Abstract
Recently, Fusarium root rot (FRR)-like symptoms were observed in Uganda’s agroecology zones, prompting the National Agricultural Organisation (NARO) to conduct a disease survey. The survey reports indicated FRR as the second most prevalent root rot disease of common bean in Uganda after Southern blight. Ninety nine Fusarium spp. strains were obtained from samples collected during the surveys. The strains were morphologically and pathogenically characterised and confirmed to cause Fusarium root rot as observed in the field. However, molecular characterization of the strains was not conducted. In this study, therefore, 80 of the strains were characterized using partial sequences of translation elongation factor 1-alpha (TEF-1α) gene, beta tubulin (β tubulin) gene and internal transcribed spacers (ITS) region of ribosomal RNA to determine species diversity. High-quality Sanger sequences from the target genes were compared to the sequences from Fusarium species available in the National Centre for Biotechnology Information coding sequences (NCBI-CDS) database to determine the most likely species the strains belonged. The sequences from our strains were deposited into the NCBI gene bank under ID#288420, 2883276, 2873058 for TEF-1α, β tubulin and ITS respectively. The Fusarium species identified included; F. oxysporum, F. solani, F. equiseti F. delphinoides, F. commune, F. subflagellisporum, F. fabacearum, F. falciforme, F. brevicaudatum, F. serpentimum, F. fredkrugeri and F. brachygibbosum. The diversity of these Fusarium species needs to be taken into consideration when developing breeding programs for management of the disease since currently there is no variety of common bean resistant to FRR in Uganda.
1. Introduction
The common bean (Phaseolus vulgaris L.) is the most widely distributed Phaseolus species grown all over Africa [1]. According to FAO [2], Uganda produced 1,008,410 tons of common bean in 2016, making it the second largest producer after Tanzania at 1,200,000 tons. The production in Uganda is mostly done by small-scale farmers with land holdings of between 0.1 and 4 hectares [3]. Common bean production faces several constraints. Among the biotic constraints, fungal root rots are key [4,5]. Many fungal pathogens such as Sclerotium rolfsii, Fusarium species, Pythium species, Macrophomina phaseolina and Rhizoctonia solani have been reported to cause bean root rot [6,7,8].
Fusarium root rot disease of common bean, hereafter referred to as FRR, was reported as the second most important bean root rot disease in Uganda after Southern blight caused by Sclerotium rolfsii Sacc. (teleomorph Arthelia rolfsii (Curzi) C. C. Tu and Kimbr.) [9]. Several different species of Fusarium have been reported to cause FRR in common bean in several countries. Fusarium cuneirostrum from Fusaium solani species complex was reported to cause FRR in several countries such as the China, United States, Brazil, Canada and Uganda [10,11,12]. Other Fusarium species in the Fusarium solani species complex that have been reported to cause Fusarium bean root rot include Fusarium equiseti, Fusarium graminearum, Fusarium rodelens and Fusarium sporochoides [13,14]. Meanwhile, Fusarium oxysporum had been documented to cause vascular wilts, however, it has been found to cause Fusarium root rot in common bean [13,15,16].
The symptoms of FRR include longitudinal reddish-brown lesions on hypocotyls accompanied by longitudinal fissures or cracks with dying root tissues turning reddish brown. Infected plants are chlorotic, beginning with the primary leaves, stunted, and plants may wilt completely or undergo premature senescence. Yield losses due to Fusarium root rot (FRR) have been reported to reach 86% in severely affected soils [17]. The legumes program of National Agricultural Research Organisation conducted a survey of seven agroecological zones that included the South Western Highland (SWH), Western Mixed Farming system (WMFS), Lake Victoria Crescent and Mbale Farmlands (LVC), Eastern Highlands (EH), Northern Mixed Farming System (NMFS), North Eastern Dry Lands (NEDL) and West Nile Mixed Farming System (WNMFS). During these surveys, wilting plants with Fusarium root rot like symptoms were collected and used for pathogen isolation. Fusarium species strains were obtained and characterised both morphologically in culture media and phenotypically through pathogenicity studies [18]. While understanding the molecular diversity among pathogen populations to facilitate the development of host plant resistance is important, genetic diversity studies were not conducted in the earlier study.
Genetic diversity among Fusarium species has been studied using DNA-based markers such as Inter Simple Sequence Repeats (ISSR) and Single Sequence Repeats (SSR) [19], Amplified Fragment Length Polymorphism (AFLP) [20], Restriction Fragment Length Polymorphism (RFLP) and Randomly Amplified Polymorphic DNA (RAPD) [21]. Internal transcribed spacers region of the ribosomal RNA (ITS), beta tubulin (β tubulin) gene region [22], calmodulin gene region [23] and sequences from translation elongation factor 1 alpha (TEF1-α) gene have been widely used as taxonomic markers for fungal species identification [5,24,25].
Several studies have reported the effectiveness of the TEF1-α gene in fungal species identification, disease diagnosis and postharvest fungal toxicity surveys in crops such as coffee (Coffea species [26], sugar beet (Beta vulgaris) [27], bread wheat (Triticum aestivum L.) [28], millet (Eleusine coracana Gaertn.), sorghum (Sorghum bicolor L. Moench.), maize (Zea mays L.), groundnuts/peanuts (Arachis hypogea L.) and sesame (Sesamum indicum L.) [29]. The TEF-1α gene was used to identify and classify dermatophytes and it provided a high degree of differentiation between species that were closely related [25]. Similarly, partial sequences of the β tubulin gene region have been used to study molecular diversity and identification of Fusarium species. Kalman et al. [22] used the β tubulin gene region to identify Fusarium species causing basal rot in Allium cepa. Several authors have used ITS for the identification of fungal species [30]. Singha et al. [31] used ITS 1 and 4 to identify Fusarium species causing wilts in tomatoes and was able to detect several Fusarium species such as F. oxysporum, F. equiseti, F. proliferatum. Other authors have used other gene regions such as clamodulin (cam), RNA polymerase second largest subunit (rpb2) genes and the Cytochrome oxydase 1 (COX 1) gene region for identification of Fusarium to species level. [23,32]. Though Calmodulin primers were able to distinguish the Fusarium species [13], in the study by Gilmore [32] many of the species of Fusarium shared similar COX 1 partial gene sequences making COX 1 barcoding in Fusarium entirely infeasible.
The study by Paparu et al. [9] showed an increasing significance of FRR in Uganda’s agroecology zones. The disease was the second most prevalent after Southern blight. However, there is limited information on the diversity of Fusarium species causing root rot in common bean. To fill the observed knowledge gap, we sought to identify Fusarium species causing Fusarium root rot in Uganda. This information is useful in the development of host plant resistance, which is a key disease management strategy for smallholder farmers in sub-Saharan Africa.
2. Materials and Methods
2.1. Origin of Fusarium Species Strains Used
A collection of 99 hyphal tipped Fusarium species strains previously stored on filter papers originated from 6 agroecological zones of Uganda. These included, South Western highland (SWH), Western mixed farming system (WMFS), Lake Victoria Crescent and Mbale farmlands (LVC), Eastern highlands (EH), Northern mixed farming system (NMFS) and North Eastern dry lands (NEDL). The Strains were reactivated by growing them on Potato Dextrose Agar (PDA, Esvee Biologicals, Mumbai, India) media (39 g PDA in 1 L distilled water) for 14 days. Strains with growth rates of less than 0.6 cm per day were selected since Fusarium species that cause root rot on common bean were reported to have low growth rates [33].
2.2. DNA Extraction from Fusarium Species Strains
DNA was extracted from two-week-old mycelia of the 99 previously mentioned strains using a modified Cetyl trimethylammonium bromide (CTAB) protocol previously used by the Joint Research Council (JRC), European commission [34]. Actively growing mycelia were harvested by scraping them off the surface of the PDA into sterile Petri dishes. The mycelia were oven-dried over night at 30 °C. About 0.02 g of the mycelia was loaded into 2 mL Eppendorf tubes containing beads. The mycelia were ground into a fine powder using an automated tissue homogenizer and cell lyser Geno Grinder (1600 MiniG, Cole-Parmer, Chicago, IL, USA) for 3 min at 1450 revolutions per minute (rpm). Seven hundred microliters (700 μL) of DNA extraction buffer (2% CTAB, 50 mM EDTA pH 8.0, 100 mM Tris-Base pH 8.0, 2% PVP-40, 1% NaSO3, 1.4 M NaCl and 1% beta 2-mercaptoethanol) was added and the mycelia homogenized for another 2 min in the Geno grinder. Samples were incubated at 65 °C for 30 min with occasional shaking. Tubes were then centrifuged at 12,000 revolutions per minute for 10 min. Five hundred microliters (500 μL) of the supernatant was picked and transferred into new 2-mL Eppendorf tubes. Four hundred and fifty microliters (450 μL) of chloroform and iso amyl alcohol at the ratio of 24:1 was added to each sample, and the tubes were shaken for 2 min. Samples were then centrifuged at 10,000 strokes per minute for 10 min. Four hundred microliters (400 μL) of supernatant containing DNA was transferred into well-labeled 1.5 mL Eppendorf tubes. Four hundred and fifty microliters (450 μL) of Isopropanol (stored at −20 °C) and 40 µL of 3 M Sodium Acetate solution were added to the DNA and incubated at −20 °C for 2 h to precipitate the DNA. The tubes were then centrifuged at 15,000 rpm for 15 min to separate the DNA from the Isopropanol. The supernatant was decanted and the pellet washed with 500 µL of 70% ethanol by centrifuging at 7000 rpm for 10 min. The supernatant was decanted and DNA pellets air dried for 1 h at room temperature (25–30 °C). DNA pellets were then resuspended in 100 µL of elution buffer, the DNA concentration was assessed using a NanoDrop (ND-1000) (Thermo Fisher, Waltham, MA, USA), and it was stored at −80 °C. Generally, the concentration of all the samples was above 500 ng/μL, while the A260/A280 ratios ranged between 1.9 to 2.1.
2.3. Fusarium Species Identification Using TEF1-α, β Tubulin and ITS Partial Sequences
A multi-gene approach was used to identify the 99 Fusarium species strains. The primer sequences used to amplify portions of the target genes included TEF1-α gene forward primer (Ef 1: 5′-ATGGGTAAGGARGACAAGAC-3′) and reverse primer (Ff 2: 5′-GGARGTACCAGTSATCATGTT-3′) [12]. ITS forward primer ITS 1 (GGAAGTAAAAGTCGTAACAAGG) and reverse primer ITS 4 (TCCTCCGCTTATTGATATGC) were used for the amplification of the ITS region of ribosomal RNA [35]. Meanwhile, the β tubulin gene region was amplified using a forward primer (T1-AACATGCGTGAGATTGTAAGT) and reverse primer (T2-TAGTGACCCTTGGCCCAGTTG) [22].
A PCR master mix (Bioneer Corporation, Daejeon, Republic of Korea) was used in the amplification reactions according to the manufacturer’s instructions. A total reaction volume of 30 µL was used and it consisted of 15 µL premix, 1 µL of each reverse and primer, 3 µL of DNA and 10 µL of DNase free water. The PCR conditions included an initial denaturation at 95 °C for 5 min followed by 35 cycles of denaturation at 95 °C for 3 min, annealing at the various annealing temperature for the respective primers for 40 s, extension at 72 °C for 1 min and a final extension at 72 °C for 5 min. The annealing temperature for each primer was as follows: TEF1-α at 55 °C, ITS at 53 °C and β tubulin at 57 °C. For quality control, 5 µL of PCR products from each sample were electrophoresed alongside the 100 bp DNA ladder in a 1.5% agarose gel containing Gel-red fluorescent dye (Botium) in 1x TBE buffer at 100 V for 40 min. Gels were documented using a bench top Transilluminator (BioDoc-It Imaging System 8. Cole-Parmer, Chicago, IL, USA). However, out of the 99 strains, 80 of the strains were able to show amplification with the various primers. These PCR products from the 80 strains were purified using the AccPrepTM purification kit (Bioneer Cooperation, Daejeon, Republic of Korea) following the manufacturer’s instructions. The products were sequenced using their respective reverse primers in an ABI13730XL Sanger sequencing machine (Applied Biosystems, Waltham, MA, USA) using the BigDye Terminator v3.1 sequencing kit (Applied biosystems, USA) at Macrogen (Amsterdam, The Netherlands).
2.4. Growth Rate, Disease Severity Index (DSI) and Morphological Characteristics of Fusarium Species Strains
The average disease severity index (DSI) and growth rate of strains was obtained from Erima et al. [18]. In the study by Erima et al., the inoculum was prepared by cutting 1-cm2 agar plugs from 2-week-old cultures on PDA and inoculating them in 50 g of sterile millet in an autoclave bag. Spore concentration could not be used to measure the inoculum because some of the isolates did not produce conidia. Bags were incubated at 25 °C for two weeks until mycelia had fully covered the millet. Wooden trays of 100 cm × 35 cm × 10 cm were used to set up the experiment in the greenhouse. Ten grams of the inoculum was mixed with about 20 kg of soil in the wooden trays. Then, 16 seeds of each of the five test lines were planted in each tray with a replicate. A control tray which was un-inoculated was also planted with the test varieties. Virulence was then assessed at 28 days after planting using a scale of 1 to 9.
Meanwhile, the growth rate was determined on PDA using Petri dishes with a 9-cm diameter. A cross was made on the bottom of the Petri dish to mark its center. Inoculum was picked from 2-week-old cultures by tapping the mycelia with a needle. The inoculum was then transferred to the center of the marked Petri dish. Each strain was replicated three times. Growth data were collected 2 days post-inoculation by using a 30-cm ruler to measure the diameter of the colony until day 8 when mycelia for some isolates had reached the edge of the Petri dish. Information on colony color was also recorded. Microscopy was then conducted using 2-week-old cultures on PDA at 40× for selected strains of the different species. The shape and sizes of the macro- and micro-conidia were recorded, and photos were taken of the different strains.
2.5. Data Analysis
Sanger sequences were imported into chromas software (Chromas 2.6) for quality assessment. Low-quality bases at the 5′- and 3′-ends were trimmed off, and high-quality sequences exported as a FASTA file. The high-quality reads representing 80 Fusarium species strains from different agro-ecology zones were obtained and used for downstream analysis. They included 60 sequences from TEF1-α, 59 sequences from β tubulin and 58 sequences from ITS. The number of sequences of various strains varied because not all primer sets amplified the genes from the same strains. To confirm the species, the strains’ sequences were compared to the coding sequences in the National Centre for Biotechnology Information coding sequences (NCBI-CDS) database using basic local alignment search tool for nucleotides (BLASTn). The sequences were analyzed for the presence of open reading frames, exons and introns. The concordance of species’ names between two independent databases as the top hit was used to assign the species’ identities to the strains. Sequences were imported into MEGA 11.0 and aligned. A phylogenetic tree was constructed using the neighbor joining method using the TEF1-α sequences since it resolved all the Fusarium species. Curated Fusarium species sequences were deposited in the NCBI database. ITS and β tubulin could not resolve some species from Fusarium solani and Fusarium oxysporum species complexes, identifying all of them as F. solani and F. oxysporum, respectively. Data on morphological characteristics such as growth rate, virulence and colony color were obtained from Erima et al. [18]. Tukey’s honestly significant difference (HSD) test was used to test the difference in virulence and growth rate between the different Fusarium species.
3. Results
Identification of Fusarium Strains Using TEF1-α Gene, β Tubulin Gene and ITS Partial Sequences
Partial sequences of about 700 bp, 580 bp and 560 pb were obtained after the PCR amplification and sequencing of the PCR products of TEF1-α gene, β tubulin gene and ITS partial sequences, respectively (Figure 1). Sequences were successfully sequenced and processed for a combined total of 80 strains (TEF1-α = 60 strains, β tubulin = 59 strains and ITS = 58 strains). The sequences were deposited at the National Centre for Biotechnology Information (NCBI) under accessions PQ363745 to PQ363805, PQ497178 to PQ497237, PQ497119 to PQ497177 for ITS, TEF1-α and β tubulin, respectively.
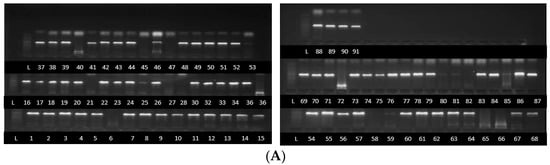
Figure 1.
PCR product bands of Fusarium species strains following amplification using ITS (A), β tubulin (B) and TEF1-α primers (C). Some strains without bands were not detected by the primers.
Comparing the high-quality, trimmed Sanger sequences with the NCBI CDS database for TEF1-α, ITS and β-tubulin, we identified 12 different Fusarium species with sequence identities ranging from 99.9% to 100% to those of the respective reference sequences of the species in the database. Thirty seven strains were most identical to F. oxysporum, 13 to F. solani, 7 to F. falciforme, 9 to F. equiseti and 4 to F. commune. Meanwhile, F. fabacearum and F. subflagellisporum were each represented by two strains. Single strains of F. delphinoides, F. brevicaudatum, F. serpentimum, F. fredkrugeri and F. brachygibbosum were identified. A strain belonging to Clonostachys rhizophaga was also identified (Table 1). The TEF1-α gene had the least number of strains with missing data, and produced the longest reads with high-quality bases after trimming. However, we used the sequences from all three genes (TEF1-α, ITS and β tubulin) to dependently identify the isolates to species level (Table 1). A strain was assigned to species if at least two of the gene sequences matched with the same species with >99% identity. Due to variation in read length from ITS and β tubulin genes some strains grouped under F. falciforme and F. serpentimum by TEF1-α could not be resolved from Fusarium solani species complex. The two genes primers were also unable to resolve F. fredkrugeri, F. commune, F. fabacearum, F. subflagellisporum and F. brachygibbosum from the Fusarium oxysporum species complex, identifying them as Fusarium oxysporum. A maximum likelihood phylogenetic tree was generated using the neighbor joining method, using only the TEF1-α sequences (Figure 2). A consensus tree could not be generated by concatenating the sequences because of the high variation in length, quality and data absence of ITS and β tubulin in sequences after trimming. One strain, MitF-487-2, identified as Clonostachys rhizophaga, could not be included in the tree because its sequences were too divergent to align with those of the Fusarium species. The Fusarium species, their agroecology of origin and accession numbers are summarized in Table 1.

Table 1.
Fusarium species strains and accession numbers in the NCBI database. Gene regions that were amplified have accession numbers, while those that failed to amplify do not have accession numbers.
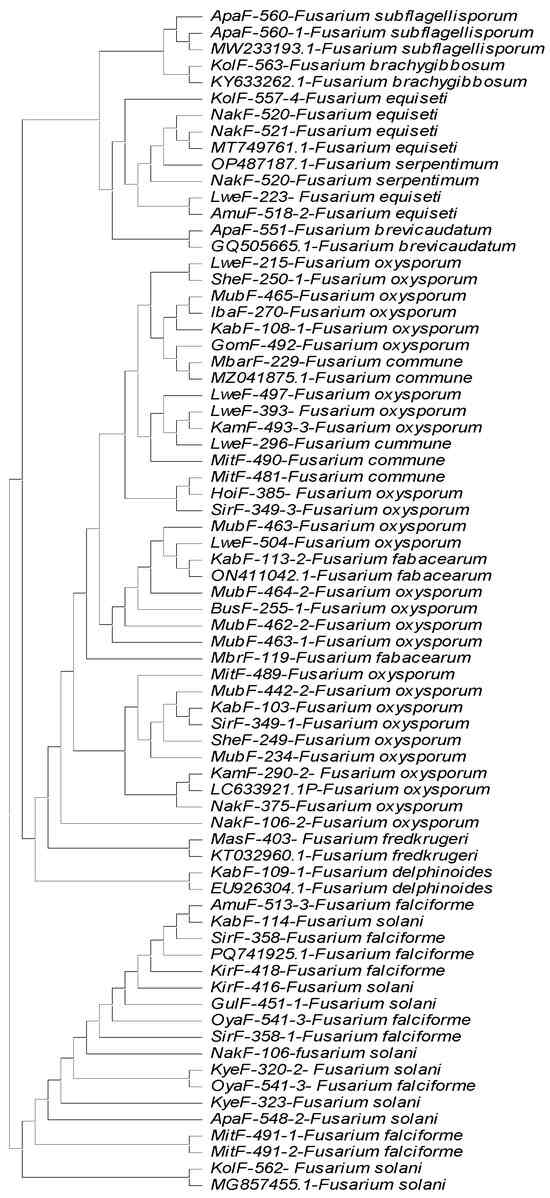
Figure 2.
The phylogenetic tree constructed using the neighbor joining method for Fusarium species strains collected from six Ugandan agroecology zones. This analysis involved 72 nucleotide sequences. All ambiguous positions were removed for each sequence pair (pairwise deletion option). There were a total of 1939 positions in the final dataset. Evolutionary analyses were conducted in MEGA11.
Fusarium species have been reported to vary in their morphological characteristics such as growth rate, virulence, and shape and sizes of microscopic structures and colony color [29,33]. The average disease severity index (DSI), growth rate and colony colors of the strains were obtained from Erima et al. [18]. The average DSI and growth rate varied among the species (Table 1 and Table S1). All the different Fusarium species varied significantly in disease severity index (DSI) caused to five common bean varieties (F = 3.6 p = 0.03). Following Tukey’s honestly significant difference (Tukey’s HSD) test, the average DSI caused by the Fusarium species were still significantly different from each other. Fusarium solani was the least pathogenic, with an average DSI of 37.2% while F. subplagellisporum was the most pathogenic with an average DSI of 66.6% (Table 2). The Fusarium species strains also varied significantly in average growth rate per day (F = 2.9 p < 0.001). Following Tukey’s HSD, the growth rates of F. brachygibosum, F. fredkrugeri, F. delphinoides and F. fabacearum were similar, while those of F. solani, F. oxysporum, F. brevicaudatum, F. serpentimum, F. falciforme and F. equiseti were also similar. The growth rates of F. subflagellisporum and F. commune were similar, while the growth rate of C. rhizophaga was different from that of all the Fusarium species strains.

Table 2.
Average disease severity index (DSI), growth rate and microscopic structures of different Fusarium species from Ugandan agroecology zones.
Many of the Fusarium species strains exhibited multiple colors. Colony colorations such as white, white/purple, white/pink and white/cream were reported for F. oxysporum and F. solani, though specific colorations such as white/yellow and white/brown were reported for F. solani. All the strains of F. equiseti were white both on the top and bottom of the Petri dish. While F. falciforme and F. commune had strains which were white/pink, white/purple and white, the strains of F. brevicaudatum, F. serpentimum, F. brachygibbosum, F. subflagellisporum, C. rhizophaga, F. delphinoides, F. fabacearum and F. fredkrugeri were colored white, white/purple, white/purple, white/brown, white/purple, white/pink, purple and white, respectively (Figure S1, Table S1). We could not present the colony pictures of the strains of F. brevicaudatum and F. serpentimum because, after DNA extraction, the filter papers in storage were depleted. However, their colony colors were obtained from Erima et al. 2024 [18]. Photos of the symptoms caused by a few Fusarium species identified above were retrieved from the archives at National Crops Resources Research Institute and were observed to vary among the species. The lesions caused by F. oxysporum were along the vascular bundle and extended from the roots to above the soil line (Figure 3a,b), while the lesions caused by other Fusarium species were restricted to the root area (Figure 3c,d). Photos were not captured for every strain phenotyped, as we were not aware at that time if they belonged to different species or not.
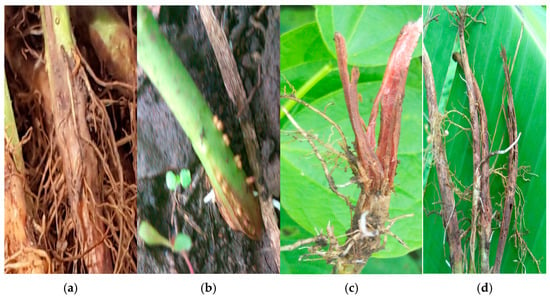
Figure 3.
Symptoms caused by some of the strains during pathogenicity by Erima et al. [18]. (a) Longitudinal dark brown lesions extending along the vascular bundle past the collar of the plant, typical of F. oxysporum. (b–d) Longitudinal reddish brown spots with cracks and fissures leading to total reduction of the main root system.
Following microscopy, all the strains were observed to have septate hyphae. They either produced micro- or macro-conidia or both. The micro-conidia were spherical while the macro-conidia were rod-shaped, oval, or sickle-shaped. The shape and sizes of micro- and macro-conidia are summarized in Figure 4 and Table 2.
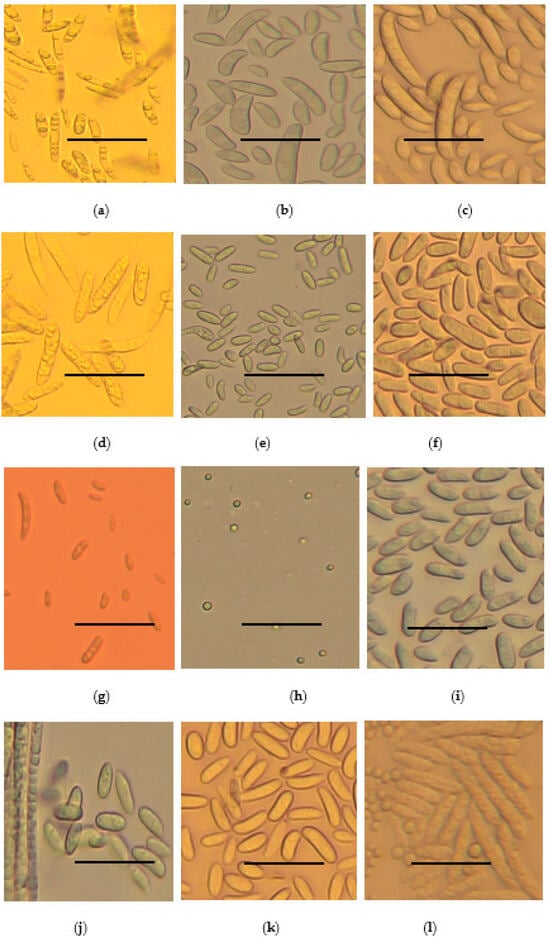
Figure 4.
Microscopic features of Fusarium species strains at 40x magnification: (a) F. oxysporum, (b) F. solani, (c) F. falciforme, (d) equiseti, (e) F. brachygibosum, (f) C. rhizophaga, (g) F. fredkrugeri, (h) F. subflagellisporum, (i) F. fabacearum, (j) F. delphinoides, (k) F. commune and (l) F. equiseti macro- and micro-conidia. The scale bar in the pictures represents 100 µm.
4. Discussion
Partial sequences of translation elongation factor 1-alpha (TEF1-α), β-tubulin, and the ITS region of ribosomal RNA were used to identify Fusarium species strains previously isolated from the roots of wilting common beans. Fusarium species identified as the main pathogens causing Fusarium root rot (FRR) in Uganda included F. oxysporum, F. solani, F. equiseti, F. falciforme, F. flagellisporum, F. commune, F. brevicaudatum, F. brachyggibosum, F. serpentimum, F. frekrugeri, F. fabacearum and F. delphinoides. The identification of Fusarium species based on plant disease symptoms is quite challenging. In both field and greenhouse settings, the early symptoms of FRR and wilt are similar (wilting and yellowing of leaves), and root rots sometimes occur as disease complexes. Morphological identification and classification continue to be used but with enormous challenges as it requires experienced mycologists to identify fungi to species level [36]. Despite this, the proper identification and classification of Fusarium spp. is important for monitoring changes in the species population and their impact on agriculture.
Previously, Fusarium species such as Fusarium solani and Fusarium cuneirostrum have been reported to cause bean root rot in Uganda [11,33]. Lately, many studies have taken place in other countries to identify Fusarium species causing common bean root rot. In China, Fusarium species such as F. equiseti, F. oxysporum, F. solani and F. cuneirostrum have been reported to cause common bean root rot [10,37,38,39]. The current study has identified additional Fusarium species such as F. falciforme, F. subflagellisporum, F. commune, F. brevicaudatum, F. brachyggibosum, F. serpentimum, F. frekrugeri, F. fabacearum and F. delphinoides as the causal agents of Fusarium bean root rot in Uganda.
Several members of the Fusarium species complex have a wide host range and diverse ecological niches, yet they also differ in their characteristics. For example, F. equiseti was reported to cause seedling wilting, root tip discoloration and necrosis in sugar beet by Khan et al. [40]. Fusarium falciforme was also reported to cause root rot in Weigelia florida in China [29]. According to Trabelsi et al. [41], both F. oxysporum and F. brachygibbisum cause die back in olive, trees and the two species clustered closely in the current study. The other species that clustered closely with Fusarium oxysporum included F. commune, F. frekrugeri, F. subflagellisporum, F. delphinoides, F. fabacearum, F. brachygibbosum and F. brevicaudatum. Namasaka [42] reported F. equiseti as a causal agent of cowpea root rot. Interestingly, the current study confirmed the species as a causal agent of common bean root rot in Uganda. Marcelo et al. [43] recovered F. frekrugeri from soil under Musa acuminata from Kruger national park in South Africa in undisturbed forest soil. In this study, F. frekrugeri caused a DSI of 40.3%. Meanwhile, the F. delphinoides strain GPK was reported to be pathogenic to chickpeas and pigeon peas by Guruprasad et al. [44].
There are at least 20 species complexes in the genus Fusarium. Chehri et al. [45] and Coleman [46] reported F. falciforme as a species under the F. solani species complex. In the current study, the strains of F. falciforme and F. solani clustered closely, supporting the above argument. However, contradicting the nomenclature of the Fusarium species continues to be a challenge, resulting in the lack of congruity between morphological and molecular phylogeny. For example, Sang et al. [11] reported F. cuneirostrum as a causal agent of FRR in common bean in Uganda, yet these strains were initially identified as F. solani f. sp. Phaseoli by Munkankusi [47], based on colony characteristics. Ji-wen et al. [48] also reported F. equiseti as a member of the F. incarnatum-equiseti species complex.
One strain, MitF-487-2, identified as Clonostachys rhizophaga, was obtained from LVC agroecology. It was detected by ITS and β tubulin, while TEF1-α did not detect it. This is the first report of C. rhizophaga causing wilts in common bean in Uganda. The pathogen is reported to be pathogenic to several crops. It reportedly causes wilts and root rot in chickpeas [49,50,51]. In water chestnut, C. rhizophaga causes longitudinal chlorotic streaks and black spots on the stem surface and vascular necrosis [52]. However, some other Clonostachys species, such as C. rosea, are reported to be mycoparasitic. They are aggressive parasites of fungi, and research on their use for plant disease control is ongoing [53].
Secondary data on DSI were obtained from Erima et al. [18]. All the species differed significantly in DSI caused on common bean. Strains identified as F. oxysporum caused more disease than F. solani. However, in an earlier study, Chehri et al. [45] observed that F. solani caused more disease than F. oxysporum on potato tubers. Differences in DSI among different Fusarium species were equally observed by Siddique et al. [54] in common bean and by Burlakoti et al. [55] in sugar beet, where F. graminearum strains were more pathogenic than F. oxysporum strains. The variation in virulence of a single Fusarium species in many different crops is an indicator that these species have their primary host on which they proliferate most. The colony colorations of the different species strains in the current study are also related to what other researchers observed. For example, Trebelsi et al. [39] reported the purple coloration of Fusarium oxysporum, causing olive trees die back. Tuiime [33] also reported white and brown coloration in Fusarium solani fsp phaseoli. All the F. equiseti isolates in this study had abundant white mycelia, and similar findings were reported by Mohamed et al. [32]. Similarly, the white colony coloration in F. falciforme was reported by Dong-Xia [38].
The Fusarium species strains in the current study were obtained from different agroecological zones of Uganda. This shows that Fusarium species can survive in a wide range of temperatures ranging from the cool humid South Western highlands to warm and less humid North Eastern Dryland. Tusiime [33] reported F. solani fsp phaseoli in the cool humid regions of the South-Western Highlands. However, in the current study, F. solani was reported in various agroecological zones, including in the Northern Mixed farming system, Western Mixed farming system, Lake Victoria crescent and Mbale farmlands, South-Western Highlands and the North-Eastern dry land, which is generally warmer and less humid.
5. Conclusions
Fusarium species causing root rots and wilts in common beans in Uganda exhibit morphological, phenotypic and genetic diversity. This research has generated information on the diversity of Fusarium species causing common bean root rot. The diversity of Fusarium species observed in our study needs to be taken into consideration when developing new varieties of breeding programs for the management of the disease. The strains of the different species that have been identified in this study need to be included during germplasm screening so that durable resistance to FRR can be achieved in the released varieties.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jof11040283/s1, Figure S1: Colony morphology of 10 day old Fusarium species strains on PDA. A- Morphology on the top of the Petri dish and B- Morphology on the bottom of the Petri dish; Table S1: Colony colour, growth rate and virulence of Fusarium spp. strains following inoculation on five common bean varieties in the screenhouse. artial sequences of Fusarium species strains can be obtained at NCBI under ID#2884260, 2883276, 2873058 for TEF-1α, β tubulin and ITS respectively.
Author Contributions
Conceptualization, P.P. and S.E.; methodology, P.P., S.E., A.N., A.C. and N.H.; formal analysis, S.E. and M.N.; resources, P.P.; writing—first draft, S.E.; writing—review and editing, M.N., P.P. and R.E.; supervision, P.P. and R.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Bill & Melinda Gates Foundation. Opportunity/Contract ID: OPP1084135.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The partial sequences of TEF 1α, βtubulin and ITS can be obtained from the NCBI database under ID; 288260, 288276 and 2873058 for TEF 1α, β tubulin and ITS, respectively. The rest of the data are within the article.
Acknowledgments
We acknowledge funding from the Bill & Melinda Gates Foundation through Grant Ref OPP1084135. We are grateful to the staff of the Legumes Program at the National Crops Resources Research Institute, who provided us with the strains. Our appreciation also goes to the management and staff of Makerere University Regional Centre for Crop Improvement who hosted and supported the molecular work.
Conflicts of Interest
The authors have no conflicts of interest. The funders did not participate in conceptualization of the research.
References
- Melotto, M.; Monteiro-Vitorello, C.B.; Bruschi, A.G.; Camargo, L.E. Comparative bioinformatic analysis of genes expressed in common bean (Phaseolus vulgaris L.) seedlings. Genome 2005, 48, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Food and Agricultural Organisation of United Nations (FAO). World Food and Agricultural Statistical Pocket Book; FAO: Rome, Italy, 2018; p. 28. [Google Scholar]
- CASA. Bean Sector Strategy-Uganda; CASA Uganda country team: Kampala, Uganda, 2020; p. 4, Unpublished. [Google Scholar]
- Miklas, P.N.; Kelly, J.D.; Beebe, S.E.; Blair, M.W. Common bean breeding for resistance against biotic and abiotic stress: From classical to MAS breeding. Euphytica 2006, 147, 105–131. [Google Scholar] [CrossRef]
- Li, Y.P.; You, M.P.; Barbetti, M.J. Species of Pythium associated with seedling root and hypocotyl disease on common bean (Phaseolus vulgaris) in Western Australia. Plant Dis. 2014, 98, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Lu, X.H.; Li, S.D.; Wu, B.M. First report of common bean (Phaseolus vulgaris) root rot caused by Plectosphaerella cucumerina in China. Plant Dis. 2018, 102, 1849. [Google Scholar] [CrossRef]
- Nzungize, J.; Geps, P.; Buruchara, R.; Buah, S.; Ragama, P.; Busogoro, J.P.; Baudoin, J.P. Pathogenic and molecular characterization of Pythium species inducing root rot symptoms of common bean in Rwanda. Afr. J. Microbiol. Res. 2011, 5, 1169–1181. [Google Scholar]
- Hall, R. Fusarium root rot. In Compendium of Bean Diseases; American Phytopathological Society Press: St. Paul, MN, USA, 1991; pp. 9–10. [Google Scholar]
- Paparu, P.; Acur, A.; Kato, F.; Acam, C.; Nakibuule, J.; Musoke, S.; Mukankusi, C. Prevalence and incidence of four common bean root rots in Uganda. J. Exp. Agric. 2018, 54, 888–900. [Google Scholar] [CrossRef]
- Deng, D.; Wu, W.; Duan, C.; Sun, S.; Zhu, Z. A novel pathogen Fusarium cuneirostrum causing common bean (Phaseolus vulgaris) root rot in China. J. Integr. Agric. 2024, 23, 166–176. [Google Scholar]
- Sang, H.; Jacods, J.L.; Wang, J.; Mukankusi, C.; Chilvers, M.I. First report of Fusarium cuneirostrum causing root rot of common bean (Phaseola vulgaris L.) in Uganda. Plant Dis. 2018, 102, 2639. [Google Scholar] [CrossRef]
- O’donell, K. Molecular phylogeny of Nectria haemococcus–Fusarium solani species complex. Mycologia 2000, 92, 919–938. [Google Scholar] [CrossRef]
- Adesemoye, A.O.; Orrell, T.; Kodati, S. Effect of virulence of root rot pathogens and cultivar resistance on disease occurrence in dry beans. Plant Health Prog. 2018, 19, 237–241. [Google Scholar]
- Bilgi, V.N.; Bradley, C.A.; Mathew, F.M.; Ali, S.; Rasmussen, J.B. Root rot of dry edible bean caused by Fusarium graminearum. Plant Health Prog. 2011, 12, 14. [Google Scholar]
- Husaini, A.M.; Sakina, A.; Ca mbay, S.R. Host–pathogen interaction in Fusarium oxysporum infections: Where do we stand? Mol. Plant-Microbe Interact. 2018, 31, 889–898. [Google Scholar] [PubMed]
- Abawi, G.S. Root rots. In Bean Problems in the Tropics. Cali, Colombia: CIAT (Centro Internacional de Agricultura Tropical); CIAT: Cali, Colombia, 1989; pp. 105–157. [Google Scholar]
- Abawi, G.S.; Pastor-Corrale, M.A. Root Rots of Beans in Latin America and Africa:Diagnosis, Research Methodologies, and Management Strategies; CIAT: Cali, Colombia, 1990; p. 114. Available online: https://hdl.handle.net/10568/54258 (accessed on 17 November 2024).
- Erima, S.; Moses, N.; Allan, N.; Cnadiru, A.; Nakibuule, J.; Edema, R.; Paparu, P. Morphological and pathogenic characterisation of Fusarium species causing common bean root rot in Uganda. J. Sci. Agric. 2024, 8, 7–14. [Google Scholar] [CrossRef]
- Zietkiewicz, E.; Rafalski, A.; Labuda, D. Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics 1994, 20, 176–183. [Google Scholar] [CrossRef]
- Puri, K.D.; Saucedo, E.S.; Zhong, S. Molecular characterisation of Fusarium head blight pathogens sampled from a naturally infected disease nursery used for wheat breeding programs in China. Plant Dis. 2012, 96, 1280–1285. [Google Scholar] [CrossRef]
- Fourie, G.; Steenkamp, E.T.; Ploetz, R.C.; Gordon, T.; Viljoen, A. Current status of the taxonomic position of Fusarium oxysporum formae specialis cubense within the Fusarium oxysporum complex. Infect. Genet. Evol. 2011, 11, 533–554. [Google Scholar] [CrossRef]
- Kalman, B.; Abraham, D.; Graph, S.; Perl-Treves, R.; Meller Harel, Y.; Degani, O. Isolation and identification of Fusarium spp., the causal agents of onion (Allium cepa) basal rot in Northeastern Israel. Biology 2020, 9, 69. [Google Scholar] [CrossRef]
- Nuangmek, W.; Kumla, J.; Khuna, S.; Lumyong, S.; Suwannarach, N. Identification and characterisation of Fusarium species causing watermelon fruit rot in northern Thailand. Plants 2023, 12, 956. [Google Scholar] [CrossRef]
- Kristensen, R.; Torp, M.; Kosiak, B.; Holst-Jensen, A. Phylogeny and toxigenic potential is correlated in Fusarium species as revealed by partial translation elongation factor 1 alpha gene sequences. Mycol. Res. 2005, 109, 173–186. [Google Scholar] [CrossRef]
- Mirhendi, H.; Makimura, K.; de Hoog, G.S.; Rezaei-Matehkolaei, A.; Najafzadeh, M.J.; Umeda, Y.; Ahmadi, B. Translation elongation factor 1-α gene as a potential taxonomic and identification marker in dermatophytes. Med. Mycol. J. 2015, 53, 215–224. [Google Scholar] [CrossRef]
- Olal, S.; Olango, N.; Kiggundu, A.; Ochwo, S.; Adriko, J.; Nanteza, A.; Matovu, E.; Lubega, G.W.; Kagezi, G.; Hakiza, G.J.; et al. Using translation elongation factor gene to specifically detect and diagnose Fusarium xylaroides, a causative agent of coffee wilt disease in Ethiopia, East and Central Africa. J. Plant Pathol. Microbiol. 2018, 9, 440. [Google Scholar] [CrossRef]
- Nitschke, E.; Nihlgard, M.; Varrelmann, M. Differentiation of eleven Fusarium spp. straind from sugar beet, using restriction fragment analysis of a polymerase chain reaction–amplified translation elongation factor 1α gene fragment. Phytopathology 2009, 99, 921–929. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Otieno, P.; Imbahake, S.S.; Okoth, S.; Otipa, M.; Wafula, W.V. Prevalence and phylogenetic diversity of pathogenic Fusarium species in genotypes of wheat seeds in three rift valley regions, Kenya. Adv. Agric. 2021, 2021, 8839147. [Google Scholar] [CrossRef]
- Wokorach, G.; Sofie, L.; Kris, A.; Echodu, R.; Haeseart, G. Genetic characterisation of fungal biodiversity in storage grains: Towards enhancing food safety in Northern Uganda. Microorganisms 2021, 9, 383. [Google Scholar] [CrossRef]
- Nurrahmi, D.F. Internal Transcribed Spacer (ITS) as DNA barcoding to identify fungal species: A review. Squalen Bull. Mar. Fish. Postharvest Biotechnol. 2017, 11, 37–44. [Google Scholar] [CrossRef]
- Singha, I.M.; Kakoty, Y.; Unni, B.G.; Das, J.; Kalita, M.C. Identification and characterisation of Fusarium sp. using ITS and RAPD causing fusarium wilt of tomato straind from Assam, North East India. J. Genet. Eng. Biotechnol. 2016, 14, 99–105. [Google Scholar] [CrossRef]
- Gilmore, S.R.; Graefenhan, T.; Louise-Sieze, G.E.; Seifert, K.A. Multiple copies of cytochrome oxidase 1 in species of the fungal genus Fusarium. Mol. Ecol. Resour. 2009, 9, 90–98. [Google Scholar] [CrossRef]
- Tusiime, G. Variation and Detection of Fusarium solani f. sp. phaseoli and Quantification of Soil Inoculum in Common Bean Fields. Ph.D. Thesis, Makerere University, Kampala, Uganda, 2004; p. 30. [Google Scholar]
- Available online: https://joint-research-centre.ec.europa.eu/tools-and-laboratories/standardisation_en (accessed on 16 June 2024).
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Fungal Barcoding Consortium. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- Raja, H.A.; Miller, A.N.; Pearce, C.J.; Oberlies, N.H. Fungal identification using molecular tools: A primer for the natural products research community. J. Nat. Prod. 2017, 80, 756–770. [Google Scholar] [CrossRef]
- Shen, D.-X.; Song, Z.-W.; Lu, Y.-M.; Fan, B. First report of Fusarium falciforme causing root rot in Weigelia florida in China. Plant Dis. 2020, 104, 610–623. [Google Scholar] [CrossRef]
- Liu, T.; Shen, Y.; Liu, Z.; Han, D.; Cui, J.; Zuo, Y. Control effect of three kinds of seed-coating formulations on the root rot of kidney beans. Plant Prot 2017, 43, 216–219. [Google Scholar]
- Wang, C.; Sun, C.; Liu, S. Preliminary study on pathogen of root rot of kidney bean and its control in Xinjiang. Ganhanqu Yanjiu (Arid. Zone Res.) 2010, 27, 380–384. [Google Scholar]
- Khan, M.F.R.; Liu, Y.; Bhuiyan, M.Z.R.; Lakshman, D.; Liu, Z.; Zhong, S. First report of Fusarium equiseti causing seedling death on sugar beets in Minesota, USA. Plant Dis. 2021, 105, 2017. [Google Scholar] [CrossRef]
- Trabelsi, R.; Sellami, H.; Gharbi, Y.; Krid, S.; Cheffi, M.; Kammoun, S.; Triki, M.A. Morphological and molecular characterisation of Fusarium spp. associated with olive trees dieback in Tunisia. 3 Biotech 2017, 7, 28. [Google Scholar] [CrossRef]
- Namasaka, R.W. 2017 Inheritence of Resistance to Fusarium Root Rot (Fusarium redolense) Disease of Cow Peases in Uganda. Master’s Thesis, Makerere University, Kampala, Uganda, 2017. [Google Scholar] [CrossRef]
- Marcelo, S.D.; Wilnad, J.; Swart, W.G. New Fusarium species from Kruger national park, South Africa. Mycokeys 2018, 34, 63–92. [Google Scholar] [CrossRef]
- Guruprasad, K.; Sajjan, S.; Kareguadas, T.B. Pathogenicity of Indole-3- acetic acid producing fungus Fusarium delphinoides strain GPK towards chicken peas and pigeon peas. Eur. J. Plant Pathol. 2011, 131, 355–369. [Google Scholar] [CrossRef]
- Chehri, K.; Mohamed, N.; Salleh, B.; Latiffah, Z. Occurrence and pathogenicity of Fusarium spp. on potato tubers in Malaysia. Afr. J. Agric. Res. 2011, 6, 3706–3712. [Google Scholar]
- Coleman, J.J. The Fusarium Solani species comples: Ubiguitous pathogens of Agricultural Importance. Mol. Plant Pathol. 2015, 17, 146–158. [Google Scholar] [CrossRef]
- Mukankusi, C.M. Improving resistance to Fusarium root rot [Fusarium solani (Mart.) Sacc. f. sp. phaseoli (Burkholder) W.C. Snyder & H.N. Hans.] in Common bean (Phaseolus vulgaris L.). Ph.D. Thesis, University of KwaZulu-Natal, Durban, South Africa, 2008; p. 200. [Google Scholar]
- Xia, J.W.; Sandoval-Denis, M.; Crous, P.W.; Zhang, X.G.; Lombard, L. Numbers to names- Restyling the Fusarium incarnatum-equiseti species complex. Phylogeny Evol. Fungi 2019, 43, 186–221. [Google Scholar] [CrossRef]
- Dugan, F.M.; Lupien, S.L.; Chen, W. Clonostachys rhizophaga and other fungi from chickpea debris in the Palouse region of the Pacific Northwest, USA. N. Am. Fungi 2012, 7, 1–11. [Google Scholar] [CrossRef][Green Version]
- Cota-Barreras, C.I.; García-Estrada, R.S.; León-Félix, J.; Valenzuela-Herrera, V.; Mora-Romero, G.A.; Leyva-Madrigal, K.Y.; Tovar-Pedraza, J.M. First report of Clonostachys chloroleuca causing chickpea wilt in Mexico. New Dis. Rep. 2022, 46, 121–123. [Google Scholar] [CrossRef]
- Gibert, S.; Edel-Hermann, V.; Gautheron, E.; Gautheron, N.; Bernaud, E.; Sol, J.M.; Capelle, G.; Galland, R.; Bardon-Debats, A.; Lambert, C.; et al. Identification, pathogenicity and community dynamics of fungi and oomycetes associated with pea root rot in northern France. Plant Pathol. 2022, 71, 1550–1569. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, A.F.; Gu, C.Y.; Zang, H.Y.; Chen, Y. First report of Clonostachys rhizophaga as a pathogen of water chestnut (Eleocharis dulcis) in Anhui Province of China. Plant Dis. 2018, 103, 151. [Google Scholar] [CrossRef]
- Funck, J.D.; Dubey, M.; Jensen, B.; Karlsson, M. Clonostachys Rosea to Control Plant Diseases. 2022. Available online: https://library.oapen.org/handle/20.500.12657/61518 (accessed on 14 November 2024). [CrossRef]
- Siddique, S.S.; Bhuiyan, M.K.; Momotaz, R.; Bari, G.M.; Rahman, M.H. Cultural characteristics, virulence and invitro chemical control of Fusarium oxysporum f. sp. Phaseoli of bush beans (Phaseola vulgaris). Agriculturista 2014, 12, 103–110. [Google Scholar] [CrossRef]
- Burlakoti, P.; Rivera, V.; Secor, G.A.; Qi, A.; Del Rio-Mendoza, L.E.; Khan, M.F.R. Comparative pathogenicity and virulence of Fusarium species on sugar beet. Plant Dis. 2012, 96, 1291–1296. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).