [Google Scholar] [CrossRef]
- Nzungize, J.; Geps, P.; Buruchara, R.; Buah, S.; Ragama, P.; Busogoro, J.P.; Baudoin, J.P. Pathogenic and molecular characterization of Pythium species inducing root rot symptoms of common bean in Rwanda. Afr. J. Microbiol. Res. 2011, 5, 1169–1181. [Google Scholar]
- Hall, R. Fusarium root rot. In Compendium of Bean Diseases; American Phytopathological Society Press: St. Paul, MN, USA, 1991; pp. 9–10. [Google Scholar]
- Paparu, P.; Acur, A.; Kato, F.; Acam, C.; Nakibuule, J.; Musoke, S.; Mukankusi, C. Prevalence and incidence of four common bean root rots in Uganda. J. Exp. Agric. 2018, 54, 888–900. [Google Scholar] [CrossRef]
- Deng, D.; Wu, W.; Duan, C.; Sun, S.; Zhu, Z. A novel pathogen Fusarium cuneirostrum causing common bean (Phaseolus vulgaris) root rot in China. J. Integr. Agric. 2024, 23, 166–176. [Google Scholar]
- Sang, H.; Jacods, J.L.; Wang, J.; Mukankusi, C.; Chilvers, M.I. First report of Fusarium cuneirostrum causing root rot of common bean (Phaseola vulgaris L.) in Uganda. Plant Dis. 2018, 102, 2639. [Google Scholar] [CrossRef]
- O’donell, K. Molecular phylogeny of Nectria haemococcus–Fusarium solani species complex. Mycologia 2000, 92, 919–938. [Google Scholar] [CrossRef]
- Adesemoye, A.O.; Orrell, T.; Kodati, S. Effect of virulence of root rot pathogens and cultivar resistance on disease occurrence in dry beans. Plant Health Prog. 2018, 19, 237–241. [Google Scholar]
- Bilgi, V.N.; Bradley, C.A.; Mathew, F.M.; Ali, S.; Rasmussen, J.B. Root rot of dry edible bean caused by Fusarium graminearum. Plant Health Prog. 2011, 12, 14. [Google Scholar]
- Husaini, A.M.; Sakina, A.; Ca mbay, S.R. Host–pathogen interaction in Fusarium oxysporum infections: Where do we stand? Mol. Plant-Microbe Interact. 2018, 31, 889–898. [Google Scholar] [PubMed]
- Abawi, G.S. Root rots. In Bean Problems in the Tropics. Cali, Colombia: CIAT (Centro Internacional de Agricultura Tropical); CIAT: Cali, Colombia, 1989; pp. 105–157. [Google Scholar]
- Abawi, G.S.; Pastor-Corrale, M.A. Root Rots of Beans in Latin America and Africa:Diagnosis, Research Methodologies, and Management Strategies; CIAT: Cali, Colombia, 1990; p. 114. Available online: https://hdl.handle.net/10568/54258 (accessed on 17 November 2024).
- Erima, S.; Moses, N.; Allan, N.; Cnadiru, A.; Nakibuule, J.; Edema, R.; Paparu, P. Morphological and pathogenic characterisation of Fusarium species causing common bean root rot in Uganda. J. Sci. Agric. 2024, 8, 7–14. [Google Scholar] [CrossRef]
- Zietkiewicz, E.; Rafalski, A.; Labuda, D. Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics 1994, 20, 176–183. [Google Scholar] [CrossRef]
- Puri, K.D.; Saucedo, E.S.; Zhong, S. Molecular characterisation of Fusarium head blight pathogens sampled from a naturally infected disease nursery used for wheat breeding programs in China. Plant Dis. 2012, 96, 1280–1285. [Google Scholar] [CrossRef]
- Fourie, G.; Steenkamp, E.T.; Ploetz, R.C.; Gordon, T.; Viljoen, A. Current status of the taxonomic position of Fusarium oxysporum formae specialis cubense within the Fusarium oxysporum complex. Infect. Genet. Evol. 2011, 11, 533–554. [Google Scholar] [CrossRef]
- Kalman, B.; Abraham, D.; Graph, S.; Perl-Treves, R.; Meller Harel, Y.; Degani, O. Isolation and identification of Fusarium spp., the causal agents of onion (Allium cepa) basal rot in Northeastern Israel. Biology 2020, 9, 69. [Google Scholar] [CrossRef]
- Nuangmek, W.; Kumla, J.; Khuna, S.; Lumyong, S.; Suwannarach, N. Identification and characterisation of Fusarium species causing watermelon fruit rot in northern Thailand. Plants 2023, 12, 956. [Google Scholar] [CrossRef]
- Kristensen, R.; Torp, M.; Kosiak, B.; Holst-Jensen, A. Phylogeny and toxigenic potential is correlated in Fusarium species as revealed by partial translation elongation factor 1 alpha gene sequences. Mycol. Res. 2005, 109, 173–186. [Google Scholar] [CrossRef]
- Mirhendi, H.; Makimura, K.; de Hoog, G.S.; Rezaei-Matehkolaei, A.; Najafzadeh, M.J.; Umeda, Y.; Ahmadi, B. Translation elongation factor 1-α gene as a potential taxonomic and identification marker in dermatophytes. Med. Mycol. J. 2015, 53, 215–224. [Google Scholar] [CrossRef]
- Olal, S.; Olango, N.; Kiggundu, A.; Ochwo, S.; Adriko, J.; Nanteza, A.; Matovu, E.; Lubega, G.W.; Kagezi, G.; Hakiza, G.J.; et al. Using translation elongation factor gene to specifically detect and diagnose Fusarium xylaroides, a causative agent of coffee wilt disease in Ethiopia, East and Central Africa. J. Plant Pathol. Microbiol. 2018, 9, 440. [Google Scholar] [CrossRef]
- Nitschke, E.; Nihlgard, M.; Varrelmann, M. Differentiation of eleven Fusarium spp. straind from sugar beet, using restriction fragment analysis of a polymerase chain reaction–amplified translation elongation factor 1α gene fragment. Phytopathology 2009, 99, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Otieno, P.; Imbahake, S.S.; Okoth, S.; Otipa, M.; Wafula, W.V. Prevalence and phylogenetic diversity of pathogenic Fusarium species in genotypes of wheat seeds in three rift valley regions, Kenya. Adv. Agric. 2021, 2021, 8839147. [Google Scholar] [CrossRef]
- Wokorach, G.; Sofie, L.; Kris, A.; Echodu, R.; Haeseart, G. Genetic characterisation of fungal biodiversity in storage grains: Towards enhancing food safety in Northern Uganda. Microorganisms 2021, 9, 383. [Google Scholar] [CrossRef]
- Nurrahmi, D.F. Internal Transcribed Spacer (ITS) as DNA barcoding to identify fungal species: A review. Squalen Bull. Mar. Fish. Postharvest Biotechnol. 2017, 11, 37–44. [Google Scholar] [CrossRef]
- Singha, I.M.; Kakoty, Y.; Unni, B.G.; Das, J.; Kalita, M.C. Identification and characterisation of Fusarium sp. using ITS and RAPD causing fusarium wilt of tomato straind from Assam, North East India. J. Genet. Eng. Biotechnol. 2016, 14, 99–105. [Google Scholar] [CrossRef]
- Gilmore, S.R.; Graefenhan, T.; Louise-Sieze, G.E.; Seifert, K.A. Multiple copies of cytochrome oxidase 1 in species of the fungal genus Fusarium. Mol. Ecol. Resour. 2009, 9, 90–98. [Google Scholar] [CrossRef]
- Tusiime, G. Variation and Detection of Fusarium solani f. sp. phaseoli and Quantification of Soil Inoculum in Common Bean Fields. Ph.D. Thesis, Makerere University, Kampala, Uganda, 2004; p. 30. [Google Scholar]
- Available online: https://joint-research-centre.ec.europa.eu/tools-and-laboratories/standardisation_en (accessed on 16 June 2024).
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Fungal Barcoding Consortium. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- Raja, H.A.; Miller, A.N.; Pearce, C.J.; Oberlies, N.H. Fungal identification using molecular tools: A primer for the natural products research community. J. Nat. Prod. 2017, 80, 756–770. [Google Scholar] [CrossRef]
- Shen, D.-X.; Song, Z.-W.; Lu, Y.-M.; Fan, B. First report of Fusarium falciforme causing root rot in Weigelia florida in China. Plant Dis. 2020, 104, 610–623. [Google Scholar] [CrossRef]
- Liu, T.; Shen, Y.; Liu, Z.; Han, D.; Cui, J.; Zuo, Y. Control effect of three kinds of seed-coating formulations on the root rot of kidney beans. Plant Prot 2017, 43, 216–219. [Google Scholar]
- Wang, C.; Sun, C.; Liu, S. Preliminary study on pathogen of root rot of kidney bean and its control in Xinjiang. Ganhanqu Yanjiu (Arid. Zone Res.) 2010, 27, 380–384. [Google Scholar]
- Khan, M.F.R.; Liu, Y.; Bhuiyan, M.Z.R.; Lakshman, D.; Liu, Z.; Zhong, S. First report of Fusarium equiseti causing seedling death on sugar beets in Minesota, USA. Plant Dis. 2021, 105, 2017. [Google Scholar] [CrossRef]
- Trabelsi, R.; Sellami, H.; Gharbi, Y.; Krid, S.; Cheffi, M.; Kammoun, S.; Triki, M.A. Morphological and molecular characterisation of Fusarium spp. associated with olive trees dieback in Tunisia. 3 Biotech 2017, 7, 28. [Google Scholar] [CrossRef]
- Namasaka, R.W. 2017 Inheritence of Resistance to Fusarium Root Rot (Fusarium redolense) Disease of Cow Peases in Uganda. Master’s Thesis, Makerere University, Kampala, Uganda, 2017. [Google Scholar] [CrossRef]
- Marcelo, S.D.; Wilnad, J.; Swart, W.G. New Fusarium species from Kruger national park, South Africa. Mycokeys 2018, 34, 63–92. [Google Scholar] [CrossRef]
- Guruprasad, K.; Sajjan, S.; Kareguadas, T.B. Pathogenicity of Indole-3- acetic acid producing fungus Fusarium delphinoides strain GPK towards chicken peas and pigeon peas. Eur. J. Plant Pathol. 2011, 131, 355–369. [Google Scholar] [CrossRef]
- Chehri, K.; Mohamed, N.; Salleh, B.; Latiffah, Z. Occurrence and pathogenicity of Fusarium spp. on potato tubers in Malaysia. Afr. J. Agric. Res. 2011, 6, 3706–3712. [Google Scholar]
- Coleman, J.J. The Fusarium Solani species comples: Ubiguitous pathogens of Agricultural Importance. Mol. Plant Pathol. 2015, 17, 146–158. [Google Scholar] [CrossRef]
- Mukankusi, C.M. Improving resistance to Fusarium root rot [Fusarium solani (Mart.) Sacc. f. sp. phaseoli (Burkholder) W.C. Snyder & H.N. Hans.] in Common bean (Phaseolus vulgaris L.). Ph.D. Thesis, University of KwaZulu-Natal, Durban, South Africa, 2008; p. 200. [Google Scholar]
- Xia, J.W.; Sandoval-Denis, M.; Crous, P.W.; Zhang, X.G.; Lombard, L. Numbers to names- Restyling the Fusarium incarnatum-equiseti species complex. Phylogeny Evol. Fungi 2019, 43, 186–221. [Google Scholar] [CrossRef]
- Dugan, F.M.; Lupien, S.L.; Chen, W. Clonostachys rhizophaga and other fungi from chickpea debris in the Palouse region of the Pacific Northwest, USA. N. Am. Fungi 2012, 7, 1–11. [Google Scholar] [CrossRef]
- Cota-Barreras, C.I.; García-Estrada, R.S.; León-Félix, J.; Valenzuela-Herrera, V.; Mora-Romero, G.A.; Leyva-Madrigal, K.Y.; Tovar-Pedraza, J.M. First report of Clonostachys chloroleuca causing chickpea wilt in Mexico. New Dis. Rep. 2022, 46, 121–123. [Google Scholar] [CrossRef]
- Gibert, S.; Edel-Hermann, V.; Gautheron, E.; Gautheron, N.; Bernaud, E.; Sol, J.M.; Capelle, G.; Galland, R.; Bardon-Debats, A.; Lambert, C.; et al. Identification, pathogenicity and community dynamics of fungi and oomycetes associated with pea root rot in northern France. Plant Pathol. 2022, 71, 1550–1569. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, A.F.; Gu, C.Y.; Zang, H.Y.; Chen, Y. First report of Clonostachys rhizophaga as a pathogen of water chestnut (Eleocharis dulcis) in Anhui Province of China. Plant Dis. 2018, 103, 151. [Google Scholar] [CrossRef]
- Funck, J.D.; Dubey, M.; Jensen, B.; Karlsson, M. Clonostachys Rosea to Control Plant Diseases. 2022. Available online: https://library.oapen.org/handle/20.500.12657/61518 (accessed on 14 November 2024). [CrossRef]
- Siddique, S.S.; Bhuiyan, M.K.; Momotaz, R.; Bari, G.M.; Rahman, M.H. Cultural characteristics, virulence and invitro chemical control of Fusarium oxysporum f. sp. Phaseoli of bush beans (Phaseola vulgaris). Agriculturista 2014, 12, 103–110. [Google Scholar] [CrossRef]
- Burlakoti, P.; Rivera, V.; Secor, G.A.; Qi, A.; Del Rio-Mendoza, L.E.; Khan, M.F.R. Comparative pathogenicity and virulence of Fusarium species on sugar beet. Plant Dis. 2012, 96, 1291–1296. [Google Scholar] [CrossRef]
| S/No | Strains | Agroecology | Species | Accession Numbers | ||
|---|---|---|---|---|---|---|
| TEF1-α | Β Tubulin | ITS | ||||
| 1 | MbrF-119 | WMFS | F. fabacearum | PQ497180 | PQ497177 | PQ363745 |
| 2 | NakF-106-2 | NEDL | F. oxysporum | PQ497191 | PQ497142 | PQ363764 |
| 3 | KabF-103 | SWH | F. oxysporum | PQ497198 | PQ497143 | PQ363757 |
| 4 | SheF-250-1 | WMFS | F. oxysporum | PQ497207 | PQ497144 | PQ363766 |
| 5 | GomF-492 | LVC | F. oxysporum | PQ497213 | PQ497145 | PQ363773 |
| 6 | KabF-108-1 | SWH | F. oxysporum | PQ497226 | PQ497146 | PQ363790 |
| 7 | KamF-290-2 | WMFS | F. oxysporum | PQ497234 | PQ497147 | PQ363797 |
| 8 | LweF-507 | LVC | F. oxysporum | - | PQ497148 | PQ363804 |
| 9 | MubF-442-2 | LVC | F. oxysporum | PQ497182 | PQ497148 | - |
| 10 | MitF-489 | LVC | F. oxysporum | PQ497181 | PQ497150 | PQ363746 |
| 11 | AmuF-513-3 | NEDL | F. falciforme | PQ497183 | PQ497151 | - |
| 12 | MubF-463 | LVC | F. oxysporum | PQ497184 | PQ497152 | PQ363747 |
| 13 | KabF-114 | SWH | F. solani | PQ497185 | PQ497153 | - |
| 14 | GulF-451-1 | NMFS | F. solani | PQ497186 | PQ497154 | - |
| 15 | SheF-249 | WMFS | F. oxysporum | PQ497187 | PQ497155 | PQ363748 |
| 16 | KolF-563 | NMFS | F. brachygibbosum | PQ497188 | PQ497138 | PQ363749 |
| 17 | LweF-504 | LVC | F. oxysporum | PQ497189 | PQ497176 | PQ363750 |
| 18 | MubF-463-1 | LVC | F. oxysporum | PQ497190 | PQ497156 | PQ363751 |
| 19 | KabF-109-1 | SWH | F. delphinoides | PQ497192 | - | PQ363752 |
| 20 | OyaF-541-3 | NMFS | F. falciforme | PQ497193 | - | - |
| 21 | MubF-234 | LVC | F. oxysporum | PQ497194 | PQ497175 | PQ363753 |
| 22 | KolF-557-4 | NMFS | F. equiseti | PQ497195 | PQ497174 | PQ363756 |
| 23 | LweF-497 | LVC | F. oxysporum | PQ497196 | PQ497173 | - |
| 24 | ApaF-548 | NMFS | F. solani | - | PQ497172 | PQ363755 |
| 25 | MitF-491-1 | LVC | F. falciforme | PQ497197 | PQ497171 | PQ363791 |
| 26 | NakF-521 | NEDL | F. equiseti | PQ497205 | PQ497164 | PQ363765 |
| 27 | MubF-462-2 | LVC | F. oxysporum | PQ497199 | PQ497170 | PQ363758 |
| 28 | ApaF-560 | NMFS | F. subflagellisporum | PQ497200 | PQ497169 | PQ363759 |
| 29 | NakF-520 | NEDL | F. equiseti | PQ497201 | PQ497168 | PQ363760 |
| 30 | MubF-465 | LVC | F. oxysporum | PQ497202 | PQ497167 | PQ363761 |
| 31 | ApaF-551 | NEDL | F. equiseti | PQ497203 | PQ497166 | PQ363762 |
| 32 | LirF-602-2 | NEDL | F. equiseti | - | - | PQ363763 |
| 33 | NakF-106 | NEDL | F. solani | PQ497204 | PQ497165 | PQ363764 |
| 34 | KyeF-323 | WMFS | F. solani | PQ497206 | PQ497163 | - |
| 35 | LweF-223 | LVC | F. equiseti | PQ497208 | - | - |
| 36 | KapF-372 | EH | F. oxysporum | - | PQ497162 | PQ363767 |
| 37 | SirF-349-1 | LVC | F. oxysporum | PQ497209 | PQ497161 | - |
| 38 | KamF-289 | WMFS | F. solani | - | - | PQ363768 |
| 39 | IbaF-270 | WMFS | F. oxysporum | PQ497210 | PQ497160 | PQ363769 |
| 40 | ApaF-546 | NMFS | F. equiseti | - | PQ497159 | PQ363770 |
| 41 | LweF-393 | LVC | F. oxysporum | PQ497211 | PQ497158 | PQ363771 |
| 42 | SirF-358 | LVC | F. falciforme | PQ497212 | PQ497157 | PQ363772 |
| 43 | KabF-113-2 | SWH | F. fabacearum | PQ497214 | PQ497141 | PQ363774 |
| 44 | BusF-258 | WMFS | F. oxysporum | - | PQ497140 | PQ363775 |
| 45 | MitF-490 | LVC | F. commune | PQ497215 | PQ497139 | PQ363776 |
| 46 | AmuF-518-2 | NEDL | F. equiseti | PQ497216 | PQ497137 | PQ363777 |
| 47 | KamF-493-3 | WMFS | F. oxysporum | PQ497217 | PQ497136 | PQ363778 |
| 48 | KirF-416 | WMFS | F. solani | PQ497218 | PQ497135 | PQ363779 |
| 49 | SirF-358-1 | LVC | F. falciforme | PQ497219 | PQ497134 | PQ363780 |
| 50 | NakF-102-2 | NEDL | F. solani | - | - | PQ363781 |
| 51 | KabF-91-1 | SWH | F. oxysporum | - | - | PQ363782 |
| 52 | BusF-255-1 | WMFS | F. oxysporum | PQ497220 | PQ497120 | PQ363783 |
| 53 | MubF-464-2 | LVC | F. oxysporum | PQ497221 | PQ497133 | PQ363784 |
| 54 | LweF-296 | LVC | F. commune | PQ497222 | PQ497119 | PQ363785 |
| 55 | NakF-520-1 | NEDL | F. serpentimum | PQ497223 | - | PQ363786 |
| 56 | MbarF-229 | WMFS | F. commune | PQ497224 | - | PQ363787 |
| 57 | ApaF-560-1 | NMFS | F. subflagellisporum | PQ497200 | PQ497121 | - |
| 58 | NakF-105-1 | NEDL | F. oxysporum | PQ497227 | - | - |
| 59 | MitF-487-2 | LVC | C. rhizophaga | PQ363792 | - | PQ363792 |
| 60 | MitF-481 | LVC | F. commune | PQ497225 | PQ497132 | PQ363789 |
| 61 | KyeF-320-2 | WMFS | F. solani | PQ497227 | PQ497131 | - |
| 62 | ApaF-548-2 | NMFS | F. solani | PQ497228 | - | - |
| 63 | SheF-250 | WMFS | F. oxysporum | PQ497207 | - | - |
| 64 | MasF-403 | WMFS | F. fredkrugeri | PQ497229 | - | - |
| 65 | MitF-491-2 | LVC | F. falciforme | PQ497230 | PQ497171 | PQ363788 |
| 66 | KirF-418 | WMFS | F. falciforme | PQ497231 | PQ497128 | PQ363793 |
| 67 | HoiF-385 | WMFS | F. oxysporum | PQ497232 | PQ497127 | PQ363794 |
| 68 | SirF-349-3 | LVC | F. oxysporum | PQ497233 | PQ497126 | PQ363795 |
| 69 | MitF-487 | LVC | F. oxysporum | - | PQ497129 | PQ363788 |
| 70 | MubF-466 | LVC | F. oxysporum | - | - | PQ363798 |
| 71 | ApaF-546 | NMFS | F. equiseti | - | PQ497159 | - |
| 72 | KolF-562 | NMFS | F. solani | PQ497236 | PQ497124 | PQ363799 |
| 73 | KamF-290 | WMFS | F. oxysporum | - | - | PQ363800 |
| 74 | LweF-496 | LVC | F. oxysporum | - | - | PQ363801 |
| 75 | KolF-562-1 | NMFS | F. solani | - | PQ497125 | - |
| 76 | NakF-375 | NEDL | F. oxysporum | PQ497237 | PQ497123 | PQ363802 |
| 77 | Apaf-551-1 | NMFS | F. brevicaudatum | PQ497233 | - | - |
| 78 | LweF-215 | LVC | F. oxysporum | PQ497179 | PQ497122 | PQ363805 |
| 79 | ApaF-560 | NMFS | F. oxysporum | PQ497178 | PQ497121 | - |
| 80 | HoiF-385-1 | WMFS | F. solani | PQ497219 | - | PQ363803 |
| S/No | Organism Name | No. of Strains | * DSI (%) | * Growth Rate (cm/Day) | Microscopic Structues at ×40 Magnification |
|---|---|---|---|---|---|
| 1 | F. delphinoides | 1 | 46.8 ± 6.9 | 0.96 ± 0.01 | Rod-shaped nonseptate macro-conidia about 5 to 50 µm lond and spherical micro-conidia |
| 2 | F. solani | 13 | 36.3 ± 5.9 | 0.79 ± 0.03 | Sickle shaped nonseptate macro-conidia about 5 to 50 µm long. |
| 3 | F. oxysporum | 37 | 44.4 ± 4.9 | 0.79 ± 0.03 | Rod-shaped septate micro-conidia about 20 to 50 µm long |
| 4 | F. equiseti | 9 | 47.3 ± 5.9 | 0.7 ± 0.03 | Rod-shaped nonseptate macro-conidia about 50 to 100 µm long. Spherical micro-conidia 2 to 10 µm long |
| 5 | C. rhizophaga | 1 | 31.3 ± 5.6 | 0.37 ± 0.05 | Oval and Rod-shaped macro-conidia about 10 to 50 µm long |
| 6 | F. subflagellisporum | 2 | 66.6 ± 10.3 | 1.2 ± 0 | Sperical micro-conidia about 2 to 5 µm long. No macro-conidia |
| 7 | F. fabacearum | 2 | 40.24 ± 6.0 | 0.7 ± 0.01 | Rod-shaped nonseptate macro-conidia about 5 to 50 µm long. Spheical micro-conidia |
| 8 | F. falciforme | 8 | 32.3 ± 5.8 | 0.74 ± 0.03 | Rod-shaped macro-conidia about 50 to 150 µm long. Spherical micro-conidia |
| 9 | F. brachygibbosum | 1 | 65.8 ± 5.7 | 0.87 ± 0.03 | Oval nonseptate macro-conidia about 10 to 30 µm long |
| 10 | F. brevicaudatum | 1 | 59.3 ± 4 | 0.6 ± 0.01 | Isolates in storage failed to regenerate for microscopy |
| 11 | F. commune | 4 | 62.5 ± 6.0 | 0.88 ± 0.03 | Rod-shaped nonsepate macro-conidia about 5 to 50 µm, |
| 12 | F. serpentimum | 1 | 45.1 ± 6.1 | 0.17 ± 0.02 | Isolates in storage failed to regenerate for microscopy |
| 13 | F. frekrugeri | 1 | 40.3 ± 4.1 | 0.87 ± 0.03 | Oval nonseptate macro-conidia up to about 40 µm long. Spherical micro-conidia 5 to 10 µm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erima, S.; Nyine, M.; Edema, R.; Nkuboye, A.; Habiba, N.; Candiru, A.; Paparu, P. Molecular Characterisation of Fusarium Species Causing Common Bean Root Rot in Uganda. J. Fungi 2025, 11, 283. https://doi.org/10.3390/jof11040283
Erima S, Nyine M, Edema R, Nkuboye A, Habiba N, Candiru A, Paparu P. Molecular Characterisation of Fusarium Species Causing Common Bean Root Rot in Uganda. Journal of Fungi. 2025; 11(4):283. https://doi.org/10.3390/jof11040283
Chicago/Turabian StyleErima, Samuel, Moses Nyine, Richard Edema, Allan Nkuboye, Nalule Habiba, Agnes Candiru, and Pamela Paparu. 2025. "Molecular Characterisation of Fusarium Species Causing Common Bean Root Rot in Uganda" Journal of Fungi 11, no. 4: 283. https://doi.org/10.3390/jof11040283
APA StyleErima, S., Nyine, M., Edema, R., Nkuboye, A., Habiba, N., Candiru, A., & Paparu, P. (2025). Molecular Characterisation of Fusarium Species Causing Common Bean Root Rot in Uganda. Journal of Fungi, 11(4), 283. https://doi.org/10.3390/jof11040283






