Invasive Fungal Infection by Scedosporium apiospermum with Cerebral Involvement in a Pediatric Patient Affected by Chronic Granulomatous Disease After Hematopoietic Cell Transplant
Abstract
1. Introduction
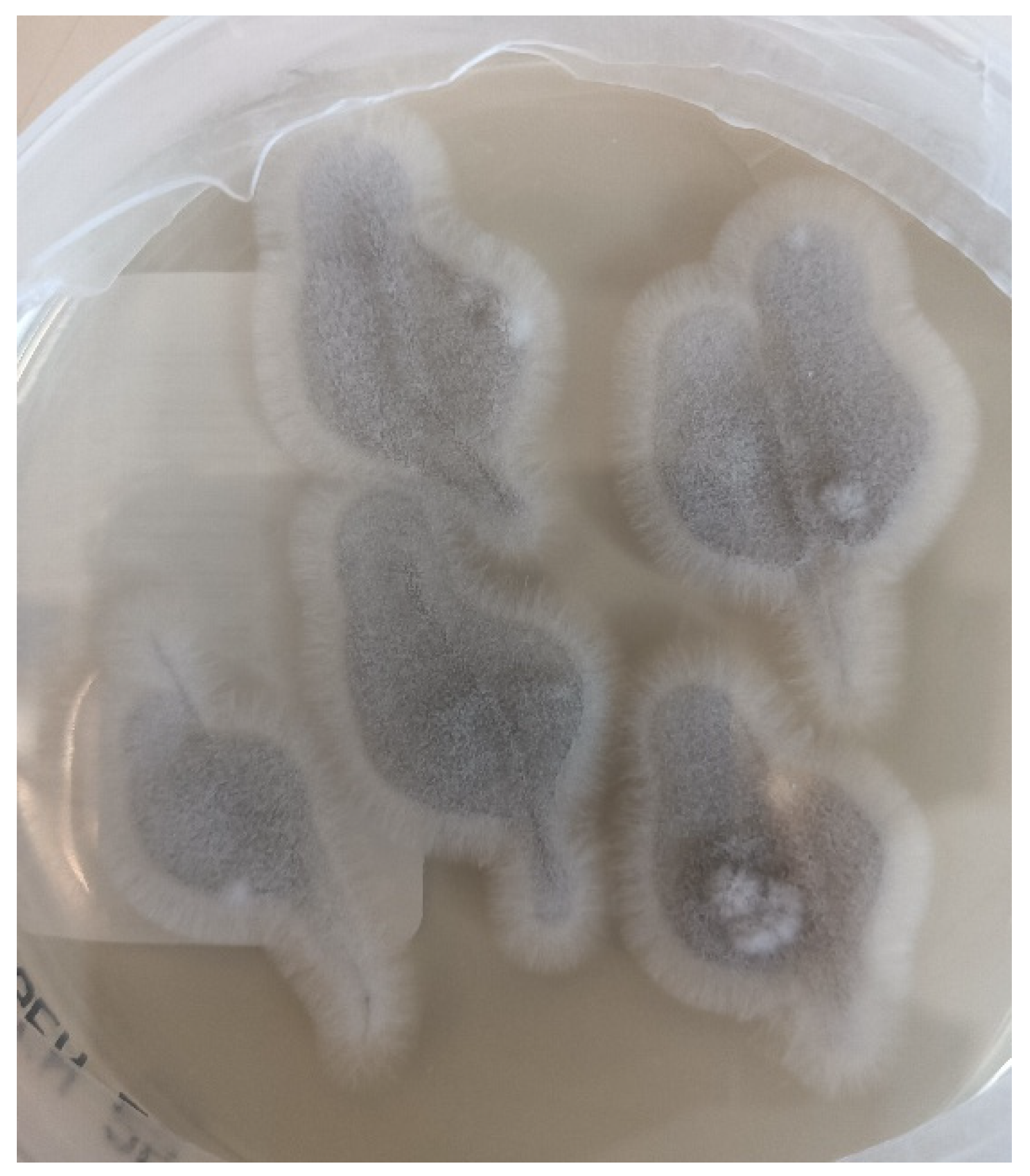
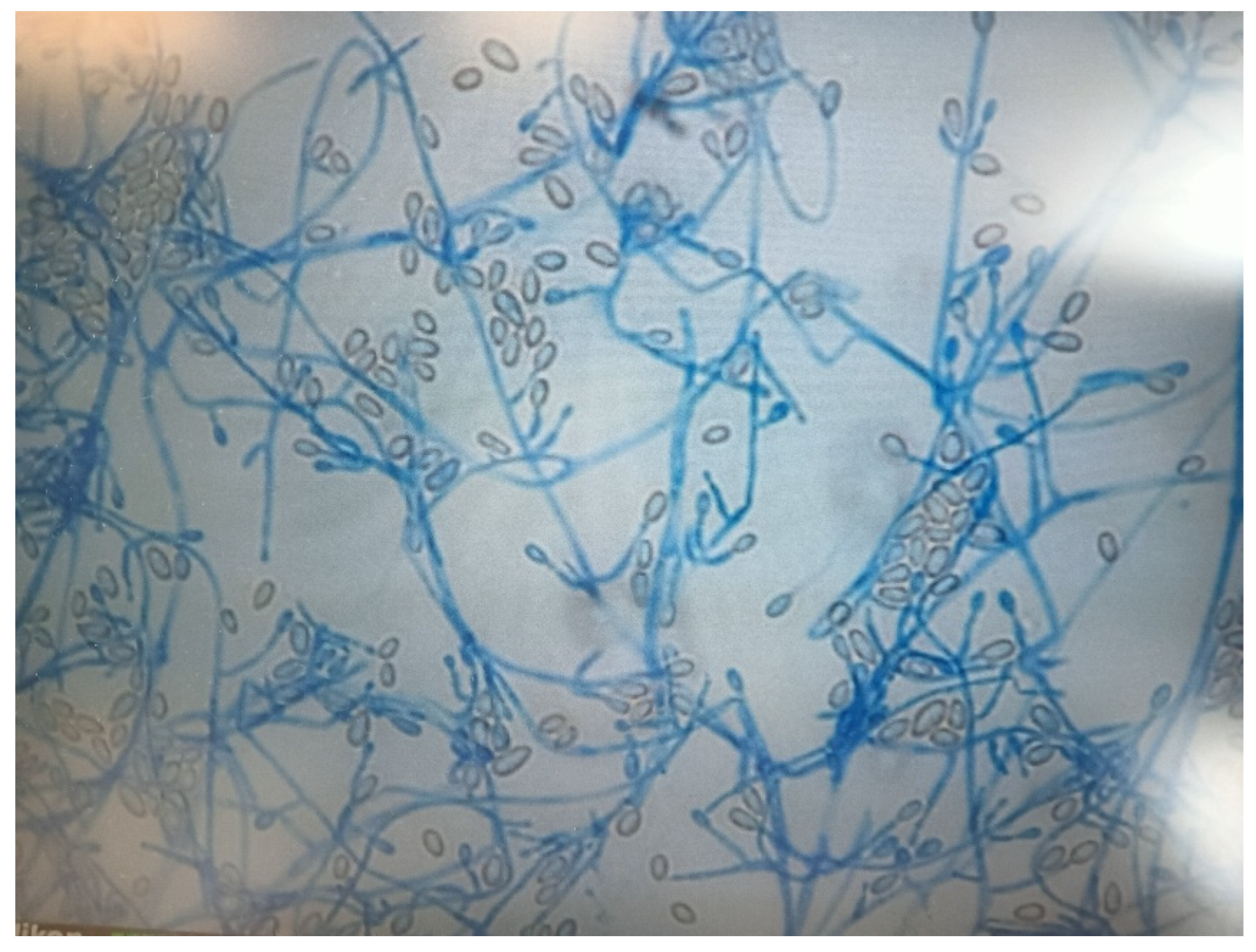
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AML | acute myeloid leukemia |
| CGD | chronic granulomatous disease |
| GF | graft failure |
| GTX | granulocyte transfusion |
| GVHD | graft-versus-host disease |
| HCT | hematopoietic cell transplant |
| S. | scedosporium |
| TDM | Therapeutic Drug Monitoring |
References
- Fang, W.; Wu, J.; Cheng, M.; Zhu, X.; Du, M.; Chen, C.; Liao, W.; Zhi, K.; Pan, W. Diagnosis of invasive fungal infections: Challenges and recent developments. J. Biomed. Sci. 2023, 30, 42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McCarthy, M.W.; Kontoyiannis, D.P.; Cornely, O.A.; Perfect, J.R.; Walsh, T.J. Novel Agents and Drug Targets to Meet the Challenges of Resistant Fungi. J. Infect. Dis. 2017, 216 (Suppl. S3), S474–S483. [Google Scholar] [CrossRef] [PubMed]
- Bupha-Intr, O.; Butters, C.; Reynolds, G.; Kennedy, K.; Meyer, W.; Patil, S.; Bryant, P.; Morrissey, C.O.; Australasian Antifungal Guidelines Steering Committee. Consensus guidelines for the diagnosis and management of invasive fungal disease due to moulds other than Aspergillus in the haematology/oncology setting, 2021. Intern. Med. J. 2021, 51 (Suppl. S7), 177–219. [Google Scholar] [CrossRef] [PubMed]
- Cortez, K.J.; Roilides, E.; Quiroz-Telles, F.; Meletiadis, J.; Antachopoulos, C.; Knudsen, T.; Buchanan, W.; Milanovich, J.; Sutton, D.A.; Fothergill, A.; et al. Infections caused by Scedosporium spp. Clin. Microbiol. Rev. 2008, 21, 157–197. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lamaris, G.A.; Chamilos, G.; Lewis, R.E.; Safdar, A.; Raad, I.I.; Kontoyiannis, D.P. Scedosporium infection in a tertiary care cancer center: A review of 25 cases from 1989–2006. Clin. Infect. Dis. 2006, 43, 1580–1584. [Google Scholar] [CrossRef]
- Seidel, D.; Meißner, A.; Lackner, M.; Piepenbrock, E.; Salmanton-García, J.; Stecher, M.; Mellinghoff, S.; Hamprecht, A.; Durán Graeff, L.; Köhler, P.; et al. Prognostic factors in 264 adults with invasive Scedosporium spp. and Lomentospora prolificans infection reported in the literature and FungiScope®. Crit. Rev. Microbiol. 2019, 45, 1–21, Erratum in: Crit. Rev. Microbiol. 2019, 45, 238. [Google Scholar] [CrossRef] [PubMed]
- Boutin, C.A.; Luong, M.L. Update on therapeutic approaches for invasive fungal infections in adults. Ther. Adv. Infect. Dis. 2024, 11, 20499361231224980. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Bellio, P.; Fagnani, L.; Nazzicone, L.; Celenza, G. New and simplified method for drug combination studies by checkerboard assay. MethodsX 2021, 8, 101543. [Google Scholar] [CrossRef]
- Husain, S.; Muñoz, P.; Forrest, G.; Alexander, B.D.; Somani, J.; Brennan, K.; Wagener, M.M.; Singh, N. Infections due to Scedosporium apiospermum and Scedosporium prolificans in transplant recipients: Clinical characteristics and impact of antifungal agent therapy on outcome. Clin. Infect. Dis. 2005, 40, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Papadopoulos, E.B.; Young, J.W. Breakthrough Scedosporium apiospermum (Pseudallescheria boydii) brain abscess during therapy for invasive pulmonary aspergillosis following high-risk allogeneic hematopoietic stem cell transplantation. Scedosporiasis and recent advances in anti-fungal therapy. Transpl. Infect. Dis. 2002, 4, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Marques, D.S.; Pinho Vaz, C.; Branca, R.; Campilho, F.; Lamelas, C.; Afonso, L.P.; Jacome, M.; Breda, E.; Monteiro, E.; Campos Júnior, A. Rhizomucor and Scedosporium infection post hematopoietic stem-cell transplant. Case Rep. Med. 2011, 2011, 830769. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marinovic, D.A.; Bhaimia, E.; Forrest, G.N.; LaRue, R.; Nathan, S.; Ustun, C.; Ward, A. Scedosporium infection disseminated “from toe to head” in allogeneic stem cell transplant recipient: A case report. BMC Infect. Dis. 2023, 23, 353. [Google Scholar] [CrossRef]
- Parta, M.; Hilligoss, D.; Kelly, C.; Kwatemaa, N.; Theobald, N.; Malech, H.; Kang, E.M. Haploidentical Hematopoietic Cell Transplantation with Post-Transplant Cyclophosphamide in a Patient with Chronic Granulomatous Disease and Active Infection: A First Report. J. Clin. Immunol. 2015, 35, 675–680. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stoyanova, E.I.; Riemens, A.; Lokhorst, H.M.; te Boome, L.; Rothova, A. Absence of intraocular infections after hematopoietic stem cell transplantation at a single center: The experience with current preventive regimens. Ocul. Immunol. Inflamm. 2014, 22, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Narita, K.; Ikeda, D.; Matsue, K. Scedosporium endophthalmitis in a patient with second allogeneic stem cell transplantation for acute myeloid leukemia. Transpl. Infect. Dis. 2024, 27, e14397. [Google Scholar] [CrossRef] [PubMed]
- Bassiri-Jahromi, S.; Doostkam, A. Fungal infection and increased mortality in patients with chronic granulomatous disease. J. Mycol. Med. 2012, 22, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Leiding, J.W.; Arnold, D.E.; Parikh, S.H.; Logan, B.R.; Marsh, R.A.; Griffith, L.M.; Wu, R.; Kidd, S.; Mallhi, K.K.; Chellapandian, D.; et al. Genotype, oxidase status, and preceding infection or autoinflammation do not affect allogeneic HCT outcomes for CGD. Blood 2023, 142, 2105–2118. [Google Scholar] [CrossRef] [PubMed]
- Güngör, T. HCT alleviates disease burden in CGD. Blood 2023, 142, 2043–2045. [Google Scholar] [CrossRef] [PubMed]
- Lamoth, F.; Nucci, M.; Fernandez-Cruz, A.; Azoulay, E.; Lanternier, F.; Bremerich, J.; Einsele, H.; Johnson, E.; Lehrnbecher, T.; Mercier, T.; et al. Performance of the beta-glucan test for the diagnosis of invasive fusariosis and scedosporiosis: A meta-analysis. Med. Mycol. 2023, 61, myad061. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoenigl, M.; Salmanton-García, J.; Walsh, T.J.; Nucci, M.; Neoh, C.F.; Jenks, J.D.; Lackner, M.; Sprute, R.; Al-Hatmi, A.M.S.; Bassetti, M.; et al. Global guideline for the diagnosis and management of rare mould infections: An initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect. Dis. 2021, 21, e246–e257, Erratum in: Lancet Infect. Dis. 2021, 21, e81. [Google Scholar] [CrossRef] [PubMed]

| Drug, Dose | Timing | |
|---|---|---|
| First HCT | ||
| Conditioning regimen | Busulfan, 1 mg/kg/dose for 16 doses over 5 days | From day −11 to day −7 |
| Fludarabine, 40 mg/m2/day for 4 days | From day −2 to −5 | |
| ATG Genzyme®, 1.5 mg/kg/dose for 6 doses in total | From day −7 to day −3 | |
| GVHD prophylaxis | Cyclophosphamide, 50 mg/kg | Days +4 and +5 post-HCT |
| Cyclosporine, 3 mg/kg/day | From day +5 | |
| Mycophenolate mofetil, 60 mg/kg/day in 3 doses | From day +5 | |
| Second HCT | ||
| Conditioning regimen | Total body irradiation, 4 Gy, single fraction | Day −7 |
| Fludarabine, 35 mg/m2/day | From day −6 to day −3 | |
| Cyclophosphamide, 30 mg/kg/day | From day −6 to day −3 | |
| ATG Neovii®, 12.5 mg/kg/day | Days −2 and −3 | |
| GVHD prophylaxis | Mycophenolate mofetil, 60 mg/kg/day | From day +1 |
| Tacrolimus, 0.1 mg/kg intravenously, modulated according to blood levels | From day −1 |
| MIC (A) | MIC (B) | Antifungal Drug |
|---|---|---|
| 0.25 | 0.25 | Voriconazole |
| 0.5 | 1 | Posaconazole |
| 1 | 1 | Itraconazole |
| 1 | 2 | Isavuconazole |
| 8 | 8 | Amphotericin |
| 8 | 8 | Micafungin |
| Reference | Outcome | Treatment | Organ Involvement | Age (Years) | N. of Cases |
|---|---|---|---|---|---|
| [10] | Mortality rate 68% | AmB/ICZ/VCZ | CNS 36% Pulmonary 40% Skin 38% Blood 25% Disseminated 69% | (mean +/− SD) 32.9 +/− 3.3 | 23 (of which 9 L. prolificans) |
| [11] | Died | AmB | CNS | 34 | 1 |
| [12] | Died | PCZ + AmB | Rhinoencephalitis | 17 | 1 |
| [13] | Survived | VCZ + AmB | Disseminated Pulmonary CNS | 65 | 1 |
| [15] | Survived | VCZ + AmB | Ocular Bone Heart CNS | 59 | 1 |
| [16] | Died | Intravitreal VCZ | Ocular CNS | 58 | 1 |
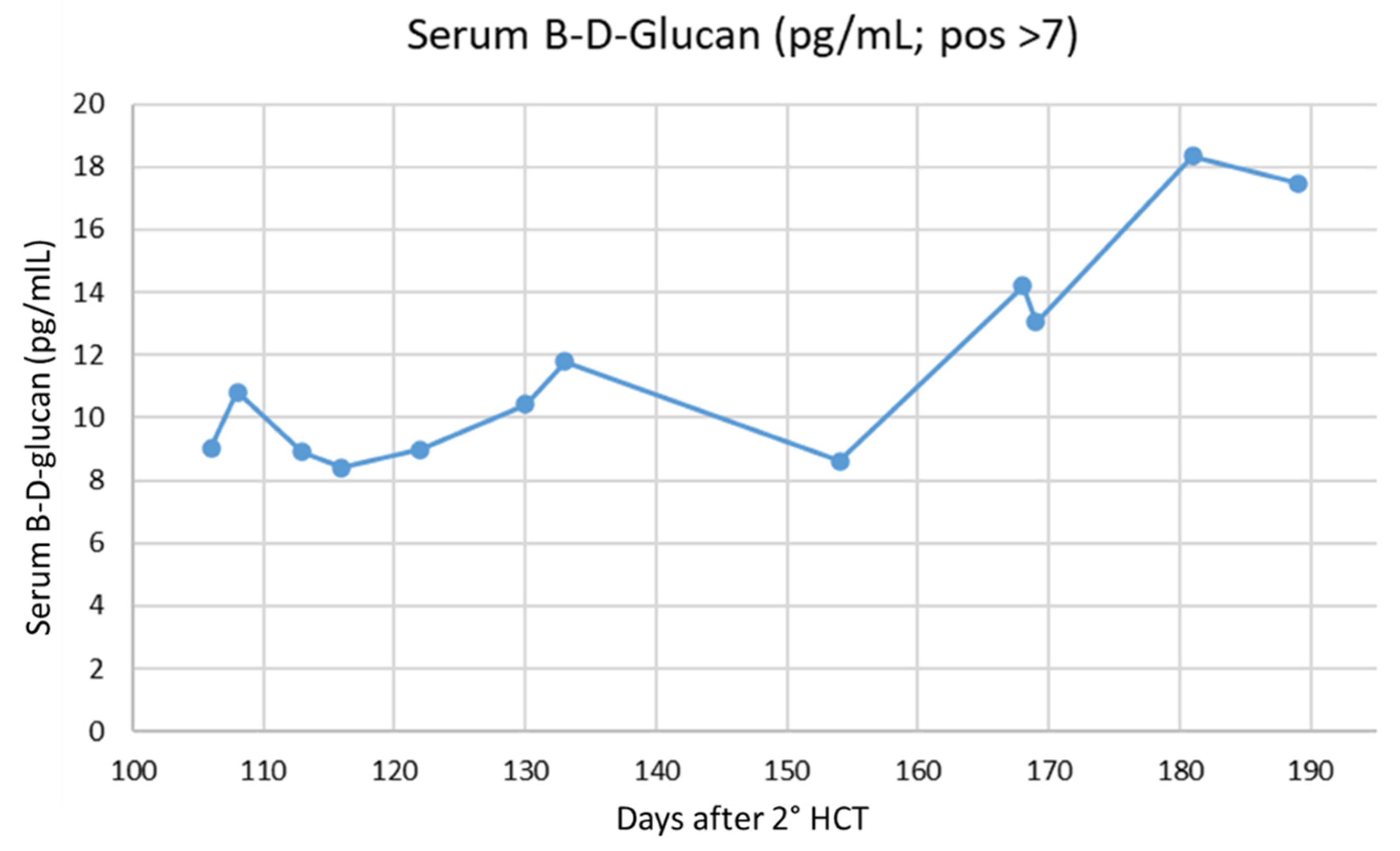
| BDG (pg/mL, Pos > 7) | Days Post-2nd HCT |
|---|---|
| 7.35 | 114 |
| 3 | 126 |
| 173 | 172 |
| 26.4 | 189 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garonzi, C.; Chinello, M.; Caddeo, G.; Bonetti, E.; Esposto, M.P.; Pezzella, V.; Vitale, V.; Zaccaron, A.; Sorrentino, A.; Gibellini, D.; et al. Invasive Fungal Infection by Scedosporium apiospermum with Cerebral Involvement in a Pediatric Patient Affected by Chronic Granulomatous Disease After Hematopoietic Cell Transplant. J. Fungi 2025, 11, 270. https://doi.org/10.3390/jof11040270
Garonzi C, Chinello M, Caddeo G, Bonetti E, Esposto MP, Pezzella V, Vitale V, Zaccaron A, Sorrentino A, Gibellini D, et al. Invasive Fungal Infection by Scedosporium apiospermum with Cerebral Involvement in a Pediatric Patient Affected by Chronic Granulomatous Disease After Hematopoietic Cell Transplant. Journal of Fungi. 2025; 11(4):270. https://doi.org/10.3390/jof11040270
Chicago/Turabian StyleGaronzi, Chiara, Matteo Chinello, Giulia Caddeo, Elisa Bonetti, Maria Pia Esposto, Vincenza Pezzella, Virginia Vitale, Ada Zaccaron, Annarita Sorrentino, Davide Gibellini, and et al. 2025. "Invasive Fungal Infection by Scedosporium apiospermum with Cerebral Involvement in a Pediatric Patient Affected by Chronic Granulomatous Disease After Hematopoietic Cell Transplant" Journal of Fungi 11, no. 4: 270. https://doi.org/10.3390/jof11040270
APA StyleGaronzi, C., Chinello, M., Caddeo, G., Bonetti, E., Esposto, M. P., Pezzella, V., Vitale, V., Zaccaron, A., Sorrentino, A., Gibellini, D., & Cesaro, S. (2025). Invasive Fungal Infection by Scedosporium apiospermum with Cerebral Involvement in a Pediatric Patient Affected by Chronic Granulomatous Disease After Hematopoietic Cell Transplant. Journal of Fungi, 11(4), 270. https://doi.org/10.3390/jof11040270








