1. Introduction
Fungi, like other eukaryotic organisms, have developed sexual reproduction to enable recombination of DNA (from genes to genomes) in populations. In addition to the standard male–female dichotomy, classes exist that have many different mating types, where specimens can mate with all other specimens of different mating types except for those that have the same mating type [
1]. In addition, there are a number of parasexual phenomena that can also ensure recombination.
The ascomycetes that belong to the true fungi, however, rely on a simple bipolar system with only two mating types [
1], recently denominated MAT1-1 and MAT1-2 [
2,
3,
4]. In principle, the loci, or the genes making up the loci, that define the mating types are allelic, but because they are very different from each other, they have been defined as idiomorphs rather than alleles [
1,
2]. For almost all populations for which sexuality has been observed, successful mating producing fertile offspring is used as an overarching criterion to define the mating specimen as belonging to the same species. This way, mating can be used to define species limits. Methods for testing species limits based on molecular phylogenies like genealogical concordance, phylogenetic species recognition, and Poisson tree processes can only substitute for this, even though they are very useful when mating is difficult to observe [
5]. Species barriers are often equivalent to mating barriers, and mating barriers can be defined in mating-type genes. Mating-type genes were observed to evolve rapidly but seem to be conserved within a species [
6], so sequence analysis of mating-type genes can also contribute to a better definition of species limits in phylogenies [
1].
For populations, including fungal populations, it makes a difference whether recombination through sexual reproduction happens. Therefore, it is interesting to study the presence of different mating types in a population. Also, specimens with different mating types might differ somewhat in their physiology, perhaps even in their virulence against different host species.
The genus
Diaporthe has the typical mating-type system for the Ascomycota [
3]. The mating types are mainly defined by the presence of either one of the genes
MAT1-1-1 for MAT1-1 or
MAT1-2-1 for MAT1-2 [
2]. These genes are coding for regulatory proteins that induce differentiation of generative tissues as well as the mating factor pheromones [
7]. Therefore, the mating types of different specimens, or rather strains or isolates, can be determined through testing for the presence of either of these two genes. Since the genes are rather different, this can be achieved by PCR with primers specific either for
MAT1-1-1 or for
MAT1-2-1. For this purpose, Santos et al. [
4] designed primers that can amplify either part of
MAT1-1-1 or
MAT1-2-1 across
Diaporthe spp.
Because
Diaporthe spp. show considerable variability in their morphological features, it is very difficult to use morphology for species identification and even more so for species definitions [
8,
9]. Because of these difficulties, association of
Diaporthe strains to different plant species has been used for species definitions, which led to a strong overestimation of
Diaporthe species numbers, since several
Diaporthe species can infect more than one plant species, and some can infect many [
10]. On the other hand, plants can be infected by different
Diaporthe species; for example, soybean can be infected by
D. aspalathi,
D. caulivora,
D. eres,
D. longicolla,
D. novem, and
D. sojae, and probably even more
Diaporthe spp. [
11,
12,
13]. Recently, molecular phylogeny has been used to define the clades in the genus, which led to a much clearer idea of species number and host associations in the genus [
5,
14]. However, a lot of work still has to be carried out before the genus is fully resolved and the present study also hopes to contribute to this.
In a previous study our group showed the presence of the four species
Diaporthe caulivora,
D. eres,
D. longicolla, and
D. novem on soybean in central Europe [
9]. Hosseini et al. [
9] gained 32 isolates of the different species and performed phylogenetic analysis with these isolates. Here, we determined the mating types of these isolates and we used sequence information on the
MAT1-1-1 and
MAT1-2-1 genes to further refine phylogenetic information for our isolates. Despite the indirect role of these genes in defining the mating type, we assumed that sequence comparisons on these genes could further contribute to defining the species limits.
D. caulivora is a homothallic species that readily forms perithecia and ascospores in vitro and on its host plants; it preferentially reproduces sexually, whereas asexual conidia are seldom found [
9]. So far, only the
MAT1-2-1 gene was found for
D. caulivora [
15], which somewhat contradicts self-fertility, for which normally both mating-type genes should be present.
D. eres is considered a species complex [
14], and we also found sequence variation in ITS,
TEF, and
TUB genes between our isolates that were classified as
D. eres [
9]. We anticipated additional information on whether our isolates do belong to the same species or whether they constitute different species by sequence comparisons and mating experiments. Therefore, we needed to first determine the mating type of our isolates before setting up mating experiments with isolates of opposing mating type.
Among the
Diaporthe species,
D. longicolla is probably the most important pathogen for soybean as it is the main causal agent of Phomopsis seed decay and is also associated with stem blight [
9,
16,
17]. For
D. longicolla, only asexual reproduction through conidia has been observed, so far. Because of this, the species was known as
Phomopsis longicolla, the name for the anamorph, for a long time. Only recently it was renamed
D. longicolla, according to the rule that there should be only one name for a species or a genus, respectively. In Hosseini et al. [
9], all
D. longicolla isolates were 100% identical, and they also showed full identity to the type strains for
D. longicolla, which indicates strong genetic homogeneity within the species. This homogeneity could be due to the lack of sexual recombination and more or less clonal reproduction. Theoretically, a genetic reason why sexual reproduction does not occur could be that only one mating type is present in the population. This famously happened to
Phytophthora infestans in Europe. Contrary to this expectation, an isolate tested for its mating type in a previous study [
15] showed the presence of both
MAT1-1-1 and
MAT1-2-1, indicating that
D. longicolla should be homothallic and self-fertile. Here, we expected to expand on these results with more isolates.
D. novem has only recently gained species status [
15]. It was found to be heterothallic. Our isolates, which clearly clustered with the
D. novem type strains, did show sequence variation, however. Hence, similar to the situation in
D. eres, successful mating between
D. novem isolates would be very helpful in determining the species limits for
D. novem.
The original aim of this study was to find further genetic differences between our Diaporthe isolates beyond what we already found in ITS, TEF, and TUB genes. These genetic differences could then be correlated to any morphological, physiological, or virulence differences between the isolates. What we describe here are mainly novel findings considering the mating-type distribution in D. longicolla and the mating-type locus in D. caulivora. In addition, we present phylogenies of our isolates based on or amended with sequences of the mating-type genes and the results of our mating experiments.
3. Results
3.1. Mating-Type Analysis Shows That D. longicolla, D. novem, and D. eres Are Heterothallic Species
After producing 32 isolates from
Diaporthe spp. corresponding to
D. caulivora,
D. eres,
D. longicolla, and
D. novem and phylogenetic analysis of the isolates [
9], we set out to further differentiate between those strains that were identical in all three genes (
TUB,
TEF, ITS) used for the phylogeny. One way to differentiate the strains was to check for different mating types. This was performed using the PCR method introduced by Santos et al. [
4]. 30 of the isolates described in [
9] and the three additional isolates listed in
Section 2.1 were included in this study.
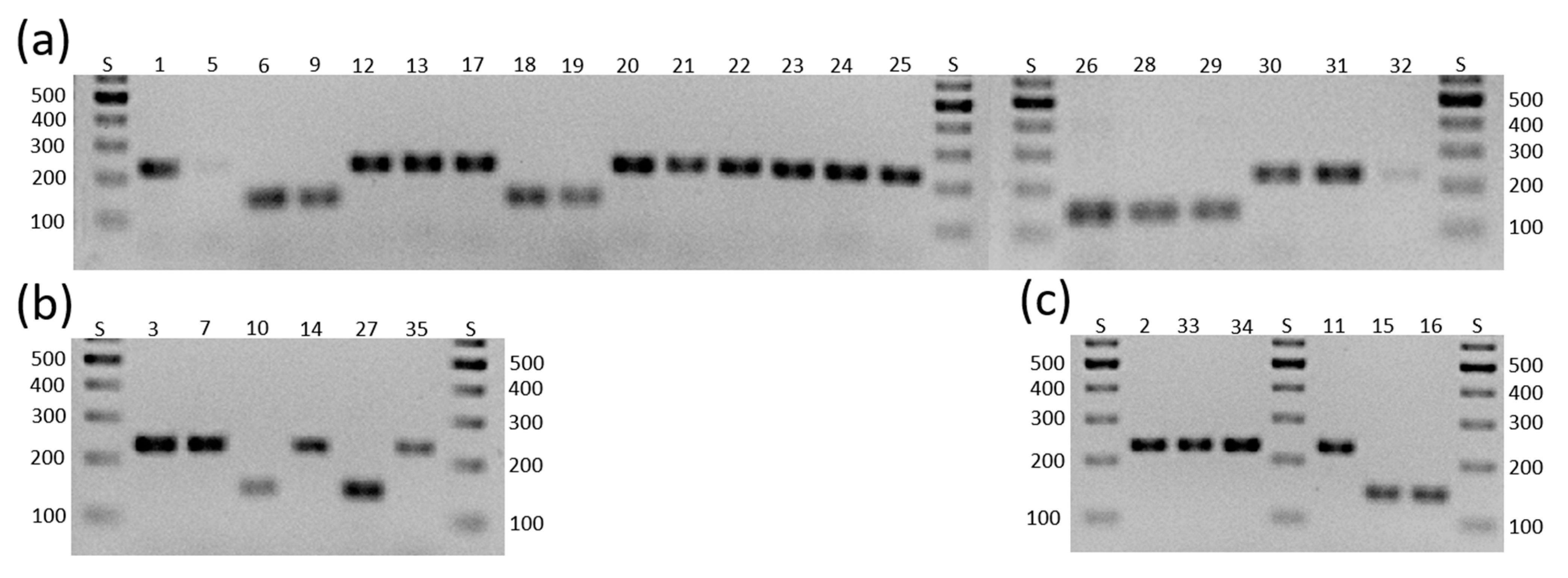
For
D. longicolla, we had successful amplification with the primers for
MAT1-1-1 in seven isolates and with the primers for
MAT1-2-1 in 14 isolates (
Figure 1a). None of the isolates had both mating types. Since for
D. longicolla (also
Phomopsis longicolla) only asexual reproduction has been observed so far, this finding that established
D. longicolla as a heterothallic species was surprising. Also, a previous study by Santos et al. [
15] found both mating-type genes in their
D. longicolla isolate. Because of this, we repeated the experiment with the same result.
To be entirely sure, we also designed primers specific to the
D. longicolla MAT1-1-1 and
MAT1-2-1 genes. For this, we first searched the
D. longicolla genome sequences published by Li et al. [
17] for the two genes and found
MAT1-1-1 in the assembly corresponding to
D. longicolla strain TWH P74 and
MAT1-2-1 in the assembly corresponding to
D. longicolla strain MSPL 10-6 78069. We resequenced parts of
MAT1-1-1 and
MAT1-2-1 in our isolates and found the sequences identical to the published sequences. Based on this, the primers DLmat111_fs and DLmat111_rs and DLmat121_fs and DLmat121_rs were designed (
Table 1). The PCR using these species-specific mating-type primers fully confirmed the finding using the genus-specific primers (
Figure S1). After our observation, it should be only a matter of time until the sexual structures of
D. longicolla will be found.
For
D. eres, two of our isolates had
MAT1-1-1 and four
MAT1-2-1; none had both mating types (
Figure 1b), corroborating
D. eres as a heterothallic species.
For
D. caulivora we could only amplify
MAT1-2-1 in all three isolates. Since Santos et al. [
15] already speculated that their primers just cannot amplify the
D. caulivora MAT1-1-1 gene, we assumed that this is also the case for our isolates and then set out to find the
MAT1-1-1 gene (see
Section 3.2).
In the three isolates representing D. novem, we could amplify MAT1-1-1 twice and MAT1-2-1 once, also in different isolates. This also corroborated D. novem as heterothallic.
3.2. Detection of MAT1-2-1 and MAT1-1-1 in D. caulivora and Sequence Analysis of the Full MAT1/2 Mating Locus of This Homothallic Self-Fertile Species
Using the primers designed by Santos et al. [
4], we were able to only detect
MAT1-2-1 in the
D. caulivora isolates DPC_HOH2, DPC_HOH33, and DPC_HOH34. This was expected, as the same result had also been achieved by this group. That the primers for
MAT1-1-1 could not bind was attributed to insufficient specificity of the primers. However, we found this finding not entirely satisfying; with self-fertile species, it is expected that they have both mating-type genes [
2]. Hence, we decided to also search for
MAT1-1-1.
To further confirm our finding and explore sequence variability within the locus, we re-sequenced the whole
MAT1/2 locus in our isolate DPC_HOH2, including the DNA lyase gene that is closely located and also used as an orientation by [
3] in their sequencing efforts. All primers for sequencing the locus were designed using the sequence by [
26].
The sequence stretch that we sequenced is 13,516 bp long. The sequence was deposited in GenBank with accession No. PP003215. Comparing the sequence from our DPC_HOH2 with that of D57 [
26], we found the sequences to be mostly identical with only a few differences. In positions 808 and 809, DPC_HOH2 has CG, whereas D57 has TC; position 3150 in DPC_HOH2 is A instead of G in D57. From 3765 to 3777 is a stretch of Cs; here DPC_HOH2 has two less than D57. In position 5965 there is a T in DPC-HOH2 instead of an A in D57; in 6074 there is a G instead of an A. After position 11,038, the D57 has 60 bp more than DPC_HOH2 (the exact position of the extra sequence is not definite; 11,038 is the most 5′ position, and the most 3′ position is 11,143; the D57 sequence is repeating itself here). From 11,263 to 11,274 is a stretch of As, which is longer by one A in DPC_HOH2; in 12,822 there is a T instead of a C. Here, the base changes can be interpreted as definite SNPs, whereas the apparent insertions or deletions could also be PCR, sequencing, or assembly (of the D57 sequence) artifacts.
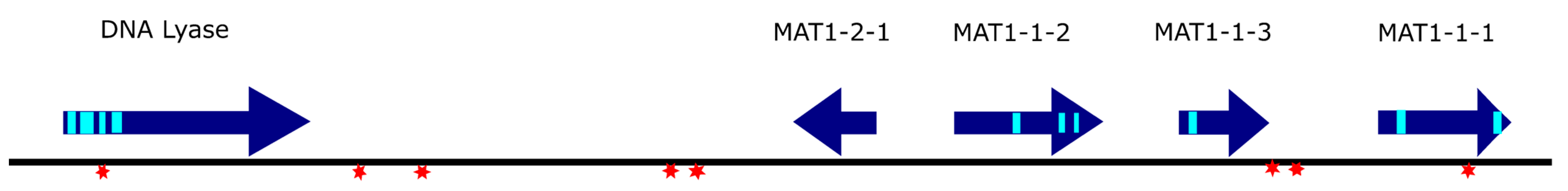
Another intriguing sequence feature came to our attention during primer design for re-sequencing—one sequence stretch of 451 bp occurs twice in the locus. The sequence is once located from 13,027 to 13,477, which means that it starts in the second intron of MAT1-1-1 and spans the third exon and 268 extra bases downstream of the gene. The same sequence is also found in the reverse complement 6953–6503, which means that it is located directly downstream of MAT1-2-1 in the reverse complement. Since MAT1-2-1 is the only gene with reverse orientation in the locus, it is tempting to speculate that this insertion was connected to the event when D. caulivora transitioned to homothallism. To check whether this sequence was also inserted anywhere else in the genome, the D57 genome was BLASTed again with that sequence, and it was found that it occurs exactly twice at those two positions in the genome that we identified. The sequence also has no obvious transposon features, which indicated that this doubling of part of the MAT locus was a singular event.
To produce a definite exon–intron structure of the genes in the locus, we also did cDNA sequencing for all the genes. These results, together with the location of the five genes in the locus, are shown in
Figure 2.
Once all the CDSs were known, we also checked the protein sequences for differences between the two isolates. For MAT1-1-1, no difference was found in the protein sequence; the SNP at position 12,822 is a synonymous exchange. For the other genes, the CDS in the DPC_HOH2 sequence was identical to the corresponding sequence in D57.
3.3. Use of Partial Sequences of MAT1-1-1 and MAT1-2-1 for Further Refinement of Phylogenies of D. caulivora, D. eres, D. longicolla, and D. novem
3.3.1. Isolates and Phylogeny Based on ITS, TEF, and TUB
As detailed in
Section 2.1, this study was conducted using our
Diaporthe strains described in [
9]. From additional isolates acquired in the meantime, we included three in this study and named them DPC_HOH33, DPC_HOH34, and DPC_HOH35. To optimally show the classification of these strains, we integrated them into the phylogeny containing our other isolates. Alignments of the ITS,
TEF, and
TUB sequences were performed and these were concatenated to a three-gene alignment and this way a three-gene phylogeny containing the new strains was built. The phylogeny is shown in
Figure S2. Of the two new
D. caulivora isolates, strain DPC_HOH33 showed 100% identity to DPC_HOH2, whereas DPC_HOH34 was identical to DPC_HOH4. The two pairs of isolates differ in one SNP in the ITS sequence. The new
D. eres isolate sequence-wise was new to our collection with differences from all our previous isolates. These differences were found in the ITS and the
TEF sequences, while in the
TUB gene all isolates have the same sequence. Here, the new isolate once more adds to the sequence variability in
D. eres.
3.3.2. Phylogeny Based on MAT1-1-1 and Combined MAT1-1-1, ITS, TEF, and TUB Phylogeny
After PCR using either the primers by Santos et al. [
4] or alternatively species-specific primers for the gene, we acquired partial sequences for
MAT1-1-1 for most of our isolates. The sequences were submitted to NCBI GenBank. The accession numbers are listed in
Table 2.
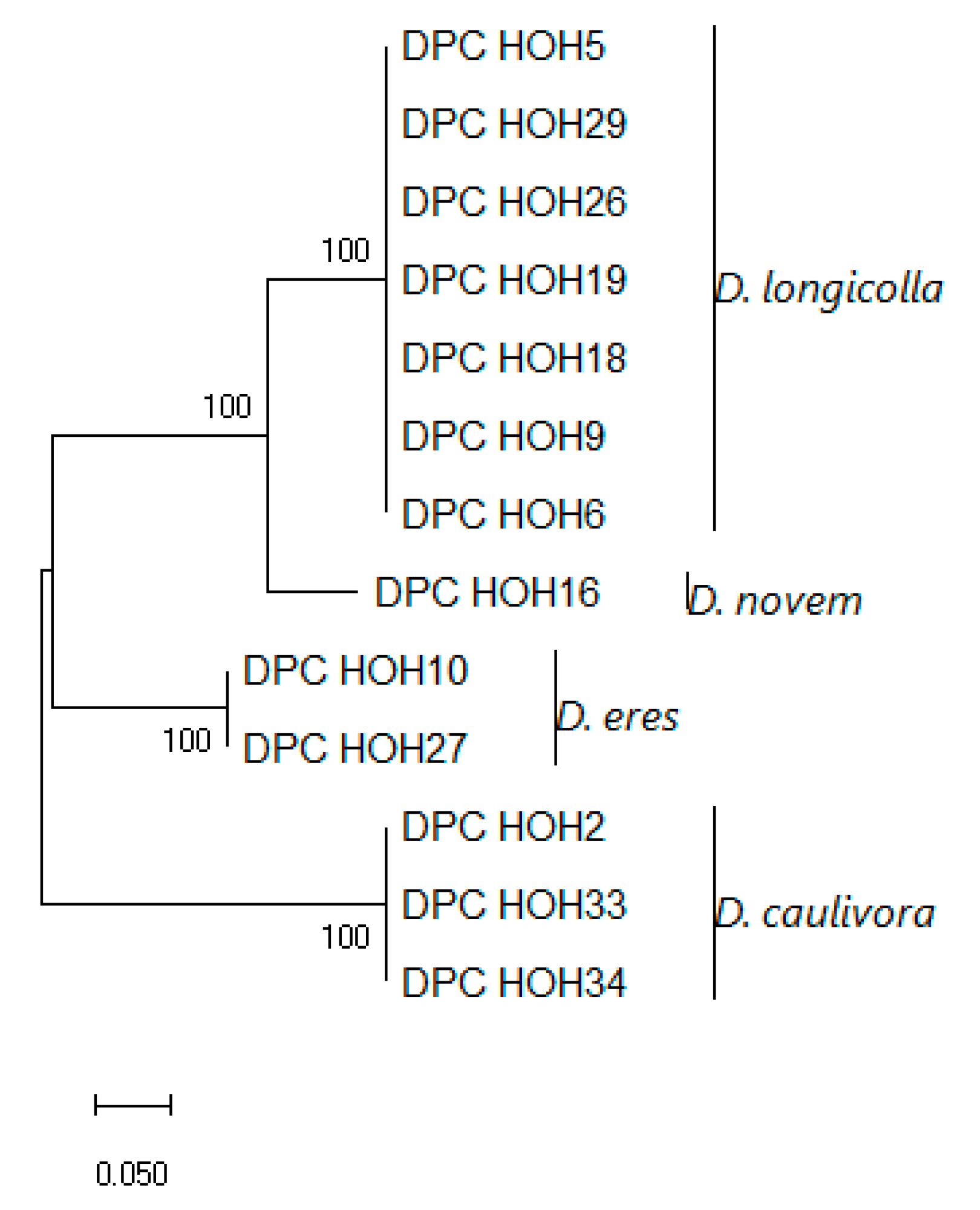
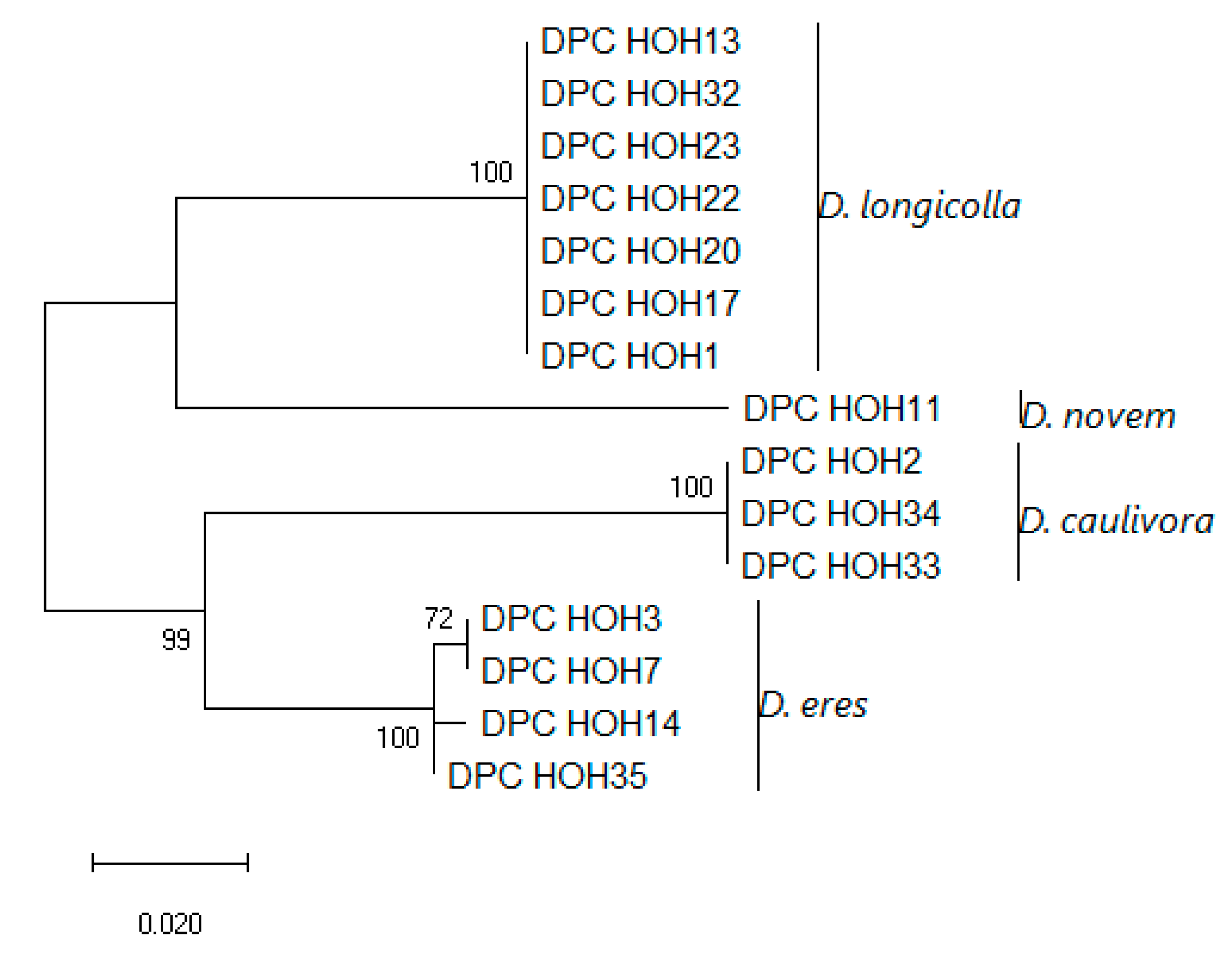
These sequences were aligned and a phylogeny was built on this alignment, which is shown in
Figure 3.
The
MAT1-1-1 sequences show clear differences between the species but are identical within the species, which might indicate that there is little intraspecific variation in these genes. To allow direct comparison to what can be learned from the other genes used for phylogenies, an analysis was also performed in which
MAT1-1-1 was combined with ITS,
TEF, and
TUB (
Figure 4).
Strikingly, the topologies of the trees are identical. Also, there seems to be very little intraspecific variation between the isolates with MAT1-1. With D. eres, it is conspicuous that two isolates that show identity in ITS, TUB, and TEF sequences also have the same mating type. Given the total number of D. eres isolates included in this study, this may be a coincidence, of course.
3.3.3. Phylogeny Based on MAT1-2-1 and Combined MAT1-2-1, ITS, TEF, and TUB Phylogeny
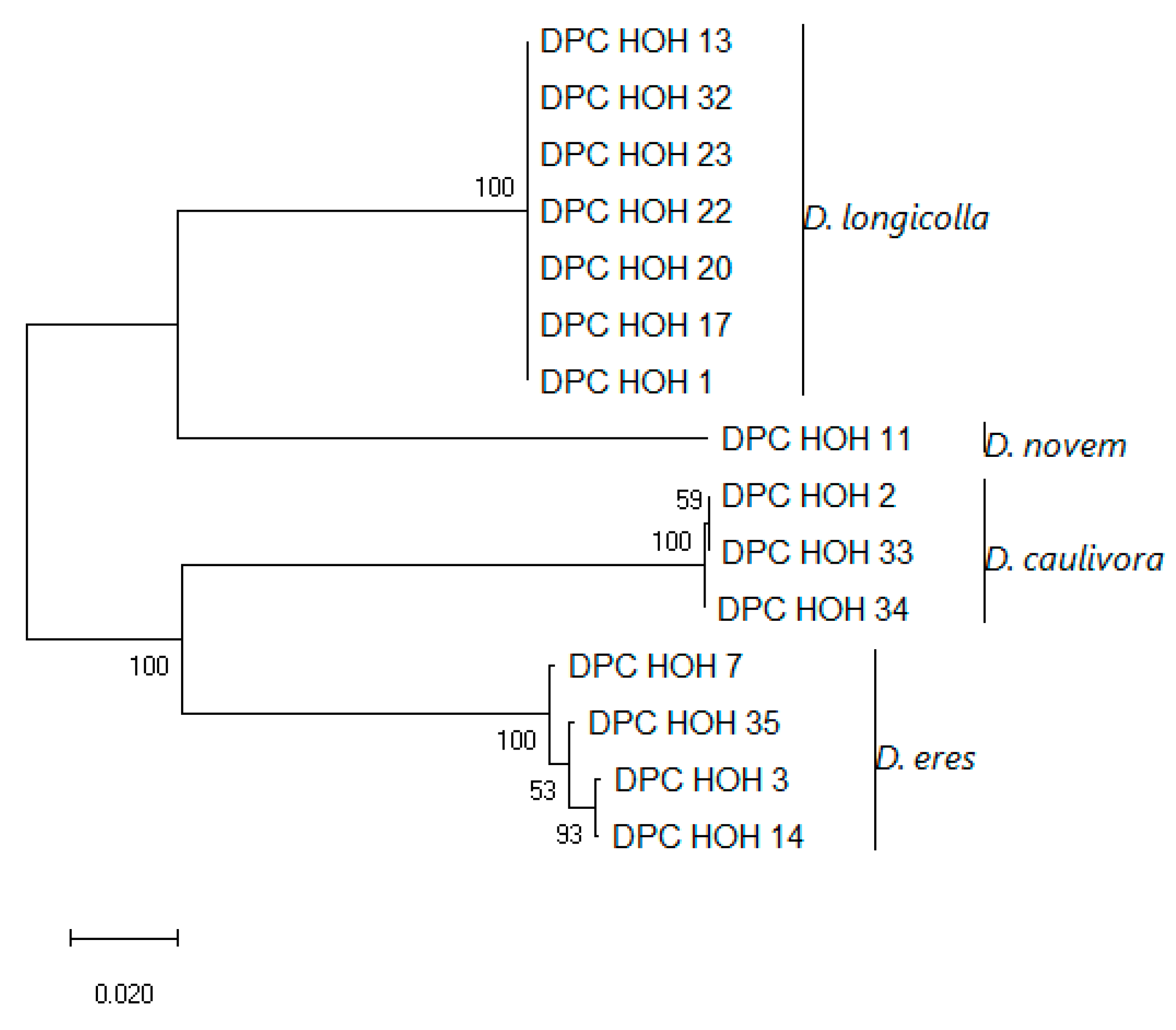
Similar to what was described in
Section 3.3.2, we also acquired partial sequences for
MAT1-2-1 for most of our isolates. The accession numbers are also listed in
Table 2. Also, as described in
Section 3.3.2 for
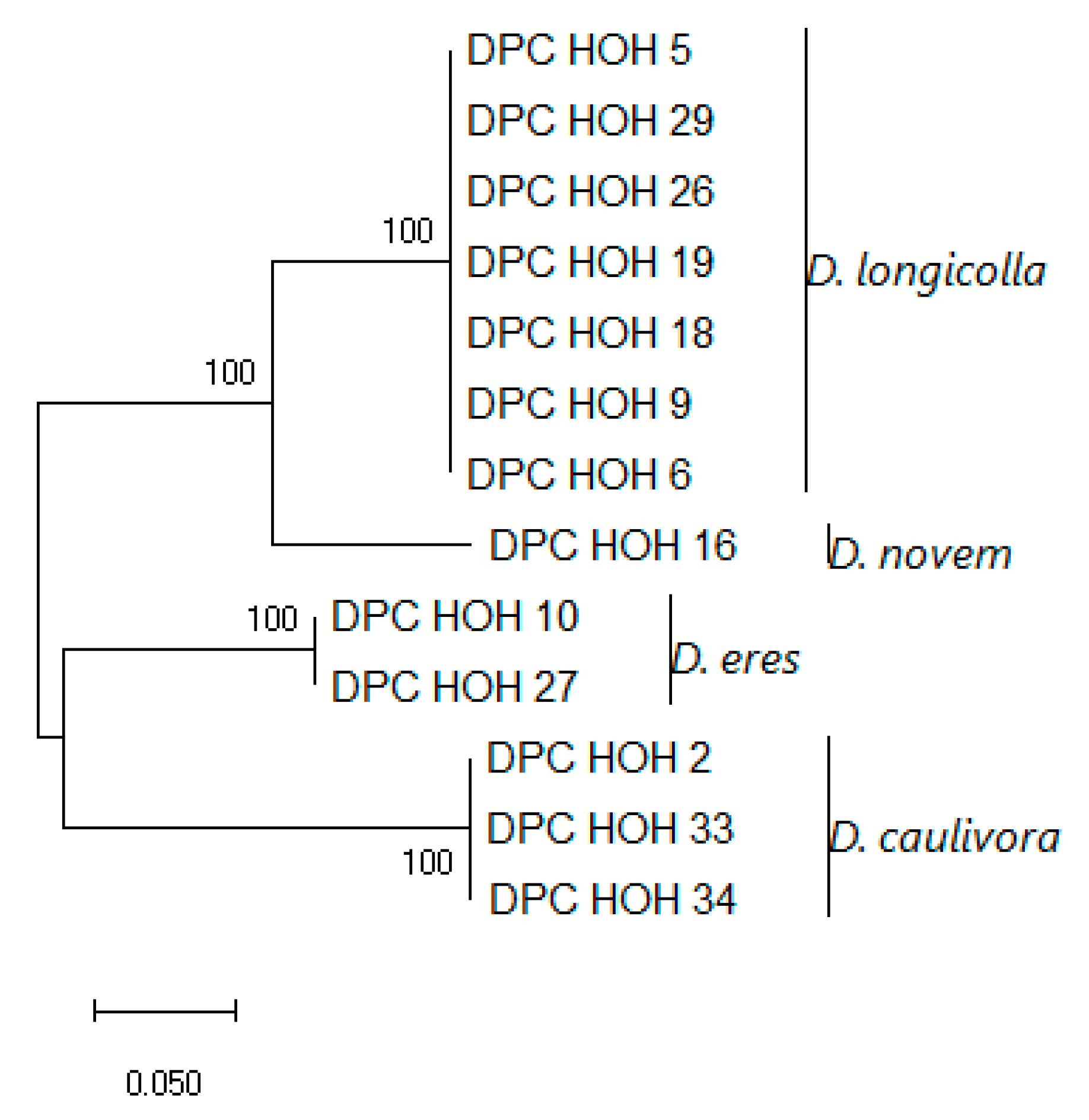
MAT1-1-1, a phylogeny was constructed for the isolates containing
MAT1-2-1 as shown in
Figure 5.
Like MAT1-1-1, MAT1-2-1 allows clear differentiation between the different species. The isolates belonging to each species cluster nicely together. However, for D. eres there exists sequence variation, which again illustrates the status of D. eres as a species complex.
Again, the topology of the combined tree (
Figure 6) is very similar to the topology of the
MAT1-2-1 tree. Except for the branch depicting
D. eres, here the isolates DPC_HOH3 and DPC_HOH7 that are identical in the
MAT1-2-1 sequence do not cluster directly but show up on different branches. According to our previous analysis [
9], the sequence difference between DPC_HOH7 and DPC_HOH3 is mainly in ITS, with only a few different bases in
TEF, whereas in
TUB the sequences are identical, as in
MAT1-2-1. Altogether, the fact that the
D. eres isolates cluster together in all four studied genes strongly indicates that they are closely related; unfortunately, our analysis does not yet resolve the issue of whether they all belong to the same species, i.e., whether
D. eres should be considered a single species or not.
3.4. Mating Experiments
Since we could identify isolates of different mating types in
D. longicolla and for the first time identified the species as heterothallic, we assumed that it should also be possible to mate appropriate isolates of opposing mating type to observe perithecia with asci and ascospores of this species. Accordingly, mating experiments were performed using several different conditions as described in
Section 2.2. In addition to the goal of observing the sexual structures of this species, isolates that successfully mate also belong to the same species, so with this experiment we also aimed to corroborate the phylogenies we inferred from sequence information and included isolates from
D. eres and
D. novem.
Table 3 shows all isolates included in the experiment.
Unfortunately, no sexual structures could be recorded from any of the matings. Only once, in an early experiment, perithecia were seen in a plate on which DPC_HOH17 and DPC_HOH29 were paired. This was a water agar plate with a soybean stem and it was incubated in the dark. Because the significance of the observation had not yet been understood during this phase of the studies, no pictures were taken, so that, as already mentioned, no records exist of any teleomorph structures based on our isolates.
In parallel incubations, D. caulivora readily formed perithecia, indicating that the conditions used by us are overall conducive for sexual reproduction. That D. longicolla did not form perithecia may be attributed to a general reluctance of this species to mate. To identify the sexual structures of this species, it will be necessary to repeat mating experiments until conditions are identified that induce D. longicolla to mate.
The fact that no sexual structures could be observed for D. eres and D. novem could theoretically have different reasons. Either here too we just were not successful in finding the correct trigger for the mating, or our isolates that showed sequence variation in ITS, TEF, and/or TUB actually do not constitute a single species and a reproductive barrier exists between them.