RNA-Seq Insight into the Impact and Mechanisms of Methyl Donor and Glycine Betaine Osmoprotectant on Polyketide Secondary Metabolism in Monascus purpureus M1
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Medium
2.3. STRAIN Activation and Fermentation
2.4. Analysis of Pigment Production
2.5. HPLC Analysis of MK Production
2.6. SEM Analysis of Monascus Mycelium and Spores
2.7. RT-qPCR Analysis of MPs and MK Biosynthesis-Related Genes
2.8. RNA-Seq Data Analysis
2.9. PPI Network Construction and the Identification of Hub Genes
2.10. Statistical Analysis
3. Results
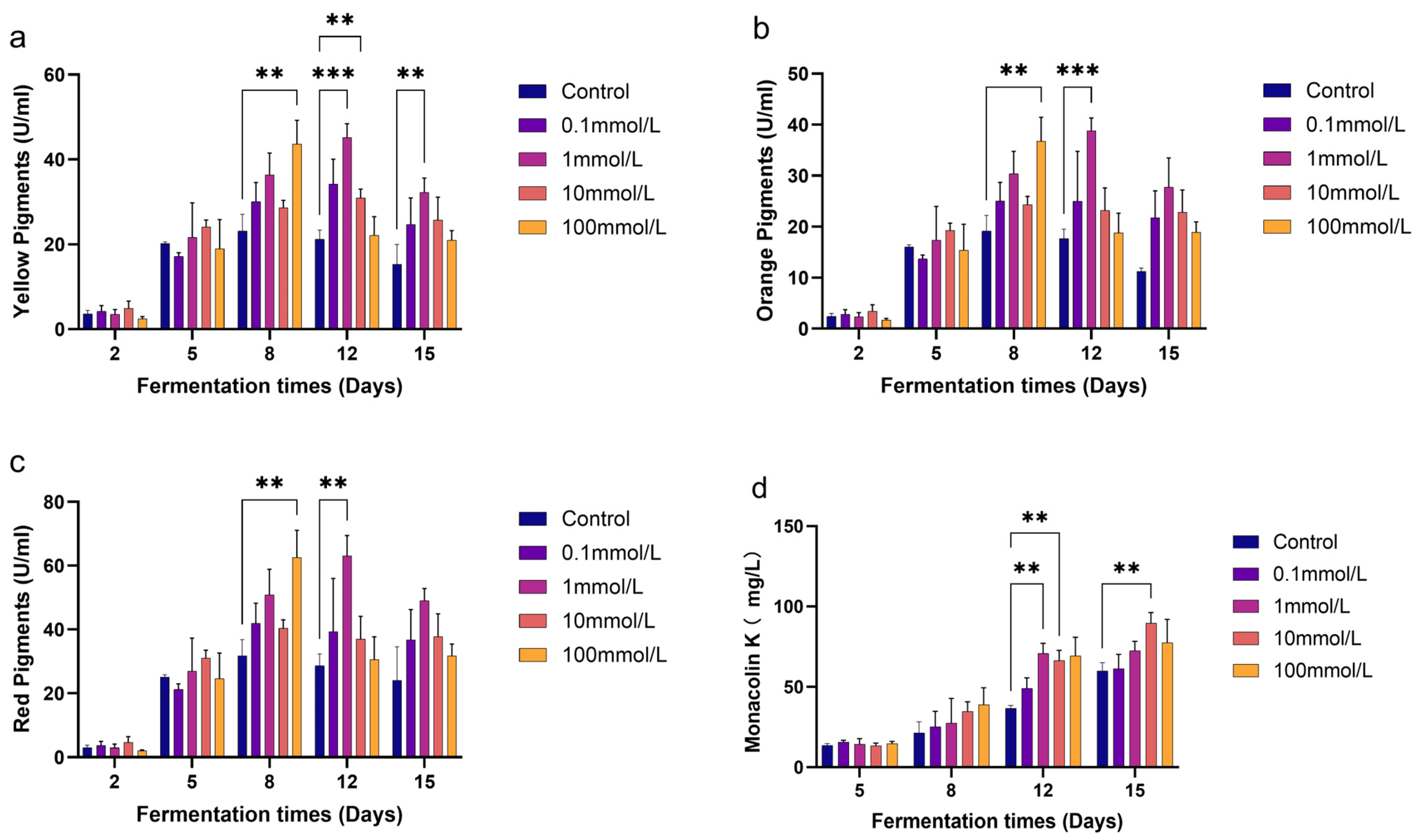
3.1. Effects of GB on the Production of MPs and MK
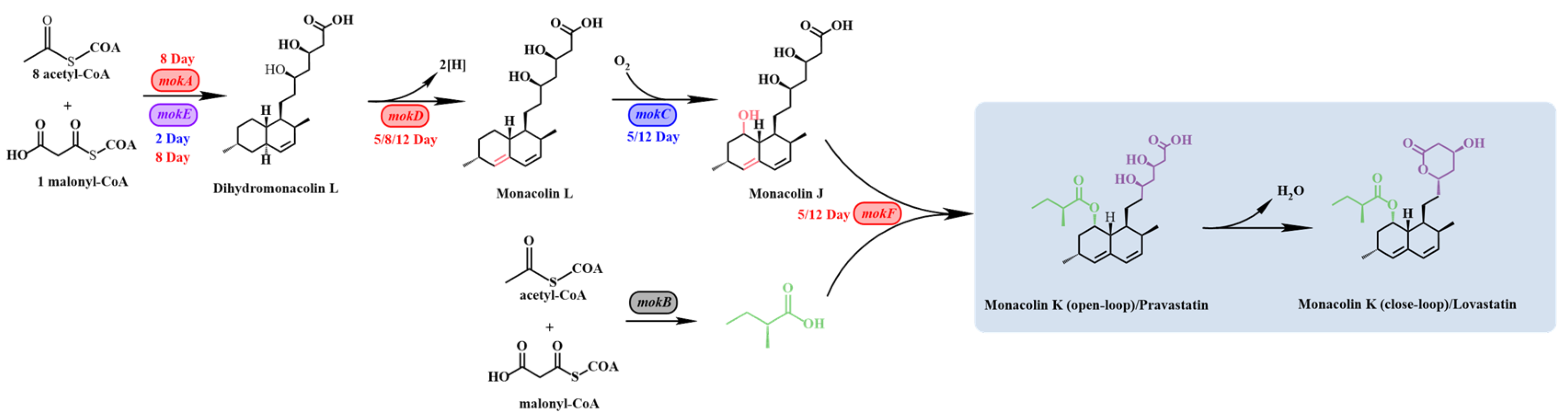
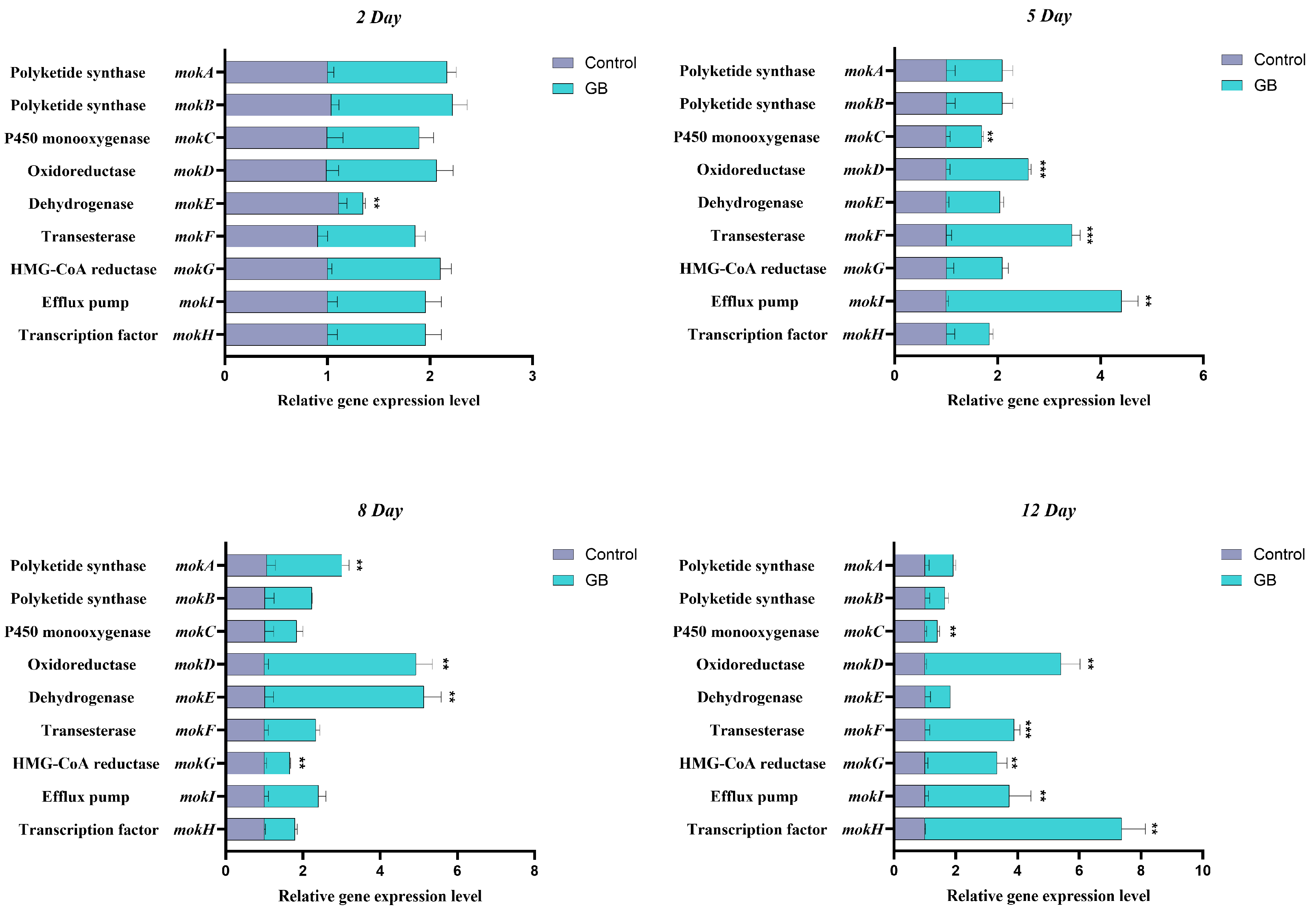
3.2. RT-qPCR Analysis of MK Biosynthesis-Related Genes
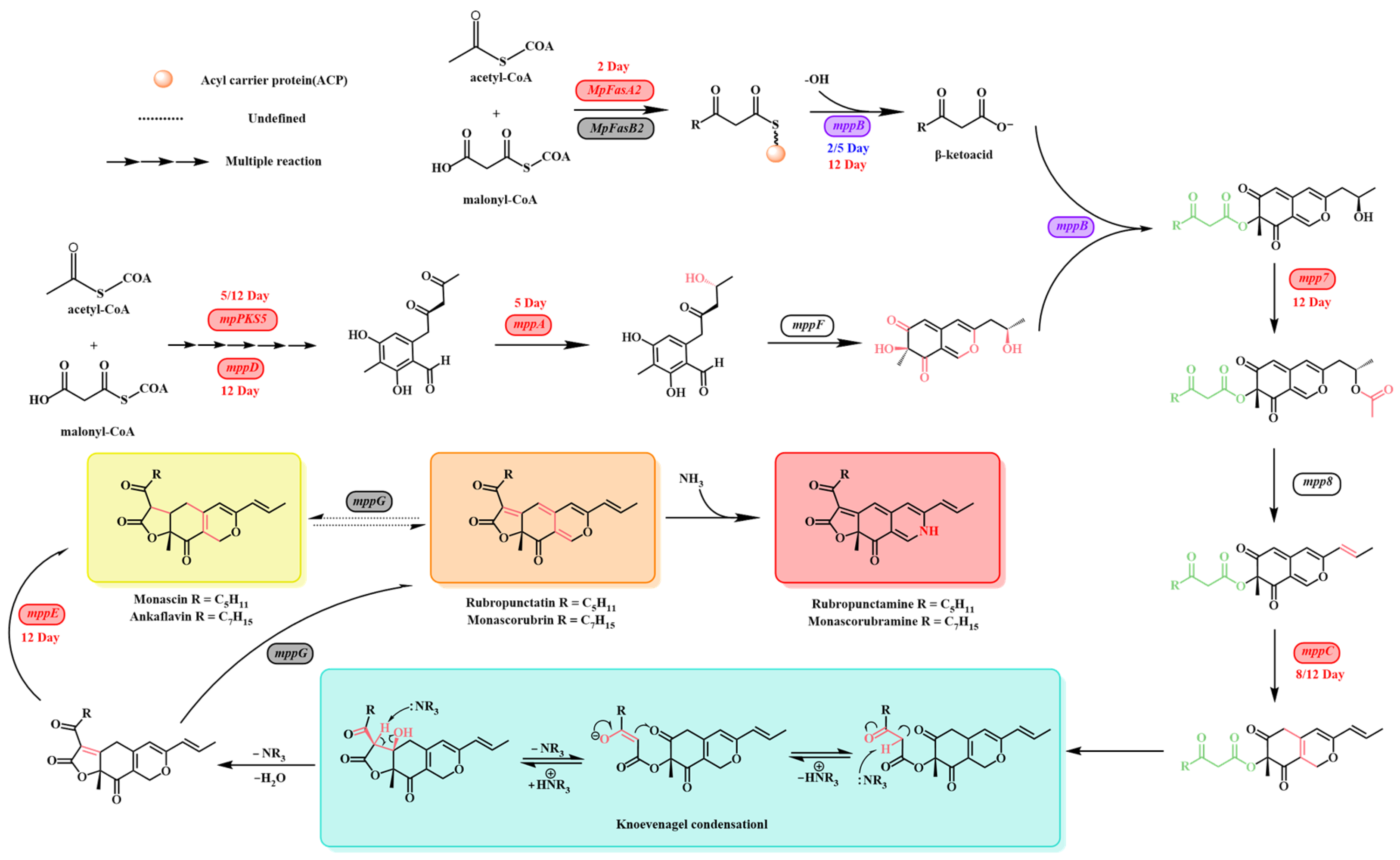
3.3. RT-qPCR Analysis of MPs Biosynthesis-Related Genes
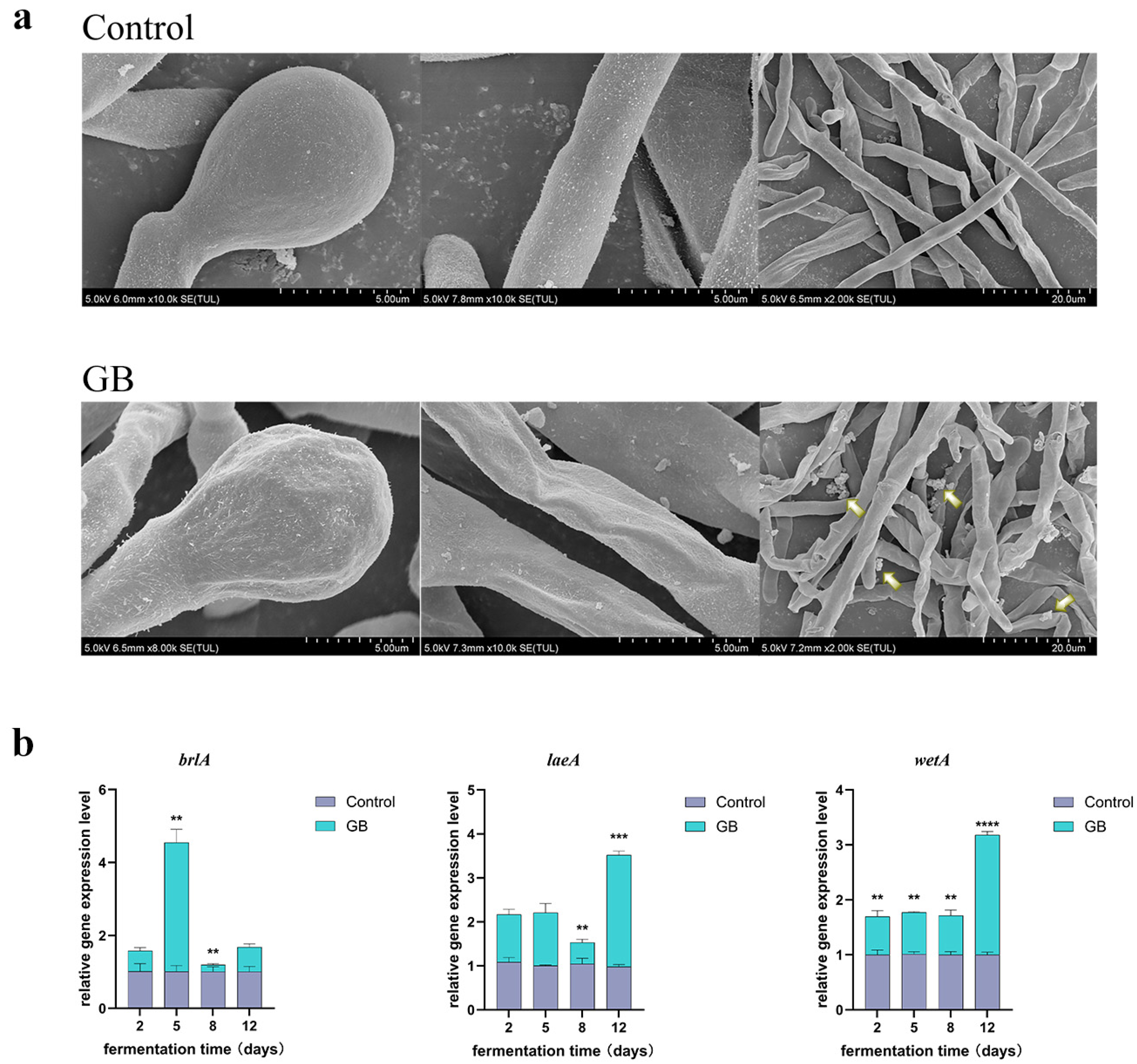
3.4. Effects of GB on the Morphology of Monascus Spores and Mycelia
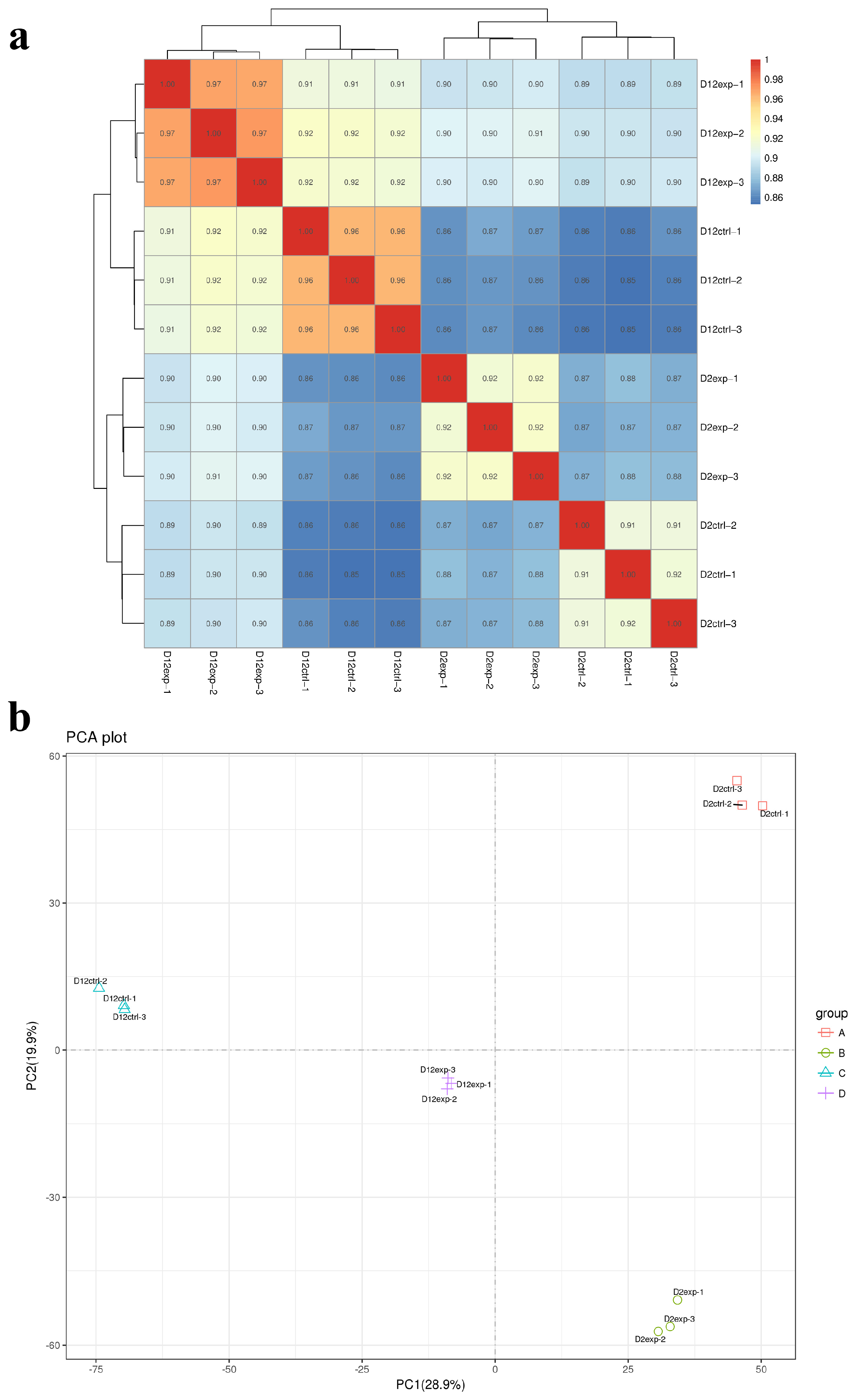
3.5. RNA-Seq Correlation Check and Principal Component Analysis
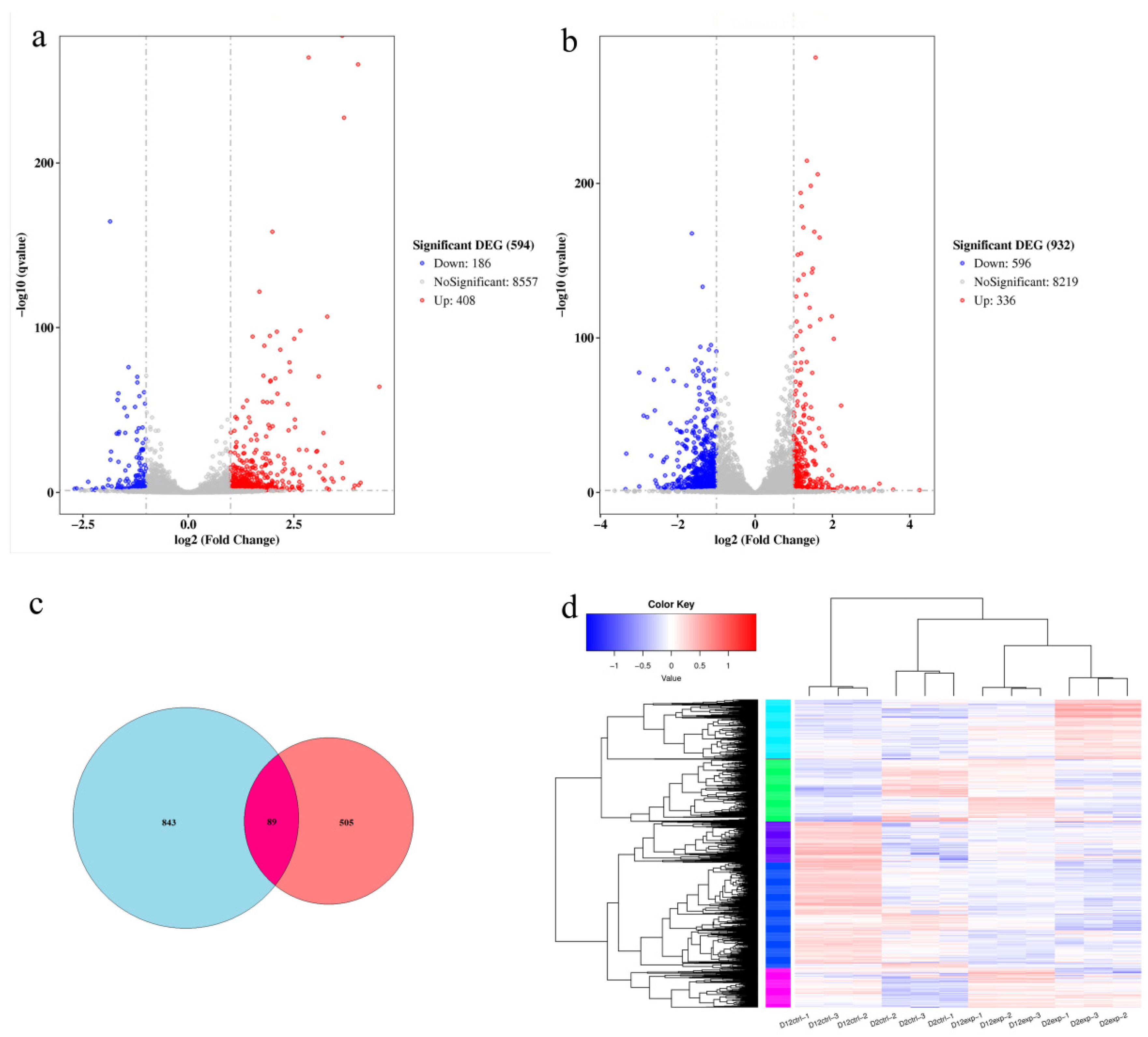
3.6. Comparison of Differential Genes
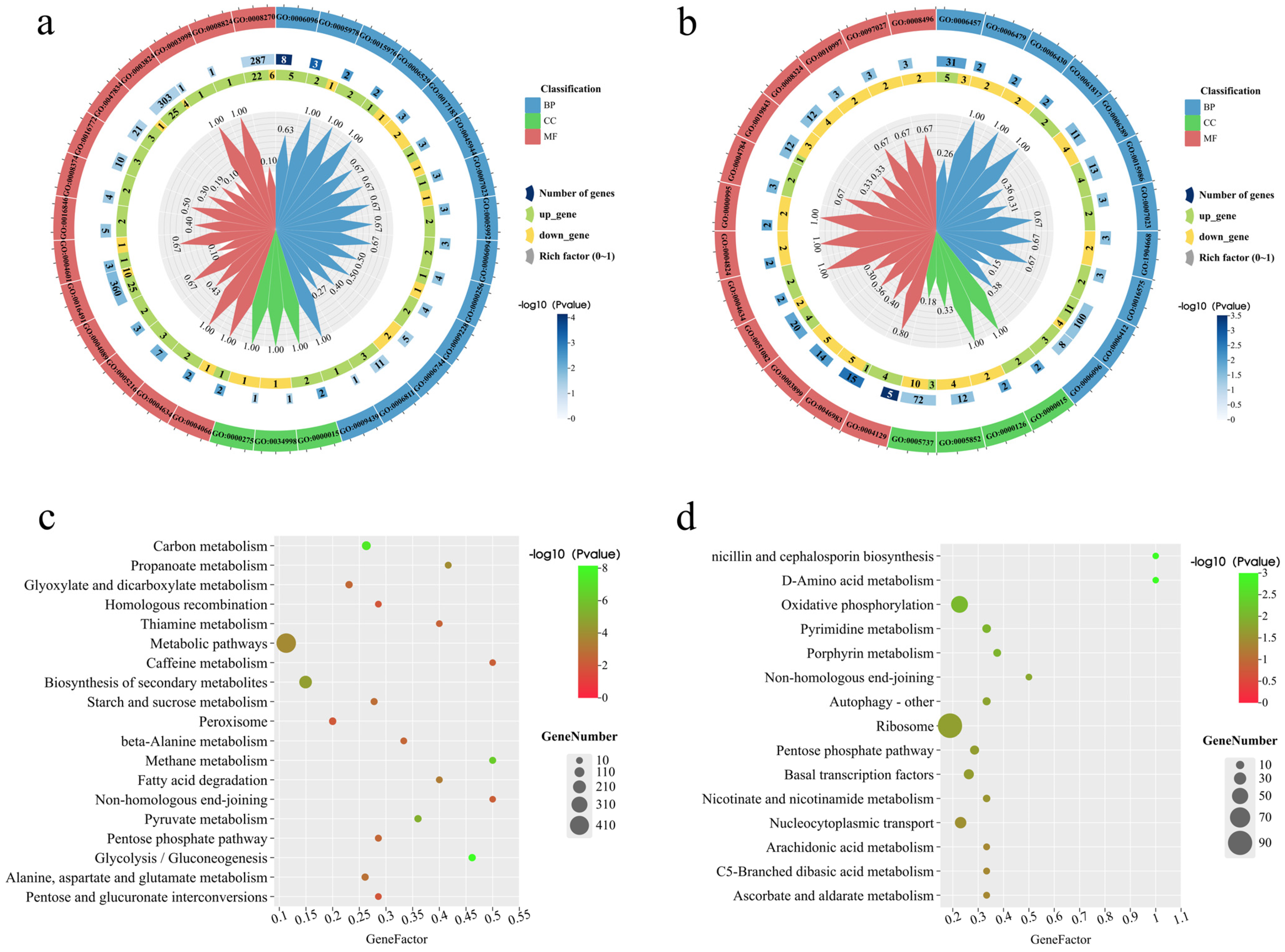
3.7. Functional Enrichment Analysis of DEGs
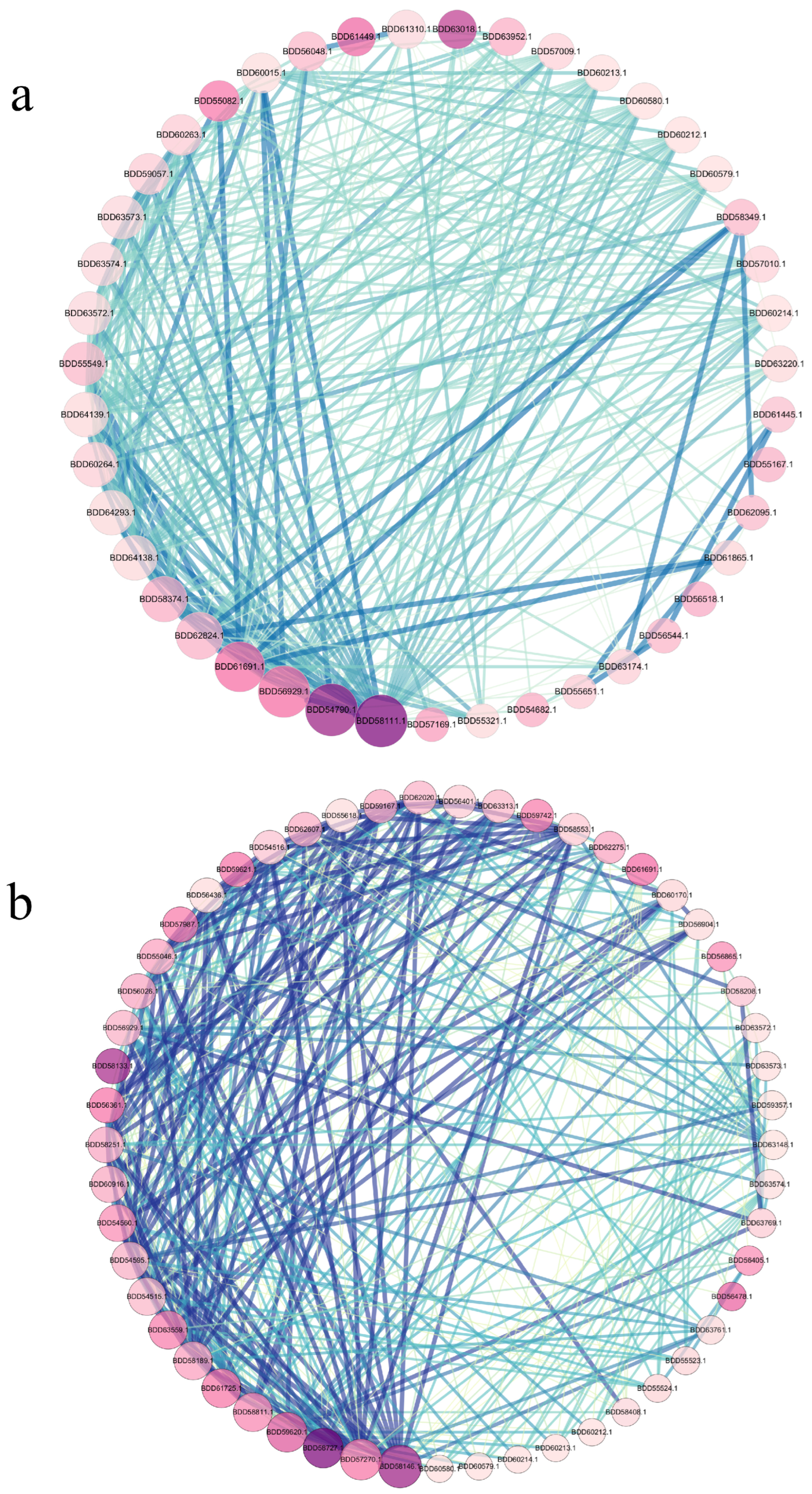
3.8. Identification of Key Genes in the PPI (Protein–Protein Interaction) Network
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pitt, J. Fungi and Food Spoilage; Springer: Cham, Switzerland, 2009. [Google Scholar]
- Zhu, B.; Qi, F.; Wu, J.; Yin, G.; Hua, J.; Zhang, Q.; Qin, L. Red Yeast Rice: A Systematic Review of the Traditional Uses, Chemistry, Pharmacology, and Quality Control of an Important Chinese Folk Medicine. Front. Pharmacol. 2019, 10, 1449. [Google Scholar] [CrossRef]
- Xu, D.; Xie, J.; Feng, X.; Zhang, X.; Ren, Z.; Zheng, Y.; Yang, J. Preparation and evaluation of a Rubropunctatin-loaded liposome anticancer drug carrier. RSC Adv. 2020, 10, 10352–10360. [Google Scholar] [CrossRef]
- Abdel-Raheam, H.E.F.; Alrumman, S.A.; Gadow, S.I.; El-Sayed, M.H.; Hikal, D.M.; Hesham, A.E.-L.; Ali, M.M.A. Optimization of Monascus purpureus for Natural Food Pigments Production on Potato Wastes and Their Application in Ice Lolly. Front. Microbiol. 2022, 13, 862080. [Google Scholar] [CrossRef]
- Liu, L.; Wu, S.; Wang, W.; Zhang, X.; Wang, Z. Sulfonation of Monascus pigments to produce water-soluble yellow pigments. Dyes Pigment. 2020, 173, 107965. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Zambon, A. Red Yeast Rice for Hypercholesterolemia: JACC Focus Seminar. J. Am. Coll. Cardiol. 2021, 77, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kang, W.; Lu, X.; Ma, S.; Dong, L.; Zou, B. Weighted gene co-expression network analysis and connectivity map identifies lovastatin as a treatment option of gastric cancer by inhibiting HDAC2. Gene 2019, 681, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Liu, T.-Y.; Huang, S.-P.; Ho, C.-T.; Huang, T.-C. Differentiation and apoptosis induction by lovastatin and γ-tocotrienol in HL-60 cells via Ras/ERK/NF-κB and Ras/Akt/NF-κB signaling dependent down-regulation of glyoxalase 1 and HMG-CoA reductase. Cell. Signal. 2015, 27, 2182–2190. [Google Scholar] [CrossRef]
- Divsalar, P.; Noorbala, A.A.; Moazen-Zadeh, E.; Jafarinia, M.; Shakiba, M.; Shahmansouri, N.; Ghazizadeh-Hashemi, M.; Etesam, F.; Akhondzadeh, S. Red yeast rice as an adjunct to sertraline for treatment of depression in patients with percutaneous coronary intervention: Placebo-controlled trial. Adv. Integr. Med. 2018, 5, 69–74. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, N.; Chen, M.; Wang, H.; Shi, J.; Wang, B.; Sun, B.; Wang, C. Metabolomics Analysis of the Effect of Glutamic Acid on Monacolin K Synthesis in Monascus purpureus. Front. Microbiol. 2020, 11, 610471. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, M.; Zang, Y.; Wang, H.; Wei, X.; Zhu, Q.; Yang, X.; Sun, B.; Wang, C. Effect of arginine supplementation on Monacolin K yield of Monascus purpureus. J. Food Compos. Anal. 2022, 106, 104252. [Google Scholar] [CrossRef]
- Tan, H.; Xing, Z.; Chen, G.; Tian, X.; Wu, Z. Evaluating Antitumor and Antioxidant Activities of Yellow Monascus Pigments from Monascus ruber Fermentation. Molecules 2018, 23, 3242. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-Z.; Jiang, W.; Zhu, Y.-Y.; Wang, C.-Z.; Zhong, W.-H.; Wu, G.; Chen, J.; Zhu, M.-N.; Wu, Q.-L.; Du, X.-L.; et al. Highland barley Monascus purpureus Went extract ameliorates high-fat, high-fructose, high-cholesterol diet induced nonalcoholic fatty liver disease by regulating lipid metabolism in golden hamsters. J. Ethnopharmacol. 2022, 286, 114922. [Google Scholar] [CrossRef] [PubMed]
- Mohankumari, H.P.; Naidu, K.A.; Narasimhamurthy, K.; Vijayalakshmi, G. Bioactive Pigments of Monascus purpureus Attributed to Antioxidant, HMG-CoA Reductase Inhibition and Anti-atherogenic Functions. Front. Sustain. Food Syst. 2021, 5, 590427. [Google Scholar] [CrossRef]
- El-Sayed, E.-S.R.; Abdelhakim, H.K.; Zakaria, Z. Extracellular biosynthesis of cobalt ferrite nanoparticles by Monascus purpureus and their antioxidant, anticancer and antimicrobial activities: Yield enhancement by gamma irradiation. Mater. Sci. Eng. C 2020, 107, 110318. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.L.; Lu, L.P.; Zhang, B.B.; Xu, G.R. Enhanced production of Monacolin K by addition of precursors and surfactants in submerged fermentation of Monascus purpureus 9901. Biotechnol. Appl. Biochem. 2014, 61, 202–207. [Google Scholar] [CrossRef]
- Huang, J.; Liao, N.; Li, H. Linoleic acid enhance the production of moncolin K and red pigments in Monascus ruber by activating mokH and mokA, and by accelerating cAMP-PkA pathway. Int. J. Biol. Macromol. 2018, 109, 950–954. [Google Scholar] [CrossRef]
- Shi, R.; Luo, Q.; Liu, Y.; Meng, G.; Chen, W.; Wang, C. Effect of γ-butyrolactone, a quorum sensing molecule, on morphology and secondary metabolism in Monascus. Lwt 2022, 172, 114225. [Google Scholar] [CrossRef]
- Yin, S.; Zhu, Y.; Zhang, B.; Huang, B.; Jia, R. Diverse Effects of Amino Acids on Monascus Pigments Biosynthesis in Monascus purpureus. Front. Microbiol. 2022, 13, 951266. [Google Scholar] [CrossRef]
- Zou, H.; Chen, N.; Shi, M.; Xian, M.; Song, Y.; Liu, J. The metabolism and biotechnological application of betaine in microorganism. Appl. Microbiol. Biotechnol. 2016, 100, 3865–3876. [Google Scholar] [CrossRef]
- Craig, S.A.S. Betaine in human nutrition. Am. J. Clin. Nutr. 2004, 80, 539–549. [Google Scholar] [CrossRef]
- Kettunen, H.; Tiihonen, K.; Peuranen, S.; Saarinen, M.T.; Remus, J.C. Dietary betaine accumulates in the liver and intestinal tissue and stabilizes the intestinal epithelial structure in healthy and coccidia-infected broiler chicks. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 130, 759–769. [Google Scholar] [CrossRef]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.-Y. Production of betalains in plant cell and organ cultures: A review. Plant Cell Tissue Organ Cult. (PCTOC) 2024, 158, 28. [Google Scholar] [CrossRef]
- Xu, K.; Xu, P. Betaine and Beet Molasses Enhance L-Lactic Acid Production by Bacillus coagulans. PLoS ONE 2014, 9, e100731. [Google Scholar] [CrossRef]
- Zou, H.; Wu, Z.; Xian, M.; Liu, H.; Cheng, T.; Cao, Y. Not only osmoprotectant: Betaine increased lactate dehydrogenase activity and l-lactate production in lactobacilli. Bioresour. Technol. 2013, 148, 591–595. [Google Scholar] [CrossRef]
- Xu, J.; Xia, X.; Zhang, J.; Guo, Y.; Zhang, W. An overlooked effect of glycine betaine on fermentation: Prevents caramelization and increases the L-lysine production. J. Microbiol. Biotechnol. 2014, 24, 1368–1376. [Google Scholar] [CrossRef]
- Kun-Tai, L.; Dong-Hong, L.; Yong-Liang, L.; Ju, C.; Yong-Hong, W.; Ying-Ping, Z.; Si-Liang, Z. Improved large-scale production of vitamin B12 by Pseudomonas denitrificans with betaine feeding. Bioresour. Technol. 2008, 99, 8516–8520. [Google Scholar] [CrossRef]
- Oren, A.; Elevi Bardavid, R.; Kandel, N.; Aizenshtat, Z.; Jehlička, J. Glycine betaine is the main organic osmotic solute in a stratified microbial community in a hypersaline evaporitic gypsum crust. Extremophiles 2013, 17, 445–451. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Sota, M.; Koumoto, K. Cryoprotective ability of betaine-type metabolite analogs during freezing denaturation of enzymes. Biotechnol. Lett. 2015, 37, 1607–1613. [Google Scholar] [CrossRef]
- Hu, X.; Li, D.; Qiao, Y.; Song, Q.; Guan, Z.; Qiu, K.; Cao, J.; Huang, L. Salt tolerance mechanism of a hydrocarbon-degrading strain: Salt tolerance mediated by accumulated betaine in cells. J. Hazard. Mater. 2020, 392, 122326. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; Fang, S.; Yang, X.; Feng, J. Betaine Improves Intestinal Functions by Enhancing Digestive Enzymes, Ameliorating Intestinal Morphology, and Enriching Intestinal Microbiota in High-salt stressed Rats. Nutrients 2018, 10, 907. [Google Scholar] [CrossRef]
- Tang, D.; Wang, X.; Wang, J.; Wang, M.; Wang, Y.; Wang, W. Choline–betaine pathway contributes to hyperosmotic stress and subsequent lethal stress resistance in Pseudomonas protegens SN15-2. J. Biosci. 2020, 45, 85. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Yuan, G.-F.; Hsieh, S.-Y.; Lin, Y.-S.; Wang, W.-Y.; Liaw, L.-L.; Tseng, C.-P. Identification of the mokH Gene Encoding Transcription Factor for the Upregulation of Monacolin K Biosynthesis in Monascus pilosus. J. Agric. Food Chem. 2009, 58, 287–293. [Google Scholar] [CrossRef]
- Shi, R.; Luo, Q.; Liu, Y.; Chen, W.; Wang, C. Effect of γ-Heptalactone on the Morphology and Production of Monascus Pigments and Monacolin K in Monascus purpureus. J. Fungi 2022, 8, 179. [Google Scholar] [CrossRef]
- Yang, X.; Xiang, L.; Zhang, C.; Cao, Y.; Wang, C. Promotion of monacolin K production in Monascus extractive fermentation: The variation in fungal morphology and in the expression levels of biosynthetic gene clusters. J. Sci. Food Agric. 2021, 101, 5652–5659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liang, J.; Zhang, A.; Hao, S.; Zhang, H.; Zhu, Q.; Sun, B.; Wang, C. Overexpression of Monacolin K Biosynthesis Genes in the Monascus purpureus Azaphilone Polyketide Pathway. J. Agric. Food Chem. 2019, 67, 2563–2569. [Google Scholar] [CrossRef]
- Shi, R.; Gong, P.; Liu, Y.; Luo, Q.; Chen, W.; Wang, C. Linoleic acid functions as a quorum-sensing molecule in Monascus purpureus–Saccharomyces cerevisiae co-culture. Yeast 2023, 40, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Embaby, A.M.; Hussein, M.N.; Hussein, A. Monascus orange and red pigments production by Monascus purpureus ATCC16436 through co-solid state fermentation of corn cob and glycerol: An eco-friendly environmental low cost approach. PLoS ONE 2018, 13, e0207755. [Google Scholar] [CrossRef]
- Zhang, C.; Chai, S.; Hao, S.; Zhang, A.; Zhu, Q.; Zhang, H.; Wang, C. Effects of glutamic acid on the production of monacolin K in four high-yield monacolin K strains in Monascus. Appl. Microbiol. Biotechnol. 2019, 103, 5301–5310. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, H.; Zhu, Q.; Hao, S.; Chai, S.; Li, Y.; Jiao, Z.; Shi, J.; Sun, B.; Wang, C. Overexpression of global regulator LaeA increases secondary metabolite production in Monascus purpureus. Appl. Microbiol. Biotechnol. 2020, 104, 3049–3060. [Google Scholar] [CrossRef]
- Raman, K. Construction and analysis of protein–protein interaction networks. Autom. Exp. 2010, 2, 2. [Google Scholar] [CrossRef]
- Talebi, R.; Ahmadi, A.; Afraz, F. Analysis of protein-protein interaction network based on transcriptome profiling of ovine granulosa cells identifies candidate genes in cyclic recruitment of ovarian follicles. J. Anim. Sci. Technol. 2018, 60, 11. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.-H.; Chen, S.-H.; Wu, H.-H.; Ho, C.-W.; Ko, M.-T.; Lin, C.-Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef]
- Seo, C.H.; Kim, J.-R.; Kim, M.-S.; Cho, K.-H. Hub genes with positive feedbacks function as master switches in developmental gene regulatory networks. Bioinformatics 2009, 25, 1898–1904. [Google Scholar] [CrossRef]
- Liu, Q.; Lin, B.; Tao, Y. Improved methylation in E. coli via an efficient methyl supply system driven by betaine. Metab. Eng. 2022, 72, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.; Li, L.; Yan, L.; Ju, X.; Hu, C.; Yao, X. Enhancement of erythritol production in Trichosporonoides oedocephalis by regulating cellular morphology with betaine. Chem. Pap. 2019, 73, 2065–2072. [Google Scholar] [CrossRef]
- Lin, L.; Wu, S.; Li, Z.; Ren, Z.; Chen, M.; Wang, C. High Expression Level of mok E Enhances the Production of Monacolin K in Monascus. Food Biotechnol. 2018, 32, 35–46. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Tseng, C.-P.; Liaw, L.-L.; Wang, C.-L.; Chen, I.C.; Wu, W.-J.; Wu, M.-D.; Yuan, G.-F. Cloning and Characterization of Monacolin K Biosynthetic Gene Cluster from Monascus pilosus. J. Agric. Food Chem. 2008, 56, 5639–5646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhu, Q.; Zhang, H.; Zhang, N.; Yang, X.; Shi, J.; Sun, B.; Wang, C. Effects on the sporulation and secondary metabolism yields of Monascus purpureus with mokH gene deletion and overexpression. Fungal Biol. 2020, 124, 661–670. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, J.; Yang, L.; Sun, B.; Wang, C. De Novo RNA Sequencing and Transcriptome Analysis of Monascus purpureus and Analysis of Key Genes Involved in Monacolin K Biosynthesis. PLoS ONE 2017, 12, e0170149. [Google Scholar] [CrossRef]
- Bizukojc, M.; Ledakowicz, S. Physiological, morphological and kinetic aspects of lovastatin biosynthesis by Aspergillus terreus. Biotechnol. J. 2009, 4, 647–664. [Google Scholar] [CrossRef]
- Kennedy, J.; Auclair, K.; Kendrew, S.G.; Park, C.; Vederas, J.C.; Richard Hutchinson, C. Modulation of Polyketide Synthase Activity by Accessory Proteins During Lovastatin Biosynthesis. Science 1999, 284, 1368–1372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Z.; Wen, Q.; Xiong, Z.; Cao, X.; Zheng, Z.; Zhang, Y.; Huang, Z. An overview on the biosynthesis and metabolic regulation of monacolin K/lovastatin. Food Funct. 2020, 11, 5738–5748. [Google Scholar] [CrossRef]
- Xiong, Z.; Cao, X.; Wen, Q.; Chen, Z.; Cheng, Z.; Huang, X.; Zhang, Y.; Long, C.; Zhang, Y.; Huang, Z. An overview of the bioactivity of monacolin K/lovastatin. Food Chem. Toxicol. 2019, 131, 110585. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; He, Y.; Zhou, Y.; Shao, Y.; Feng, Y.; Li, M.; Chen, F. Edible Filamentous Fungi from the Species Monascus: Early Traditional Fermentations, Modern Molecular Biology, and Future Genomics. Compr. Rev. Food Sci. Food Saf. 2015, 14, 555–567. [Google Scholar] [CrossRef]
- Moore, R.N.; Bigam, G.; Chan, J.K.; Hogg, A.M.; Nakashima, T.T.; Vederas, J.C. Biosynthesis of the hypocholesterolemic agent mevinolin by Aspergillus terreus. Determination of the origin of carbon, hydrogen, and oxygen atoms by carbon-13 NMR and mass spectrometry. J. Am. Chem. Soc. 1985, 107, 3694–3701. [Google Scholar] [CrossRef]
- Xie, L.; Zhu, G.; Shang, J.; Chen, X.; Zhang, C.; Ji, X.; Zhang, Q.; Wei, Y. An overview on the biological activity and anti-cancer mechanism of lovastatin. Cell. Signal. 2021, 87, 110122. [Google Scholar] [CrossRef]
- Jirasatid, S.; Nopharatana, M.; Kitsubun, P.; Vichitsoonthonkul, T.; Tongta, A. Statistical optimization for Monacolin K and yellow pigment production and citrinin reduction by Monascus purpureus in solid-state fermentation. J. Microbiol. Biotechnol. 2013, 23, 364–374. [Google Scholar] [CrossRef]
- Hying, Z.T.; Miller, T.J.; Loh, C.Y.; Bazurto, J.V. Glycine betaine metabolism is enabled in Methylorubrum extorquens PA1 by alterations to dimethylglycine dehydrogenase. Appl. Environ. Microbiol. 2024, 90, e02090-23. [Google Scholar] [CrossRef]
- Boysen, A.K.; Durham, B.P.; Kumler, W.; Key, R.S.; Heal, K.R.; Carlson, L.T.; Groussman, R.D.; Armbrust, E.V.; Ingalls, A.E. Glycine betaine uptake and metabolism in marine microbial communities. Environ. Microbiol. 2022, 24, 2380–2403. [Google Scholar] [CrossRef]
- Chen, W.; Chen, R.; Liu, Q.; He, Y.; He, K.; Ding, X.; Kang, L.; Guo, X.; Xie, N.; Zhou, Y.; et al. Orange, red, yellow: Biosynthesis of azaphilone pigments in Monascus fungi. Chem. Sci. 2017, 8, 4917–4925. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Kim, H.-J.; Suh, J.-W.; Chen, C.-C.; Liu, K.-H.; Park, S.-H.; Kwon, H.-J. Monascus azaphilone pigment biosynthesis employs a dedicated fatty acid synthase for short chain fatty acyl moieties. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 191–196. [Google Scholar] [CrossRef]
- Zabala, A.O.; Xu, W.; Chooi, Y.-H.; Tang, Y. Characterization of a Silent Azaphilone Gene Cluster from Aspergillus niger ATCC 1015 Reveals a Hydroxylation-Mediated Pyran-Ring Formation. Chem. Biol. 2012, 19, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B.; Karki, S.; Chiu, S.-H.; Kim, H.-J.; Suh, J.-W.; Nam, B.; Yoon, Y.-M.; Chen, C.-C.; Kwon, H.-J. Genetic localization and in vivo characterization of a Monascus azaphilone pigment biosynthetic gene cluster. Appl. Microbiol. Biotechnol. 2013, 97, 6337–6345. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, Y.; Zhang, R.; Yu, J.; Ma, X.; Chen, M.; Wang, Y. Transcriptional regulation contributes more to Monascus pigments diversity in different strains than to DNA sequence variation. World J. Microbiol. Biotechnol. 2019, 35, 138. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, Y.; Yi, T.; Zhao, M.; Xie, N.; Lei, M.; Liu, Q.; Shao, Y.; Chen, F. Identification and role analysis of an intermediate produced by a polygenic mutant of Monascus pigments cluster in Monascus ruber M7. Appl. Microbiol. Biotechnol. 2016, 100, 7037–7049. [Google Scholar] [CrossRef]
- Bijinu, B.; Suh, J.-W.; Park, S.-H.; Kwon, H.-J. Delineating Monascus azaphilone pigment biosynthesis: Oxidoreductive modifications determine the ring cyclization pattern in azaphilone biosynthesis. RSC Adv. 2014, 4, 59405–59408. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Chen, C.-C.; Pan, T.-M.; Kwon, H.-J. Mpp7 controls regioselective Knoevenagel condensation during the biosynthesis of Monascus azaphilone pigments. Tetrahedron Lett. 2014, 55, 1640–1643. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Park, S.-H.; Kwon, H.-J. Inactivation of the oxidase gene mppG results in the selective loss of orange azaphilone pigments in Monascus purpureus. Appl. Biol. Chem. 2017, 60, 437–446. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Park, S.-H.; Kwon, H.-J. A reductase gene mppE controls yellow component production in azaphilone polyketide pathway of Monascus. Biotechnol. Lett. 2017, 39, 163–169. [Google Scholar] [CrossRef]
- Meng, F.; Bi, S.; Sun, Z.; Jiang, B.; Wu, D.; Chen, J.-S.; Zhang, F. Synthesis of Ionic Vinylene-Linked Covalent Organic Frameworks through Quaternization-Activated Knoevenagel Condensation. Angew. Chem. Int. Ed. 2021, 60, 13614–13620. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Y.; Jiang, W.; Zhu, C.; Yao, S. Transdermal release behaviors of bioactive deep eutectic solvents as natural skin care and mechanism. J. Mol. Liq. 2022, 367, 120412. [Google Scholar] [CrossRef]
- Li, T.; Zhao, W.; Wang, C.; Shi, K.; Chen, G. Regulated synthesis and metabolism of Monascus pigments in a unique environment. World J. Microbiol. Biotechnol. 2022, 39, 46. [Google Scholar] [CrossRef]
- Naresh, K.; Diksha, D. Recent Developments in Knoevenagel Condensation Reaction: A Review. J. Adv. Sci. Res. 2022, 13, 17–25. [Google Scholar] [CrossRef]
- Borkovich, K.; Ebbole, D.J. Cellular and Molecular Biology of Filamentous Fungi; American Society for Microbiology Press: Washington, DC, USA, 2010. [Google Scholar]
- Adams, T.H.; Boylan, M.T.; Timberlake, W.E. brlA is necessary and sufficient to direct conidiophore development in Aspergillus nidulans. Cell 1988, 54, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Sewall, T.C.; Mims, C.W.; Timberlake, W.E. Conidium differentiation in Aspergillus nidulans wild-type and wet-white (wetA) mutant strains. Dev. Biol. 1990, 138, 499–508. [Google Scholar] [CrossRef]
- Bok Jin, W.; Balajee, S.A.; Marr Kieren, A.; Andes, D.; Nielsen Kristian, F.; Frisvad Jens, C.; Keller Nancy, P. LaeA, a Regulator of Morphogenetic Fungal Virulence Factors. Eukaryot. Cell 2005, 4, 1574–1582. [Google Scholar] [CrossRef]
- Laksanalamai, P.; Robb, F.T. Small heat shock proteins from extremophiles: A review. Extremophiles 2004, 8, 1–11. [Google Scholar] [CrossRef]
- Deshaies, R.J.; Koch, B.D.; Schekman, R. The role of stress proteins in membrane biogenesis. Trends Biochem. Sci. 1988, 13, 384–388. [Google Scholar] [CrossRef]
- Ban, N.; Nissen, P.; Hansen, J.; Moore, P.B.; Steitz, T.A. The complete atomic structure of the large ribosomal subunit at 2.4 A resolution. Science 2000, 289, 905–920. [Google Scholar] [CrossRef]
- Lanier, K.A.; Roy, P.; Schneider, D.M.; Williams, L.D. Ancestral Interactions of Ribosomal RNA and Ribosomal Proteins. Biophys. J. 2017, 113, 268–276. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Zhang, H.; Xue, F.; Niu, L.; Qi, C.; Chen, W.; Zheng, J.; Wang, C. RNA-Seq Insight into the Impact and Mechanisms of Methyl Donor and Glycine Betaine Osmoprotectant on Polyketide Secondary Metabolism in Monascus purpureus M1. J. Fungi 2025, 11, 273. https://doi.org/10.3390/jof11040273
Liu Z, Zhang H, Xue F, Niu L, Qi C, Chen W, Zheng J, Wang C. RNA-Seq Insight into the Impact and Mechanisms of Methyl Donor and Glycine Betaine Osmoprotectant on Polyketide Secondary Metabolism in Monascus purpureus M1. Journal of Fungi. 2025; 11(4):273. https://doi.org/10.3390/jof11040273
Chicago/Turabian StyleLiu, Zheng, Haijing Zhang, Furong Xue, Lidan Niu, Chenchen Qi, Wei Chen, Jie Zheng, and Chengtao Wang. 2025. "RNA-Seq Insight into the Impact and Mechanisms of Methyl Donor and Glycine Betaine Osmoprotectant on Polyketide Secondary Metabolism in Monascus purpureus M1" Journal of Fungi 11, no. 4: 273. https://doi.org/10.3390/jof11040273
APA StyleLiu, Z., Zhang, H., Xue, F., Niu, L., Qi, C., Chen, W., Zheng, J., & Wang, C. (2025). RNA-Seq Insight into the Impact and Mechanisms of Methyl Donor and Glycine Betaine Osmoprotectant on Polyketide Secondary Metabolism in Monascus purpureus M1. Journal of Fungi, 11(4), 273. https://doi.org/10.3390/jof11040273