Evaluation of the DendrisKIT®DP for the Diagnosis of Superficial Fungal Infections
Abstract
1. Introduction
2. Materials and Methods
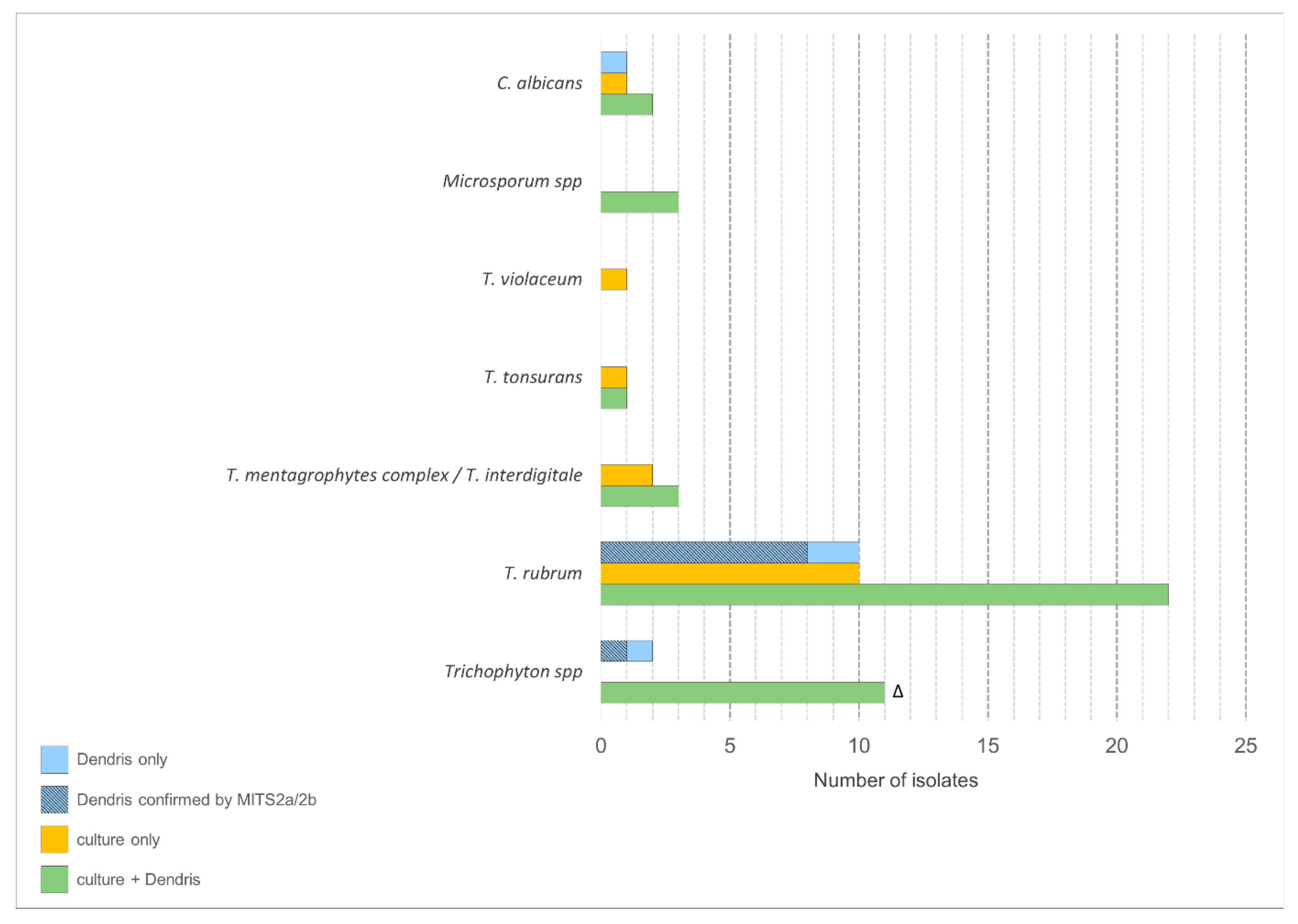
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Havlickova, B.; Czaika, V.A.; Friedrich, M. Epidemiological Trends in Skin Mycoses Worldwide. Mycoses 2008, 51 (Suppl. 4), 2–15. [Google Scholar] [CrossRef]
- Kayarkatte, M.N.; Singal, A.; Pandhi, D.; Das, S. Clinico-Mycological Study of Onychomycosis in a Tertiary Care Hospital-A Cross-Sectional Study. Mycoses 2020, 63, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Menotti, J.; Machouart, M.; Benderdouche, M.; Cetre-Sossah, C.; Morel, P.; Dubertret, L.; Derouin, F.; Feuilhade De Chauvin, M.; Lacroix, C. Polymerase Chain Reaction for Diagnosis of Dermatophyte and Scytalidium Spp. Onychomycosis. Br. J. Dermatol. 2004, 151, 518–519. [Google Scholar] [CrossRef] [PubMed]
- Paugam, A.; L’ollivier, C.; Viguié, C.; Anaya, L.; Mary, C.; de Ponfilly, G.; Ranque, S. Comparison of Real-Time PCR with Conventional Methods to Detect Dermatophytes in Samples from Patients with Suspected Dermatophytosis. J. Microbiol. Methods 2013, 95, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Hafirassou, A.Z.; Valero, C.; Gassem, N.; Mihoubi, I.; Buitrago, M.J. Usefulness of Techniques Based on Real Time PCR for the Identification of Onychomycosis-Causing Species. Mycoses 2017, 60, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Kabtani, J.; Diongue, K.; Dione, J.-N.; Delmas, A.; L’Ollivier, C.; Amoureux, M.-C.; Ndiaye, D.; Ranque, S. Real-Time PCR Assay for the Detection of Dermatophytes: Comparison between an In-House Method and a Commercial Kit for the Diagnosis of Dermatophytoses in Patients from Dakar, Senegal. J. Fungi 2021, 7, 949. [Google Scholar] [CrossRef] [PubMed]
- Harel, F.; Robert-Gangneux, F.; Gangneux, J.-P.; Guegan, H. Monocentric Evaluation of the Novaplex Dermatophyte Multiplex qPCR Assay in the Diagnosis of Dermatophytoses. J. Clin. Microbiol. 2024, 62, e0089424. [Google Scholar] [CrossRef]
- Evrard, S.; Minon, C.; Lamtiri Laarif, M.; De Backer, B.; Paridaens, H.; Hayette, M.-P.; Frère, J.; Senterre, J.-M.; Minon, J.-M. New Diagnostic Strategy for Onychomycosis: First-Line Utilization of DermaGenius® PCR and Calcofluor Microscopy Combined with Selective Culturing. J. Fungi 2024, 10, 515. [Google Scholar] [CrossRef] [PubMed]
- Anton, A.; Plinet, M.; Peyret, T.; Cazaudarré, T.; Pesant, S.; Rouquet, Y.; Tricoteaux, M.-A.; Bernier, M.; Bayette, J.; Fournier, R.; et al. Rapid and Accurate Diagnosis of Dermatophyte Infections Using the DendrisCHIP® Technology. Diagnostics 2023, 13, 3430. [Google Scholar] [CrossRef] [PubMed]
- Normand, A.-C.; Blaize, M.; Imbert, S.; Packeu, A.; Becker, P.; Fekkar, A.; Stubbe, D.; Piarroux, R. Identification of Molds with Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry: Performance of the Newly Developed MSI-2 Application in Comparison with the Bruker Filamentous Fungi Database and MSI-1. J. Clin. Microbiol. 2021, 59, e0129921. [Google Scholar] [CrossRef]
- Papanikolaou, Y.; Tsoumakas, G.; Katakis, I. Hierarchical Partitioning of the Output Space in Multi-Label Data. Data Knowl. Eng. 2018, 116, 42–60. [Google Scholar]
- Abarenkov, K.; Zirk, A.; Piirmann, T.; Pöhönen, R.; Ivanov, F.; Nilsson, R.H.; Kõljalg, U. UNITE General FASTA Release for Fungi. 2022. Available online: http://unite.ut.ee/index.php (accessed on 16 October 2022).
- Sasso, M.; Barrot, A.; Carles, M.-J.; Griffiths, K.; Rispail, P.; Crampette, L.; Lallemant, B.; Lachaud, L. Direct Identification of Molds by Sequence Analysis in Fungal Chronic Rhinosinusitis. J. Mycol. Medicale 2017, 27, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Trave, I.; Cozzani, E.; Canepa, P.; Verdiani, S.; Parodi, A. Real-Life Applicability of the Euroarray Dermatomycosis Kit in the Diagnosis of Onychomycosis. Mycoses 2022, 65, 317–322. [Google Scholar] [CrossRef] [PubMed]
| Culture Category | Total (n) | Skin (n) | Nail (n) | Hair (n) | |
|---|---|---|---|---|---|
| Samples with dermatophytes and/or C. albicans culture | 57 | 40 | 11 | 6 | |
| Kit positive | 44 * | 28 * | 10 | 6 | |
| ME pos | 38 * | 25 * | 9 | 4 | |
| ME neg | 4 | 1 | 1 | 2 | |
| ME:IQ | 2 | 2 | 0 | 0 | |
| Kit negative | 10 | 10 | 0 | 0 | |
| ME pos | 9 | 9 | |||
| ME neg | 1 | 1 | |||
| ME:IQ | 0 | 0 | |||
| Invalid PCR | 3 | 2 | 1 | 0 | |
| ME pos | 2 | 1 | 1 | ||
| ME neg | 0 | 0 | 0 | ||
| ME:IQ | 1 | 1 | 0 | ||
| Samples with non-dermatophytes/non-C. albicans culture | 8 | 5 | 3 | 0 | |
| Kit negative | 5 | 2 | 3 | 0 | |
| ME pos | 3 | 0 | 3 | ||
| ME neg | 2 | 2 | 0 | ||
| ME:IQ | 0 | 0 | 0 | ||
| Kit positive | 2 | 2 | 0 | 0 | |
| ME pos | 1 | 1 | |||
| ME neg | 1 | 1 | |||
| ME:IQ | 0 | 0 | |||
| Invalid PCR | 1 | 1 | 0 | 0 | |
| ME pos | 0 | 0 | |||
| ME neg | 1 | 1 | |||
| ME:IQ | 0 | 0 | |||
| Samples with sterile culture | 20 | 7 | 13 | 0 | |
| Kit negative | 11 | 5 | 6 | 0 | |
| ME pos | 4 | 2 | 2 | ||
| ME neg | 7 | 3 | 4 | ||
| ME:IQ | 0 | 0 | 0 | ||
| Kit positive | 8 | 2 | 6 | 0 | |
| ME pos | 6 | 1 | 5 | ||
| ME neg | 2 | 1 | 1 | ||
| ME:IQ | 0 | 0 | 0 | ||
| Invalid PCR | 1 | 0 | 1 | 0 | |
| ME pos | 0 | 0 | |||
| ME neg | 1 | 1 | |||
| ME:IQ | 0 | 0 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirard-Collet, P.; Durupt, F.; Hérault, M.; Miossec, C.; Lemoine, J.-P.; Wallon, M.; Dupont, D.; Persat, F.; Menotti, J. Evaluation of the DendrisKIT®DP for the Diagnosis of Superficial Fungal Infections. J. Fungi 2025, 11, 269. https://doi.org/10.3390/jof11040269
Tirard-Collet P, Durupt F, Hérault M, Miossec C, Lemoine J-P, Wallon M, Dupont D, Persat F, Menotti J. Evaluation of the DendrisKIT®DP for the Diagnosis of Superficial Fungal Infections. Journal of Fungi. 2025; 11(4):269. https://doi.org/10.3390/jof11040269
Chicago/Turabian StyleTirard-Collet, Pauline, François Durupt, Marion Hérault, Charline Miossec, Jean-Philippe Lemoine, Martine Wallon, Damien Dupont, Florence Persat, and Jean Menotti. 2025. "Evaluation of the DendrisKIT®DP for the Diagnosis of Superficial Fungal Infections" Journal of Fungi 11, no. 4: 269. https://doi.org/10.3390/jof11040269
APA StyleTirard-Collet, P., Durupt, F., Hérault, M., Miossec, C., Lemoine, J.-P., Wallon, M., Dupont, D., Persat, F., & Menotti, J. (2025). Evaluation of the DendrisKIT®DP for the Diagnosis of Superficial Fungal Infections. Journal of Fungi, 11(4), 269. https://doi.org/10.3390/jof11040269







