Aspergillus fumigatus in the Food Production Chain and Azole Resistance: A Growing Concern for Consumers
Abstract
1. Introduction
2. Contamination by Aspergillus fumigatus in Agricultural Crops and Food Products: Sources and Risk Factors
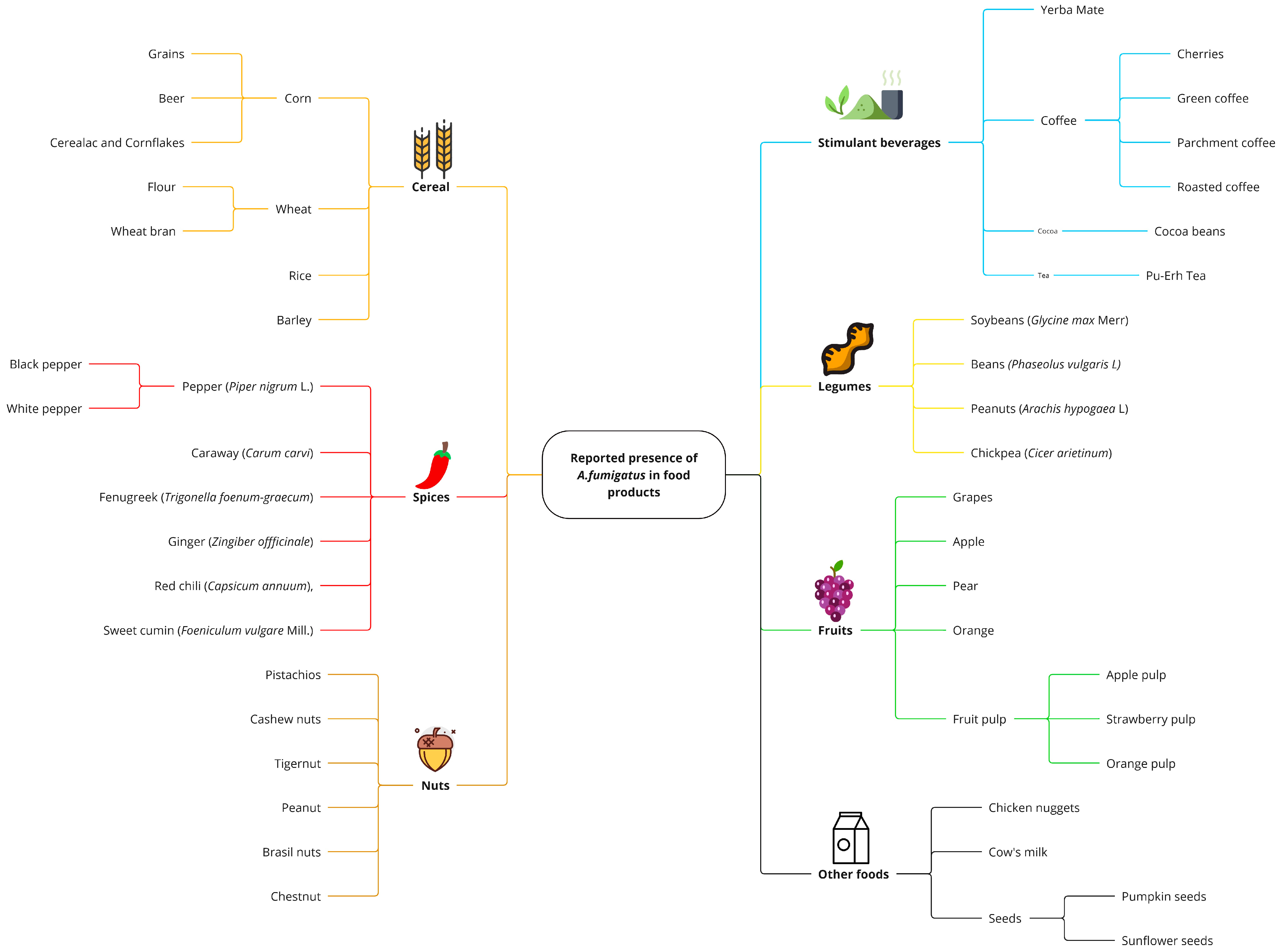
2.1. Presence of Aspergillus fumigatus in Food Products
2.1.1. Cereals
2.1.2. Spices
2.1.3. Coffee (Coffea)
2.1.4. Cocoa (Theobroma cacao)
2.1.5. Pu-Erh Tea and Yerba Mate
2.1.6. Legumes
2.1.7. Nuts and Edible Seeds
2.1.8. Fruits and Vegetables
2.1.9. Other Foods
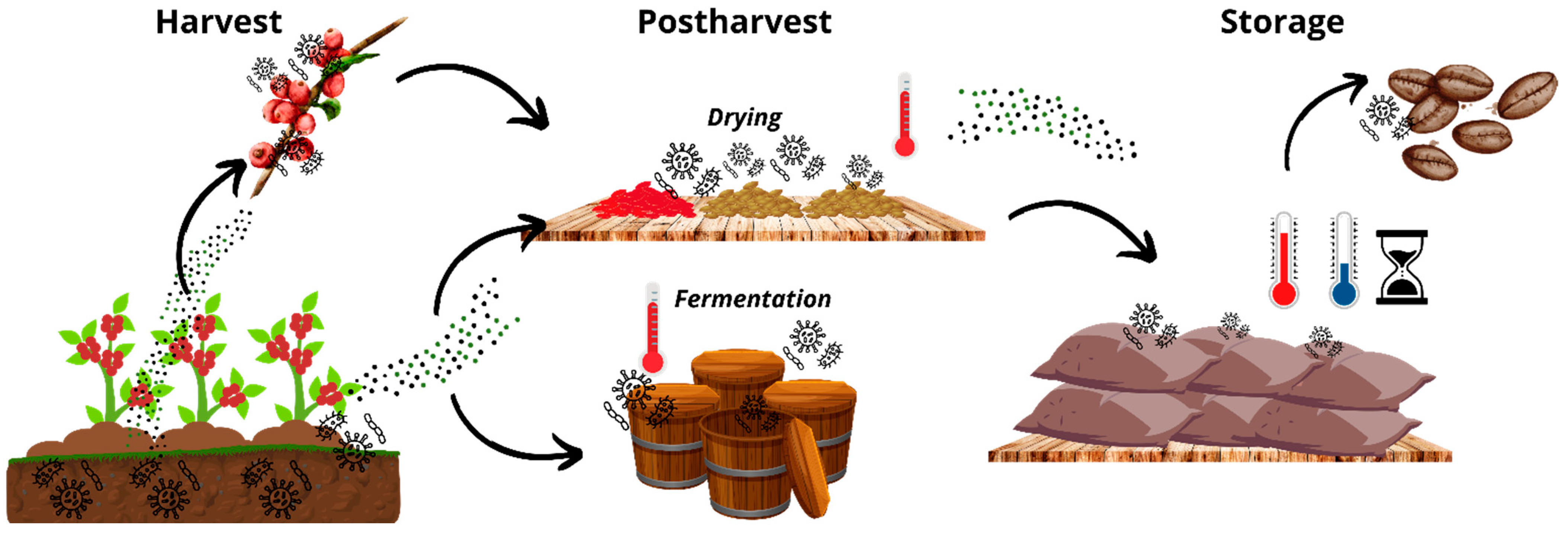
3. Possible Pathways of Aspergillus fumigatus Contamination in the Food Chain: From Production to Consumption
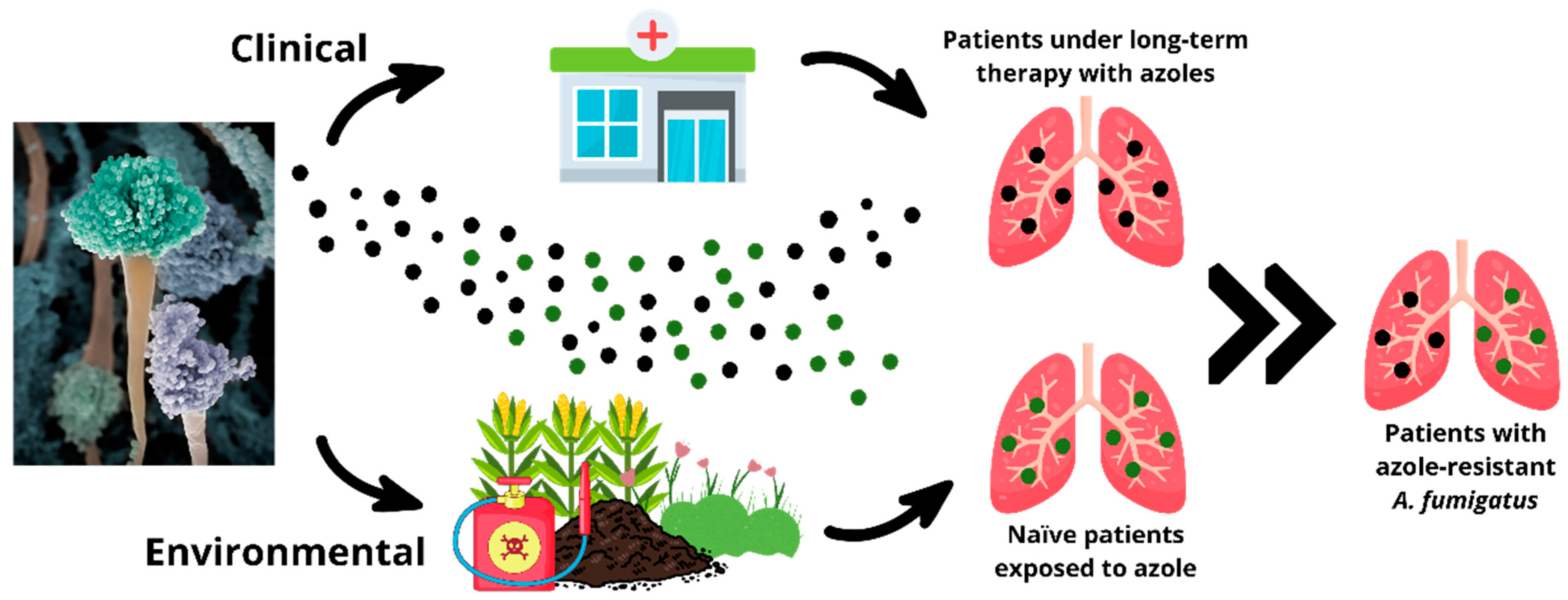
4. Azole Resistance in Aspergillus fumigatus
5. Future Perspectives on the Evaluation and Control of A. fumigatus in Food and Consumer Health Risks
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Navale, V.; Vamkudoth, K.R.; Ajmera, S.; Dhuri, V. Aspergillus Derived Mycotoxins in Food and the Environment: Prevalence, Detection, and Toxicity. Toxicol. Rep. 2021, 8, 1008–1030. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.A.; Visagie, C.M.; Houbraken, J.; Hong, S.-B.; Hubka, V.; Klaassen, C.H.W.; Perrone, G.; Seifert, K.A.; Susca, A.; Tanney, J.B.; et al. Phylogeny, Identification and Nomenclature of the Genus Aspergillus. Stud. Mycol. 2014, 78, 141–173. [Google Scholar] [CrossRef] [PubMed]
- Taniwaki, M.H.; Pitt, J.I.; Magan, N. Aspergillus Species and Mycotoxins: Occurrence and Importance in Major Food Commodities. Curr. Opin. Food Sci. 2018, 23, 38–43. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 3rd ed.; Springer: New York, NY, USA, 2022; ISBN 978-1-4899-8409-8. [Google Scholar]
- Burks, C.; Darby, A.; Gómez Londoño, L.; Momany, M.; Brewer, M.T. Azole-Resistant Aspergillus fumigatus in the Environment: Identifying Key Reservoirs and Hotspots of Antifungal Resistance. PLoS Pathog. 2021, 17, e1009711. [Google Scholar] [CrossRef]
- Meis, J.F.; Chowdhary, A.; Rhodes, J.L.; Fisher, M.C.; Verweij, P.E. Clinical Implications of Globally Emerging Azole Resistance in Aspergillus fumigatus. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150460. [Google Scholar] [CrossRef]
- Cadena, J.; Thompson, G.R.; Patterson, T.F. Aspergillosis: Epidemiology, Diagnosis, and Treatment. Infect. Dis. Clin. N. Am. 2021, 35, 415–434. [Google Scholar] [CrossRef]
- Latgé, J.-P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, e00140-18. [Google Scholar] [CrossRef]
- Caggiano, G.; Apollonio, F.; Consiglio, M.; Gasparre, V.; Trerotoli, P.; Diella, G.; Lopuzzo, M.; Triggiano, F.; Stolfa, S.; Mosca, A.; et al. Tendency in Pulmonary Aspergillosis Investigation During the COVID-19 Era: What Is Changing? Int. J. Environ. Res. Public. Health 2022, 19, 7079. [Google Scholar] [CrossRef]
- Lai, C.-C.; Yu, W.-L. COVID-19 Associated with Pulmonary Aspergillosis: A Literature Review. J. Microbiol. Immunol. Infect. 2021, 54, 46–53. [Google Scholar] [CrossRef]
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-006024-1. [Google Scholar]
- Pena, G.A.; Monge, M.P.; González Pereyra, M.L.; Dalcero, A.M.; Rosa, C.A.R.; Chiacchiera, S.M.; Cavaglieri, L.R. Gliotoxin Production by Aspergillus fumigatus Strains from Animal Environment. Micro-Analytical Sample Treatment Combined with a LC-MS/MS Method for Gliotoxin Determination. Mycotoxin Res. 2015, 31, 145–150. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC); European Chemicals Agency (ECHA); European Environment Agency (EEA); European Medicines Agency (EMA); European Commission’s Joint Research Centre (JRC). Impact of the Use of Azole Fungicides, Other than as Human Medicines, on the Development of Azole-Resistant Aspergillus spp. EFSA J. 2025, 23, e9200. [Google Scholar] [CrossRef]
- van Dijk, M.A.M.; Buil, J.B.; Tehupeiory-Kooreman, M.; Broekhuizen, M.J.; Broens, E.M.; Wagenaar, J.A.; Verweij, P.E. Azole Resistance in Veterinary Clinical Aspergillus fumigatus Isolates in the Netherlands. Mycopathologia 2024, 189, 50. [Google Scholar] [CrossRef]
- Berger, S.; El Chazli, Y.; Babu, A.F.; Coste, A.T. Azole Resistance in Aspergillus fumigatus: A Consequence of Antifungal Use in Agriculture? Front. Microbiol. 2017, 8, 1024. [Google Scholar] [CrossRef]
- Rivelli Zea, S.M.; Toyotome, T. Azole-Resistant Aspergillus fumigatus as an Emerging Worldwide Pathogen. Microbiol. Immunol. 2022, 66, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Molinero, R.L.; Hermida Alava, K.S.; Brito Devoto, T.; Sautua, F.; Carmona, M.; Cuestas, M.L.; Pena, G.A. Prevalence of Azole-Resistant Aspergillus fumigatus and Other Aspergilli in the Environment from Argentina. Med. Mycol. 2024, 62, myae098. [Google Scholar] [CrossRef]
- Chowdhary, A.; Sharma, C.; van den Boom, M.; Yntema, J.B.; Hagen, F.; Verweij, P.E.; Meis, J.F. Multi-Azole-Resistant Aspergillus fumigatus in the Environment in Tanzania. J. Antimicrob. Chemother. 2014, 69, 2979–2983. [Google Scholar] [CrossRef]
- Kano, R.; Kohata, E.; Tateishi, A.; Murayama, S.Y.; Hirose, D.; Shibata, Y.; Kosuge, Y.; Inoue, H.; Kamata, H.; Hasegawa, A. Does Farm Fungicide Use Induce Azole Resistance in Aspergillus fumigatus? Med. Mycol. 2015, 53, 174–177. [Google Scholar] [CrossRef]
- Ren, J.; Jin, X.; Zhang, Q.; Zheng, Y.; Lin, D.; Yu, Y. Fungicides Induced Triazole-Resistance in Aspergillus fumigatus Associated with Mutations of TR46/Y121F/T289A and Its Appearance in Agricultural Fields. J. Hazard. Mater. 2017, 326, 54–60. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Han, X.; Tian, S.; Zhao, J.; Chen, F.; Su, X.; Zhao, J.; Zou, Z.; Gong, Y.; et al. Elevated MIC Values of Imidazole Drugs Against Aspergillus fumigatus Isolates with TR34/L98H/S297T/F495I Mutation. Antimicrob. Agents Chemother. 2018, 62, e01549-17. [Google Scholar] [CrossRef]
- Cui, N.; He, Y.; Yao, S.; Zhang, H.; Ren, J.; Fang, H.; Yu, Y. Tebuconazole Induces Triazole-Resistance in Aspergillus fumigatus in Liquid Medium and Soil. Sci. Total Environ. 2019, 648, 1237–1243. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, F.; Zhao, J.; Fan, H.; Qin, C.; Li, R.; Verweij, P.E.; Zheng, Y.; Han, L. High Azole Resistance in Aspergillus fumigatus Isolates from Strawberry Fields, China, 2018. Emerg. Infect. Dis. 2020, 26, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Fraaije, B.A.; Atkins, S.L.; Santos, R.F.; Hanley, S.J.; West, J.S.; Lucas, J.A. Epidemiological Studies of Pan-Azole Resistant Aspergillus fumigatus Populations Sampled during Tulip Cultivation Show Clonal Expansion with Acquisition of Multi-Fungicide Resistance as Potential Driver. Microorganisms 2021, 9, 2379. [Google Scholar] [CrossRef]
- Fraaije, B.; Atkins, S.; Hanley, S.; Macdonald, A.; Lucas, J. The Multi-Fungicide Resistance Status of Aspergillus fumigatus Populations in Arable Soils and the Wider European Environment. Front. Microbiol. 2020, 11, 599233. [Google Scholar] [CrossRef]
- Cao, D.; Wu, R.; Dong, S.; Wang, F.; Ju, C.; Yu, S.; Xu, S.; Fang, H.; Yu, Y. Triazole Resistance in Aspergillus fumigatus in Crop Plant Soil after Tebuconazole Applications. Environ. Pollut. 2020, 266, 115124. [Google Scholar] [CrossRef]
- Resendiz-Sharpe, A.; Dewaele, K.; Merckx, R.; Bustamante, B.; Vega-Gomez, M.C.; Rolon, M.; Jacobs, J.; Verweij, P.E.; Maertens, J.; Lagrou, K. Triazole-Resistance in Environmental Aspergillus fumigatus in Latin American and African Countries. J. Fungi 2021, 7, 292. [Google Scholar] [CrossRef]
- Kang, S.E.; Sumabat, L.G.; Melie, T.; Mangum, B.; Momany, M.; Brewer, M.T. Evidence for the Agricultural Origin of Resistance to Multiple Antimicrobials in Aspergillus fumigatus, a Fungal Pathogen of Humans. G3 2021, 12, jkab427. [Google Scholar] [CrossRef]
- Eucast: Breakpoints for Antifungals. Available online: https://www.eucast.org/astoffungi/clinicalbreakpointsforantifungals (accessed on 8 February 2025).
- Viegas, C.; Simões, A.B.; Faria, M.; Gomes, B.; Cervantes, R.; Dias, M.; Carolino, E.; Twaruzek, M.; Kosicki, R.; Viegas, S.; et al. Tea Contamination by Mycotoxins and Azole-Resistant Mycobiota—The Need of a One Health Approach to Tackle Exposures. Int. J. Food Microbiol. 2023, 385, 110015. [Google Scholar] [CrossRef]
- Caetano, L.A.; Faria, T.; Batista, A.C.; Viegas, S.; Viegas, C. Assessment of Occupational Exposure to Azole Resistant Fungi in 10 Portuguese Bakeries. AIMS Microbiol. 2017, 3, 960–975. [Google Scholar] [CrossRef]
- Viegas, C.; Pacífico, C.; Faria, T.; de Oliveira, A.C.; Caetano, L.A.; Carolino, E.; Gomes, A.Q.; Viegas, S. Fungal Contamination in Green Coffee Beans Samples: A Public Health Concern. J. Toxicol. Environ. Health Part A 2017, 80, 719–728. [Google Scholar] [CrossRef]
- Pouris, J.; Kolyva, F.; Bratakou, S.; Vogiatzi, C.A.; Chaniotis, D.; Beloukas, A. The Role of Fungi in Food Production and Processing. Appl. Sci. 2024, 14, 5046. [Google Scholar] [CrossRef]
- Njobeh, P.B.; Dutton, M.F.; Koch, S.H.; Chuturgoon, A.; Stoev, S.; Seifert, K. Contamination with Storage Fungi of Human Food from Cameroon. Int. J. Food Microbiol. 2009, 135, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Sandona, K.; Billingsley Tobias, T.L.; Hutchinson, M.I.; Natvig, D.O.; Porras-Alfaro, A. Diversity of Thermophilic and Thermotolerant Fungi in Corn Grain. Mycologia 2019, 111, 719–729. [Google Scholar] [CrossRef]
- Ekwomadu, T.I.; Gopane, R.E.; Mwanza, M. Occurrence of Filamentous Fungi in Maize Destined for Human Consumption in South Africa. Food Sci. Nutr. 2018, 6, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Ekpakpale, D.O.; Kraak, B.; Meijer, M.; Ayeni, K.I.; Houbraken, J.; Ezekiel, C.N. Fungal Diversity and Aflatoxins in Maize and Rice Grains and Cassava-Based Flour (Pupuru) from Ondo State, Nigeria. J. Fungi 2021, 7, 635. [Google Scholar] [CrossRef]
- Salisu, B.; Anua, S.M.; Wan Ishak, W.R.; Mazlan, N. Mycotoxigenic Fungi Contamination of Grains and Peanuts from Open Markets in Kelantan, Malaysia. Food Res. 2022, 6, 69–77. [Google Scholar] [CrossRef]
- Tabuc, C.; Marin, D.; Guerre, P.; Sesan, T.; Bailly, J.D. Molds and Mycotoxin Content of Cereals in Southeastern Romania. J. Food Prot. 2009, 72, 662–665. [Google Scholar] [CrossRef]
- Reddy, K.R.N.; Farhana, N.I.; Wardah, A.R.; Salleh, B. Morphological Identification of Foodborne Pathogens Colonizing Rice Grains in South Asia. Pak. J. Biol. Sci. 2010, 13, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Douksouna, Y.; Masanga, J.; Nyerere, A.; Runo, S.; Ambang, Z. Towards Managing and Controlling Aflatoxin Producers within Aspergillus Species in Infested Rice Grains Collected from Local Markets in Kenya. Toxins 2019, 11, 544. [Google Scholar] [CrossRef]
- Saleemi, M.K.; Khan, M.Z.; Khan, A.; Hameed, M.R.; Khatoon, A.; ul Abadin, Z.; Hassan, Z.U. Study of Fungi and Their Toxigenic Potential Isolated from Wheat and Wheat Bran. Toxin Rev. 2017, 36, 80–88. [Google Scholar] [CrossRef]
- Yassein, A.S.; El-Said, A.H.M.; El-Dawy, E.G.A. Biocontrol of Toxigenic Aspergillus Strains Isolated from Baby Foods by Essential Oils. Flavour Fragr. J. 2020, 35, 182–189. [Google Scholar] [CrossRef]
- Saeed, I.; Shaheen, S.; Hussain, K.; Khan, M.A.; Jaffer, M.; Mahmood, T.; Khalid, S.; Sarwar, S.; Tahir, A.; Khan, F. Assessment of Mold and Yeast in Some Bakery Products of Lahore, Pakistan Based on LM and SEM. Microsc. Res. Tech. 2019, 82, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Adekoya, I.; Obadina, A.; Adaku, C.C.; De Boevre, M.; Okoth, S.; De Saeger, S.; Njobeh, P. Mycobiota and Co-Occurrence of Mycotoxins in South African Maize-Based Opaque Beer. Int. J. Food Microbiol. 2018, 270, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Yogendrarajah, P.; Deschuyffeleer, N.; Jacxsens, L.; Sneyers, P.-J.; Maene, P.; De Saeger, S.; Devlieghere, F.; De Meulenaer, B. Mycological Quality and Mycotoxin Contamination of Sri Lankan Peppers (Piper nigrum L.) and Subsequent Exposure Assessment. Food Control 2014, 41, 219–230. [Google Scholar] [CrossRef]
- Man, A.; Mare, A.; Toma, F.; Curticăpean, A.; Santacroce, L. Health Threats from Contamination of Spices Commercialized in Romania: Risks of Fungal and Bacterial Infections. Endocr. Metab. Immune Disord. Drug Targets 2016, 16, 197–204. [Google Scholar] [CrossRef]
- Gatti, M.J.; Fraga, M.E.; Magnoli, C.; Dalcero, A.M.; da Rocha Rosa, C.A. Mycological Survey for Potential Aflatoxin and Ochratoxin Producers and Their Toxicological Properties in Harvested Brazilian Black Pepper. Food Addit. Contam. 2003, 20, 1120–1126. [Google Scholar] [CrossRef]
- Hashem, M.; Alamri, S. Contamination of Common Spices in Saudi Arabia Markets with Potential Mycotoxin-Producing Fungi. Saudi J. Biol. Sci. 2010, 17, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Kouadio, I.A.; Koffi, L.B.; Nemlin, J.G.; Dosso, M.B. Effect of Robusta (Coffea canephora P.) Coffee Cherries Quantity Put out for Sun Drying on Contamination by Fungi and Ochratoxin A (OTA) under Tropical Humid Zone (Côte d‘Ivoire). Food Chem. Toxicol. 2012, 50, 1969–1979. [Google Scholar] [CrossRef] [PubMed]
- Culliao, A.G.L.; Barcelo, J.M. Fungal and Mycotoxin Contamination of Coffee Beans in Benguet Province, Philippines. Food Addit. Contam. Part A 2015, 32, 250–260. [Google Scholar] [CrossRef]
- Suárez-Quiroz, M.; González-Rios, O.; Barel, M.; Guyot, B.; Schorr-Galindo, S.; Guiraud, J.-P. Study of Ochratoxin A-Producing Strains in Coffee Processing. Int. J. Food Sci. Technol. 2004, 39, 501–507. [Google Scholar] [CrossRef]
- Alvindia, D.G.; de Guzman, M.F. Survey of Philippine Coffee Beans for the Presence of Ochratoxigenic Fungi. Mycotoxin Res. 2016, 32, 61–67. [Google Scholar] [CrossRef]
- Nganou, N.D.; Tchinda, E.S.; Noumo, T.N.; Mouafo, H.T.; Sokamte, A.T.; Tatsadjieu, L.N. Fungal Diversity and Evaluation of Ochratoxin A Content of Coffee from Three Cameroonian Regions. J. Food Qual. 2020, 2020, 8884514. [Google Scholar] [CrossRef]
- Casas-Junco, P.P.; Ragazzo-Sánchez, J.A.; Ascencio-Valle, F.d.J.; Calderón-Santoyo, M. Determination of Potentially Mycotoxigenic Fungi in Coffee (Coffea arabica L.) from Nayarit. Food Sci. Biotechnol. 2017, 27, 891–898. [Google Scholar] [CrossRef]
- Barišić, V.; Jozinović, A.; Flanjak, I.; Šubarić, D.; Babić, J.; Milicević, B.; Doko, K.; Ackar, D. Difficulties with Use of Cocoa Bean Shell in Food Production and High Voltage Electrical Discharge as a Possible Solution. Sustainability 2020, 12, 3981. [Google Scholar] [CrossRef]
- Praseptiangga, D.; Zambrano, J.M.G.; Sanjaya, A.P.; Muhammad, D.R.A. Challenges in the Development of the Cocoa and Chocolate Industry in Indonesia: A Case Study in Madiun, East Java. AIMS Agric. Food 2020, 5, 920–937. [Google Scholar] [CrossRef]
- Niles, E.V. Mycoflora of Imported Cocoa Beans. J. Stored Prod. Res. 1981, 17, 147–150. [Google Scholar]
- Mounjouenpou, P.; Gueule, D.; Fontana-Tachon, A.; Guyot, B.; Tondje, P.R.; Guiraud, J.P. Filamentous Fungi Producing Ochratoxin a during Cocoa Processing in Cameroon. Int. J. Food Microbiol. 2008, 121, 234–241. [Google Scholar] [CrossRef]
- Sánchez-Hervás, M.; Gil, J.V.; Bisbal, F.; Ramón, D.; Martínez-Culebras, P.V. Mycobiota and Mycotoxin Producing Fungi from Cocoa Beans. Int. J. Food Microbiol. 2008, 125, 336–340. [Google Scholar] [CrossRef]
- Copetti, M.V.; Iamanaka, B.T.; Frisvad, J.C.; Pereira, J.L.; Taniwaki, M.H. Mycobiota of Cocoa: From Farm to Chocolate. Food Microbiol. 2011, 28, 1499–1504. [Google Scholar] [CrossRef]
- Ma, Y.; Ling, T.; Su, X.; Jiang, B.; Nian, B.; Chen, L.; Liu, M.; Zhang, Z.; Wang, D.; Mu, Y. Integrated Proteomics and Metabolomics Analysis of Tea Leaves Fermented by Aspergillus Niger, Aspergillus Tamarii and Aspergillus fumigatus. Food Chem. 2021, 334, 127560. [Google Scholar]
- Wang, Q.; Gong, J.; Chisti, Y.; Sirisansaneeyakul, S. Fungal Isolates from a Pu-Erh Type Tea Fermentation and Their Ability to Convert Tea Polyphenols to Theabrownins. J. Food Sci. 2015, 80, M809–M817. [Google Scholar] [CrossRef] [PubMed]
- Haas, D.; Pfeifer, B.; Reiterich, C.; Partenheimer, R.; Reck, B.; Buzina, W. Identification and Quantification of Fungi and Mycotoxins from Pu-Erh Tea. Int. J. Food Microbiol. 2013, 166, 316–322. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Tong, H.R.; Zhou, L.; Wang, E.X.; Liu, Q.J. Fungal Colonization of Pu-Erh Tea in Yunnan. J. Food Saf. 2010, 30, 769–784. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, R.; Fang, W.; Yan, L.; Lu, J.; Sheng, J.; Lv, J. Characterization of Thermophilic Fungal Community Associated with Pile Fermentation of Pu-Erh Tea. Int. J. Food Microbiol. 2016, 227, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Riachi, L.G.; De Maria, C.A.B. Yerba Mate: An Overview of Physiological Effects in Humans. J. Funct. Foods 2017, 38, 308–320. [Google Scholar] [CrossRef]
- Castrillo, M.L.; Horianski, M.A.; Jerke, G. Aislamiento de Cepas de Aspergillus Sección Nigri En La Yerba Mate Comercializada En Posadas (Misiones, Argentina) y Evaluación de Su Potencial Ocratoxigénico. Rev. Argent. Microbiol. 2013, 45, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Arrúa Alvarenga, A.A.; Peralta López, I.P.; Rojas Abraham, C.M.; Reyes Caballero, Y.M.; Toledo Popoff, C.; Vazquez, L.; Moura Mendes Arrua, J. Presencia de Hongos Filamentosos En Yerba Mate Compuesta y Eficiencia de Medios de Cultivo Para El Aislamiento de Aspergillus. Investig. Agrar. 2016, 18, 49–55. [Google Scholar] [CrossRef]
- Di Pentima, M.C.; Steele-Moore, L.; Muehlbauer, L.; Klein, J.D. Are Your Patients at Risk? Fungal Contamination of Ilex Paraguariensis St. Hil (Yerba Maté). Transpl. Infect. Dis. 2005, 7, 47–48. [Google Scholar] [CrossRef]
- El-Maghraby, O.M.O.; Mohamed El-Maraghy, S.S. Mycoflora and Mycotoxins of Peanut (Arachis hypogaea, L.) Seeds in Egypt. 1-Sugar Fungi and Natural Occurrence of Mycotoxins. Mycopathologia 1987, 98, 165–170. [Google Scholar] [CrossRef]
- Youssef, M.; El-Maghraby, O.; Ibrahim, Y. Mycobiota and Mycotoxins of Egyptian Peanut (Arachis hypogeae L.) Seeds. Int. J. Bot. 2008, 4, 349–360. [Google Scholar] [CrossRef]
- Kazemi, A.; Ostadrahimi, A.; Ashrafnejad, F.; Sargheini, N.; Mahdavi, R.; Farshchian, M.; Mahluji, S. Mold Contamination of Untreated and Roasted with Salt Nuts (Walnuts, Peanuts and Pistachios) Sold at Markets of Tabriz, Iran. Jundishapur J. Microbiol. 2014, 7, e8751. [Google Scholar] [CrossRef]
- Lima, R.; de Oliveira, D.M.F.; Rufino, L.R.A.; Boriolo, M.F.G.; Silvério, A.C.P.; Garcia, J.A.D.; Fiorini, J.E.; Oliveira, N.M.S. Mycological and Toxicological Analysis of Peanuts and Derivatives. Rev. Ciências Farm. Básica E Apl. 2012, 33, 395–400. [Google Scholar]
- Adetunji, M.C.; Ezeokoli, O.T.; Ngoma, L.; Mwanza, M. Phylogenetic Diversity and Prevalence of Mycoflora in Ready-to-Eat Supermarket and Roadside-Vended Peanuts. Mycologia 2021, 113, 1–11. [Google Scholar] [CrossRef]
- Ramirez, M.L.; Cendoya, E.; Nichea, M.J.; Zachetti, V.G.L.; Chulze, S.N. Impact of Toxigenic Fungi and Mycotoxins in Chickpea: A Review. Curr. Opin. Food Sci. 2018, 23, 32–37. [Google Scholar] [CrossRef]
- Shamsi, S.; Khatun, A. Prevalence of Fungi in Different Varieties of Chickpea (Cicer arietinum L.) Seeds in Storage. J. Bangladesh Acad. Sci. 2016, 40, 37–44. [Google Scholar] [CrossRef]
- Kumar, N. Food Seed Health of Chick Pea (Cicer arietinum L.) at Panchgaon, Gurgaon, India. Adv. Crop Sci. Technol. 2016, 4, 2. [Google Scholar] [CrossRef]
- Alwakeel, S.S.; Nasser, L.A. Microbial Contamination and Mycotoxins from Nuts in Riyadh, Saudi Arabia. Am. J. Food Technol. 2011, 6, 613–630. [Google Scholar] [CrossRef]
- Adebajo, L.O.; Diyaolu, S.A. Mycology and Spoilage of Retail Cashew Nuts. Afr. J. Biotechnol. 2003, 2, 369–373. [Google Scholar] [CrossRef]
- Kačániová, M.; Sudzinová, J.; Kádasi-Horáková, M.; Valšíková, M.; Kráčmar, S. The Determination of Microscopic Fungi from Chestnut (Castanea sativa Mill.) Fruits, Leaves, Crust and Pollen. Acta Univ. Agric. Silvic. Mendel. Brun. 2014, 58, 73–78. [Google Scholar] [CrossRef]
- Freire, F.D.C.O.; Kozakiewicz, Z.; Paterson, R. Mycoflora and Mycotoxins in Brazilian Black Pepper, White Pepper and Brazil Nuts. Mycopathologia 2000, 149, 13–19. [Google Scholar] [CrossRef]
- Adebanjo, A.; Shopeju, E. Sources and Mycoflora Associated with Some Sundried Vegetables in Storage. Int. Biodeterior. Biodegrad. 1993, 31, 255–263. [Google Scholar] [CrossRef]
- Serra, R.; Abrunhosa, L.; Kozakiewicz, Z.; Venâncio, A. Black Aspergillus Species as Ochratoxin A Producers in Portuguese Wine Grapes. Int. J. Food Microbiol. 2003, 88, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Sardiñas, N.; Vázquez, C.; Gil-Serna, J.; González-Jaén, M.T.; Patiño, B. Specific Detection and Quantification of Aspergillus Flavus and Aspergillus Parasiticus in Wheat Flour by SYBR® Green Quantitative PCR. Int. J. Food Microbiol. 2011, 145, 121–125. [Google Scholar] [CrossRef]
- Paixão Dos Santos, J.L.; Samapundo, S.; Biyikli, A.; Van Impe, J.; Akkermans, S.; Höfte, M.; Abatih, E.N.; Sant’Ana, A.S.; Devlieghere, F. Occurrence, Distribution and Contamination Levels of Heat-Resistant Moulds throughout the Processing of Pasteurized High-Acid Fruit Products. Int. J. Food Microbiol. 2018, 281, 72–81. [Google Scholar] [CrossRef]
- Asci, F.; Aydin, B.; Akkus, G.U.; Unal, A.; Erdogmus, S.F.; Korcan, S.E.; Jahan, I. Fatty Acid Methyl Ester Analysis of Aspergillus fumigatus Isolated from Fruit Pulps for Biodiesel Production Using GC-MS Spectrometry. Bioengineered 2020, 11, 408–415. [Google Scholar] [CrossRef]
- Wigmann, É.F.; Saccomori, F.; Bernardi, A.O.; Frisvad, J.C.; Copetti, M.V. Toxigenic Penicillia Spoiling Frozen Chicken Nuggets. Food Res. Int. 2015, 67, 219–222. [Google Scholar] [CrossRef]
- Pena, G.A.; Alonso, V.; Manini, M.V.; Pellegrino, M.; Cavaglieri, L.R. Molecular Characterization of Aspergillus fumigatus Isolated from Raw Cow Milk in Argentina: Molecular Typing of A. Fumigatus from Raw Cow Milk. Int. J. Food Microbiol. 2018, 275, 1–7. [Google Scholar] [CrossRef]
- El Mahgubi, A.; Puel, O.; Bailly, S.; Tadrist, S.; Querin, A.; Ouadia, A.; Oswald, I.P.; Bailly, J.D. Distribution and Toxigenicity of Aspergillus Section Flavi in Spices Marketed in Morocco. Food Control 2013, 32, 143–148. [Google Scholar] [CrossRef]
- Delgado-Ospina, J.; Molina-Hernández, J.B.; Chaves-López, C.; Romanazzi, G.; Paparella, A. The Role of Fungi in the Cocoa Production Chain and the Challenge of Climate Change. J. Fungi 2021, 7, 202. [Google Scholar] [CrossRef]
- Kofoed, V.C.; Campion, C.; Rasmussen, P.U.; Møller, S.A.; Eskildsen, M.; Nielsen, J.L.; Madsen, A.M. Exposure to Resistant Fungi across Working Environments and Time. Sci. Total Environ. 2024, 923, 171189. [Google Scholar] [CrossRef]
- Malik, A.; Ali, S.; Shahid, M.; Bhargava, R. Occupational Exposure to Aspergillus and Aflatoxins among Food-Grain Workers in India. Int. J. Occup. Environ. Health 2014, 20, 189–193. [Google Scholar] [CrossRef]
- Gómez-Salazar, J.A.; Ruiz-Hernández, K.; Martínez-Miranda, M.M.; Castro-Ríos, K. Postharvest Strategies for Decontamination of Aflatoxins in Cereals. Food Rev. Int. 2023, 39, 3635–3662. [Google Scholar] [CrossRef]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahomoodally, M.F. A Systematic Review on Black Pepper (Piper nigrum L.): From Folk Uses to Pharmacological Applications. Crit. Rev. Food Sci. Nutr. 2019, 59, S210–S243. [Google Scholar] [CrossRef] [PubMed]
- International Coffee Organization. Historical Data on the Global Coffee Trade; International Coffee Organization (ICO): London, UK, 2021. [Google Scholar]
- ICCO. Cocoa Market Report; International Cocoa Organization: London, UK, 2022; p. 3. [Google Scholar]
- Vieira, N.O.; Peres, A.; Aquino, V.R.; Pasqualotto, A.C. Drinking Yerba Mate Infusion: A Potential Risk Factor for Invasive Fungal Diseases? Transpl. Infect. Dis. 2010, 12, 565–569. [Google Scholar] [CrossRef]
- Argyropoulos, C.D.; Skoulou, V.; Efthimiou, G.; Michopoulos, A.K. Airborne Transmission of Biological Agents within the Indoor Built Environment: A Multidisciplinary Review. Air Qual. Atmos. Health 2023, 16, 477–533. [Google Scholar] [CrossRef]
- Dijksterhuis, J. Fungal Spores: Highly Variable and Stress-Resistant Vehicles for Distribution and Spoilage. Food Microbiol. 2019, 81, 2–11. [Google Scholar] [CrossRef]
- Stensvold, C.R.; Jørgensen, L.N.; Arendrup, M.C. Azole-Resistant Invasive Aspergillosis: Relationship to Agriculture. Curr. Fungal Infect. Rep. 2012, 6, 178–191. [Google Scholar] [CrossRef]
- Abdel-Azeem, A.M.; Salem, F.M.; Abdel-Azeem, M.A.; Nafady, N.A.; Mohesien, M.T.; Soliman, E.A. Biodiversity of the Genus Aspergillus in Different Habitats. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 3–28. ISBN 978-0-444-63505-1. [Google Scholar]
- Verweij, P.E.; Lucas, J.A.; Arendrup, M.C.; Bowyer, P.; Brinkmann, A.J.F.; Denning, D.W.; Dyer, P.S.; Fisher, M.C.; Geenen, P.L.; Gisi, U.; et al. The One Health Problem of Azole Resistance in Aspergillus fumigatus: Current Insights and Future Research Agenda. Fungal Biol. Rev. 2020, 34, 202–214. [Google Scholar] [CrossRef]
- Toepfer, S.; Keniya, M.V.; Lackner, M.; Monk, B.C. Azole Combinations and Multi-Targeting Drugs That Synergistically Inhibit Candidozyma auris. J. Fungi 2024, 10, 698. [Google Scholar] [CrossRef]
- Schuster, M.; Kilaru, S.; Steinberg, G. Azoles Activate Type I and Type II Programmed Cell Death Pathways in Crop Pathogenic Fungi. Nat. Commun. 2024, 15, 4357. [Google Scholar] [CrossRef] [PubMed]
- Arastehfar, A.; Carvalho, A.; Houbraken, J.; Lombardi, L.; Garcia-Rubio, R.; Jenks, J.D.; Rivero-Menendez, O.; Aljohani, R.; Jacobsen, I.D.; Berman, J.; et al. Aspergillus fumigatus and Aspergillosis: From Basics to Clinics. Stud. Mycol. 2021, 100, 100115. [Google Scholar] [CrossRef]
- Denning, D.W.; Venkateswarlu, K.; Oakley, K.L.; Anderson, M.J.; Manning, N.J.; Stevens, D.A.; Warnock, D.W.; Kelly, S.L. Itraconazole Resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 1997, 41, 1364–1368. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.J.; Cerar, D.; Anderson, M.J.; Albarrag, A.; Fisher, M.C.; Pasqualotto, A.C.; Laverdiere, M.; Arendrup, M.C.; Perlin, D.S.; Denning, D.W. Frequency and Evolution of Azole Resistance in Aspergillus fumigatus Associated with Treatment Failure. Emerg. Infect. Dis. 2009, 15, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Snelders, E.; van der Lee, H.A.L.; Kuijpers, J.; Rijs, A.J.M.M.; Varga, J.; Samson, R.A.; Mellado, E.; Donders, A.R.T.; Melchers, W.J.G.; Verweij, P.E. Emergence of Azole Resistance in Aspergillus fumigatus and Spread of a Single Resistance Mechanism. PLoS Med. 2008, 5, e219. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.E.; Warrilow, A.G.S.; Price, C.L.; Mullins, J.G.L.; Kelly, D.E.; Kelly, S.L. Resistance to Antifungals That Target CYP51. J. Chem. Biol. 2014, 7, 143–161. [Google Scholar] [CrossRef]
- Buied, A.; Moore, C.B.; Denning, D.W.; Bowyer, P. High-Level Expression of cyp51B in Azole-Resistant Clinical Aspergillus fumigatus Isolates. J. Antimicrob. Chemother. 2013, 68, 512–514. [Google Scholar] [CrossRef]
- Camps, S.M.T.; Dutilh, B.E.; Arendrup, M.C.; Rijs, A.J.M.M.; Snelders, E.; Huynen, M.A.; Verweij, P.E.; Melchers, W.J.G. Discovery of a hapE Mutation That Causes Azole Resistance in Aspergillus fumigatus through Whole Genome Sequencing and Sexual Crossing. PLoS ONE 2012, 7, e50034. [Google Scholar] [CrossRef]
- Fraczek, M.G.; Bromley, M.; Buied, A.; Moore, C.B.; Rajendran, R.; Rautemaa, R.; Ramage, G.; Denning, D.W.; Bowyer, P. The cdr1B Efflux Transporter Is Associated with Non-Cyp51a-Mediated Itraconazole Resistance in Aspergillus fumigatus. J. Antimicrob. Chemother. 2013, 68, 1486–1496. [Google Scholar] [CrossRef]
- Hagiwara, D.; Arai, T.; Takahashi, H.; Kusuya, Y.; Watanabe, A.; Kamei, K. Non-cyp51A Azole-Resistant Aspergillus fumigatus Isolates with Mutation in HMG-CoA Reductase. Emerg. Infect. Dis. J. 2018, 24, 1889. [Google Scholar] [CrossRef]
- Wei, X.; Chen, P.; Gao, R.; Li, Y.; Zhang, A.; Liu, F.; Lu, L. Screening and Characterization of a Non-cyp51A Mutation in an Aspergillus fumigatus Cox10 Strain Conferring Azole Resistance. Antimicrob. Agents Chemother. 2017, 61, e02101-16. [Google Scholar] [CrossRef]
- Lazzarini, C.; Esposto, M.C.; Prigitano, A.; Cogliati, M.; De Lorenzis, G.; Tortorano, A.M. Azole Resistance in Aspergillus fumigatus Clinical Isolates from an Italian Culture Collection. Antimicrob. Agents Chemother. 2015, 60, 682–685. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Gil, V.G.; Gutierrez, F.; Lindner, J.R.; Albataineh, M.T.; McCarthy, D.I.; Sanders, C.; Fan, H.; Fothergill, A.W.; Sutton, D.A. First Detection of TR34 L98H and TR46 Y121F T289A Cyp51 Mutations in Aspergillus fumigatus Isolates in the United States. J. Clin. Microbiol. 2016, 54, 168–171. [Google Scholar] [CrossRef]
- van der Linden, J.W.M.; Snelders, E.; Kampinga, G.A.; Rijnders, B.J.A.; Mattsson, E.; Debets-Ossenkopp, Y.J.; Van Tiel, F.H.; Melchers, W.J.G.; Verweij, P.E. Clinical Implications of Azole Resistance in Aspergillus fumigatus, The Netherlands, 2007–2009. Emerg. Infect. Dis. 2011, 17, 1846–1854. [Google Scholar] [CrossRef] [PubMed]
- Hollomon, D. Does Agricultural Use of Azole Fungicides Contribute to Resistance in the Human Pathogen Aspergillus fumigatus? Pest Manag. Sci. 2017, 73, 1987–1993. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Chowdhary, A.; Melchers, W.J.G.; Meis, J.F. Azole Resistance in Aspergillus fumigatus: Can We Retain the Clinical Use of Mold-Active Antifungal Azoles? Clin. Infect. Dis. 2016, 62, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Gisi, U. Assessment of Selection and Resistance Risk for Demethylation Inhibitor Fungicides in Aspergillus Fumigatus in Agriculture and Medicine: A Critical Review. Pest Manag. Sci. 2014, 70, 352–364. [Google Scholar] [CrossRef]
- Bowyer, P.; Denning, D.W. Environmental Fungicides and Triazole Resistance in Aspergillus. Pest Manag. Sci. 2014, 70, 173–178. [Google Scholar] [CrossRef]
- Wang, F.; Yao, S.; Cao, D.; Ju, C.; Yu, S.; Xu, S.; Fang, H.; Yu, Y. Increased Triazole-Resistance and cyp51A Mutations in Aspergillus fumigatus After Selection with a Combination of the Triazole Fungicides Difenoconazole and Propiconazole. J. Hazard. Mater. 2020, 400, 123200. [Google Scholar] [CrossRef]
- Eggimann, P.; Chevrolet, J.-C.; Starobinski, M.; Majno, P.; Totsch, M.; Chapuis, B.; Pittet, D. Primary Invasive Aspergillosis of the Digestive Tract: Report of Two Cases and Review of the Literature. Infection 2006, 34, 333–338. [Google Scholar] [CrossRef]
- Schoustra, S.E.; Debets, A.J.M.; Rijs, A.J.M.M.; Zhang, J.; Snelders, E.; Leendertse, P.C.; Melchers, W.J.G.; Rietveld, A.G.; Zwaan, B.J.; Verweij, P.E. Environmental Hotspots for Azole Resistance Selection of Aspergillus fumigatus, the Netherlands. Emerg. Infect. Dis. 2019, 25, 1347–1353. [Google Scholar] [CrossRef]
| Samples | Origin of Isolates | Number of A. fumigatus Isolates | Type of Fungicides Azoles Evaluated | Concentration of Azoles Fungicides (mg/L) | MIC (mg/L) * | CYP51A Aminoacid Substitutions Associated with Azole Resistance | References |
|---|---|---|---|---|---|---|---|
| Soil and woody debris | Tanzania | 15 | itraconazole | 0.03 to 16 | 1 to 2 | TR34/L98A and TR46/Y121F/T298A | [18] |
| voriconazole | 0.03 to 16 | >16 | |||||
| posaconazole | 0.015 to 8 | 0.25 to 0.5 | |||||
| isavuconazole | 0.015 to 8 | 8 | |||||
| bromuconazole | 0.06 to 32 | >32 | |||||
| cyproconazole | 0.06 to 32 | >32 | |||||
| difenoconazole | 0.06 to 32 | >32 | |||||
| epoxiconazole | 0.06 to 32 | >32 | |||||
| penconazole | 0.06 to 32 | >32 | |||||
| tebuconazole | 0.06 to 32 | >32 | |||||
| triadimefon | 0.06 to 32 | >32 | |||||
| metconazole | 0.06 to 32 | >32 | |||||
| hexaconazole | 0.06 to 32 | >32 | |||||
| tricyclazole | 0.06 to 32 | >32 | |||||
| pumpkin farm (air samples from agricultural field) | Japan | 50 | itraconazole | 0.19 to 1.5 | 0.74 | NR | [19] |
| posaconazole | 0.19 to 1.5 | 0.006 to 0.16 | |||||
| voriconazole | 0.19 to 1.5 | 0.47 to 0.64 | |||||
| tetraconazole | 580 to 1132 | ≤36.25 | |||||
| Soil samples from greenhouses for vegetables and fruits | China | 73 | itraconazole | NR | 0.5 to 16 | TR46/Y121F/T289A; A284T; G448S; P222Q and TR34/L98H/S297T/F495I | [20] |
| posaconazole | NR | 0.25 to 1 | |||||
| voriconazole | NR | 1 to 16 | |||||
| epoxiconazole | 1 to 16 | NR | |||||
| tebuconazole | 1 to 16 | NR | |||||
| propiconazole | 1 to 16 | NR | |||||
| hexaconazole | 1 to 16 | NR | |||||
| metconazole | 1 to 16 | NR | |||||
| Environmental isolates | China | 24 | itraconazole | NR | 0.25 to ≥16 | TR34/L98H/S297T/F495I; G54R; G54V and TR46/Y121F/T289A | [21] |
| voriconazole | NR | 0.25 to ≥16 | |||||
| posaconazole | NR | 0.125 to ≥8 | |||||
| epoxiconazole | 0.06 to 32 | 0.5 to ≥32 | |||||
| bromuconazole | 0.06 to 32 | 0.25 to ≥32 | |||||
| tebuconazole | 0.06 to 32 | 0.5 to ≥32 | |||||
| difenoconazole | 0.06 to 32 | 0.25 to ≥32 | |||||
| propiconazole | 0.06 to 32 | 1 to ≥32 | |||||
| imazalil | 0.06 to 32 | 0.06 to ≥32 | |||||
| prochloraz | 0.06 to 32 | 0.125 to ≥32 | |||||
| Soil samples | China | 2 | itraconazole | NR | 0.5 to 16 | TR46/Y121F/T289A | [22] |
| voriconazole | NR | 0.25 to >16 | |||||
| posaconazole | NR | 0.125 to 0.5 | |||||
| tebuconazole | 0.5 to 5 | 0.5 to >16 | |||||
| Soil samples | China | 21 | itraconazole | 4 | 1 to >16 | TR34/L98H; TR34/L98H/S297T/F495I and TR46/Y121F/T289A | [23] |
| voriconazole | 2 | 1 to >16 | |||||
| posaconazole | 0.5 | 1 to 2 | |||||
| epoxiconazole | 0.06 to 32 | 2 to >32 | |||||
| bromuconazole | 0.06 to 32 | 1 to >32 | |||||
| tebuconazole | 0.06 to 32 | 2 to >32 | |||||
| difenoconazole | 0.06 to 32 | 0.5 to >32 | |||||
| propiconazole | 0.06 to 32 | 2 to >32 | |||||
| prochloraz | 0.06 to 32 | 0.25 to >32 | |||||
| imazalil | 0.06 to 32 | 0.125 to >32 | |||||
| voriconazole | 0.03 to 32 | 0.25 to >32 | |||||
| isavuconazole | 0.03 to 32 | 0.5 to >32 | |||||
| posaconazole | 0.03 to 32 | 0.125 to 2 | |||||
| prothioconazole | 0.016 to 16 | 4 to >32 | |||||
| paclobutrazole | 0.016 to 16 | 16 to >32 | |||||
| epoxiconazole | 0.016 to 16 | 4 to >32 | |||||
| propiconazole | 0.016 to 16 | 4 to 32 | |||||
| tebuconazole | 0.016 to 16 | 2 to >32 | |||||
| difenoconazole | 0.016 to 16 | 1 a >32 | |||||
| metconazole | 0.016 to 16 | 0.25 to 16 | |||||
| prothiozonacole-desthio | 0.016 to 16 | ||||||
| imazalil | 0.016 to 16 | 0.125 to 32 | |||||
| prochloraz | 0.016 to 16 | 0.25 to 32 | |||||
| mefentriflfluconazole | 0.016 to 16 | >32 | |||||
| Tulip field soils, flower bulbs, tulip peel waste heaps (decaying material), flower waste compost sites, clinical isolates | Netherlands and UK | 200 | voriconazole | 0.1 to 19.120 | 0.292 to >19.120 | TR46/Y121F/T289A; TR34/L98H/T289A/I364V/G448S; F46Y/M172V/E427K; TR34/L98H/S297T/F495I and TR34/L98H | [24] |
| imazalil | 0.25 to 43.153 | 0.714 to >43.153 | |||||
| tebuconazole | 0.1 to 17.349 | 0.643 to >17.349 | |||||
| Soil samples and wheat straw | UK | 692 | voriconazole | 0.1 to 19.120 | 0.342 to >19.120 | TR46/Y121F/T289A; TR46/Y121F/M172V/T289A/G448S and TR46/Y121F/T289A/S363P/I364V/G448S | [25] |
| itraconazole | 0.025 to 22.324 | 1.056 to >22.234 | |||||
| imazalil | 0.25 to 43.153 | 0.978 to >43.153 | |||||
| tebuconazole | 0.1 to 17.349 | 0.829 to >17.349 | |||||
| Soil samples | China | 162 | itraconazole | NR | 1 to >16 | TR34/L98H and TR34/L98H/S297T/F495I | [26] |
| voriconazole | NR | 0.5 to 8 | |||||
| posaconazole | NR | 0.25 to 2 | |||||
| tebuconazole | NR | 8 to 16 | |||||
| Soil samples from rural and urban locations | México, Paraguay, Perú, Benin and Nigeria | NR | tebuconazole | NR | 0.5 to 32 | TR34/L98H and TR46/Y121/T289A | [27] |
| itraconazole | 4 | >16 | |||||
| voriconazole | 2 | 1 | |||||
| Soil, plant debris, and compost | USA | 748 | tebuconazole | 3 | 1 to >16 | TR46/Y121F/T289A; I242V and Y46F/V172M/T248N/E255D/K427E | [28] |
| itraconazole | 0.015 to 16 | 0.5 to 2 | |||||
| voriconazole | 0.015 to 16 | 0.25 to >16 | |||||
| posaconazole | 0.015 to 16 | 0.25 to 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Ríos, K.; Buri, M.C.S.; Ramalho da Cruz, A.D.; Ceresini, P.C. Aspergillus fumigatus in the Food Production Chain and Azole Resistance: A Growing Concern for Consumers. J. Fungi 2025, 11, 252. https://doi.org/10.3390/jof11040252
Castro-Ríos K, Buri MCS, Ramalho da Cruz AD, Ceresini PC. Aspergillus fumigatus in the Food Production Chain and Azole Resistance: A Growing Concern for Consumers. Journal of Fungi. 2025; 11(4):252. https://doi.org/10.3390/jof11040252
Chicago/Turabian StyleCastro-Ríos, Katherin, Maria Clara Shiroma Buri, Arla Daniela Ramalho da Cruz, and Paulo Cezar Ceresini. 2025. "Aspergillus fumigatus in the Food Production Chain and Azole Resistance: A Growing Concern for Consumers" Journal of Fungi 11, no. 4: 252. https://doi.org/10.3390/jof11040252
APA StyleCastro-Ríos, K., Buri, M. C. S., Ramalho da Cruz, A. D., & Ceresini, P. C. (2025). Aspergillus fumigatus in the Food Production Chain and Azole Resistance: A Growing Concern for Consumers. Journal of Fungi, 11(4), 252. https://doi.org/10.3390/jof11040252







